Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Marino B. Arnao and Version 2 by Camila Xu.
Melatonin (N-acetyl-5-methoxytryptamine) is a biogenic amine discovered in 1958 in the pineal gland of cow, and later in humans.
- low nitrogen use
- melatonin
- nitrogen uptake
- nitrogen metabolism
1. Introduction
Nitrogen is the main element that limits plant productivity in crops, with nitrate being the main nitrogen form for plants. The massive use of nitrates and other nitrogen compounds, such as ammonium, has led to serious problems in agricultural soils, such as the high salinity and contamination of aquifers with nitrogen [1][2][3][4][1,2,3,4]. Excess nitrogen in soil usually has negative consequences on plant physiology, such as a lower photosynthetic rate, osmotic stress, nitrogen metabolism disorders, and the excessive appearance of ROS (reactive oxygen species) and RNS (reactive nitrogen species). Furthermore, alterations in the assimilation of other elements such as Ca and Mg cause a lower response in defense against pathogens [5]. Either directly or indirectly, excess nitrates increase ammonium levels in the soil, affecting the overall growth of the plant, both in aerial and root systems [6][7][8][9][6,7,8,9]. Therefore, overuse of nitrogen can result in decreased crop productivity. Therefore, producing more whilst using less nitrogen, the so-called Nitrogen Use Efficiency (NUE), is a recommended practice [10][11][12][10,11,12]. Currently, the use of “smart fertilizers”, such as coated fertilizers, and the application of plant-growth-promoting rhizobacteria (PGPR) to the soil has ostensibly improved the nutrition of nitrogen in plants [13][14][15][13,14,15]. On the other hand, the deficit of nitrogen in soils usually entails great limitations in plant growth and serious nitrogen and carbon metabolic dysfunctions, reducing the photosynthesis and biosynthesis of amino acids and proteins [16][17][16,17].
Melatonin (N-acetyl-5-methoxytryptamine) is a biogenic amine discovered in 1958 in the pineal gland of cow [18], and later in humans [19][20][19,20]. Its properties as a hormone which regulates light/dark cycles and other endogenous rhythms in mammals have been extensively studied. Remarkably, melatonin was identified in plants in 1995 simultaneously by three groups of researchers [21][22][23][21,22,23]. Although initially there were many doubts about the presence of melatonin in plant tissues, today it is one of the most prolific and exciting areas of study within plant physiology [24]. In plants, melatonin has a multi-regulatory role, behaving as a “plant master regulator”, stimulating processes such as seed germination, photosynthesis, growth, and rooting, whilst inhibiting leaf senescence, and regulating fruit ripening and senescence. In addition, melatonin considerably increases tolerance to biotic (bacteria, fungi, and viruses) agents and to abiotic stressors, such as water deficit, extreme temperatures, salinity, contaminants, etc. These aspects are of great interest in its application in crops [25][26][27][28][29][30][31][25,26,27,28,29,30,31].
In plant metabolism, melatonin regulates many primary metabolism pathways, mainly in carbohydrates (starch and sucrose) [32], lipids, and nitrogen compound routes, and also in secondary metabolism pathways (phenolics, flavonoids, and terpenoids) [24]. The broad functions and regulatory capacities of melatonin in plant and animal cells have been studied and their analogies and differences have been compared [33][34][33,34].
2. Melatonin Biosynthesis
In plants, melatonin is synthetized from chorismic acid, which is generated from shikimic acid (a condensation product of phosphoenolpyruvate from glycolysis and erythrose 4-phosphate from the pentose phosphate pathway (Figure 1)). Chorismic acid, a precursor to aromatic amino acids (phenylalanine, tyrosine, and tryptophan), is transformed through the anthranilate/indole pathway to tryptophan [35]. Tryptophan is the origin of the melatonin biosynthesis pathway in both animal and plant cells [33][36][37][33,36,37]. In animals, tryptophan is converted to 5-hydroxytryptophan by TPH, an enzyme that apparently has not been identified in plants. Tryptophan is mainly transformed into tryptamine by TDC present in cytoplasm of plant cells, and then up to serotonin (5-hydroxytryptamine) by T5H (in endoplasmic reticulum) (Figure 1). The transformation of serotonin into melatonin is produced in the chloroplast or cytoplasm depending on the enzyme involved. Thus, serotonin can first be acetylated by SNAT to N-acetylserotonin (in the chloroplast) and subsequently hydroxylated to melatonin (in the cytoplasm) by ASMT/COMT. Under conditions of stress or excess serotonin, 5-methoxytryptamine is formed preferentially by the action of ASMT/COMT [38], and finally melatonin is generated by SNAT (in the chloroplast) [39][40][41][39,40,41]. Melatonin is usually hydroxylated at different positions on the indole ring, with 2-hydroxymelatonin being the major catabolite in plants, showing interesting regulatory properties [42][43][44][42,43,44].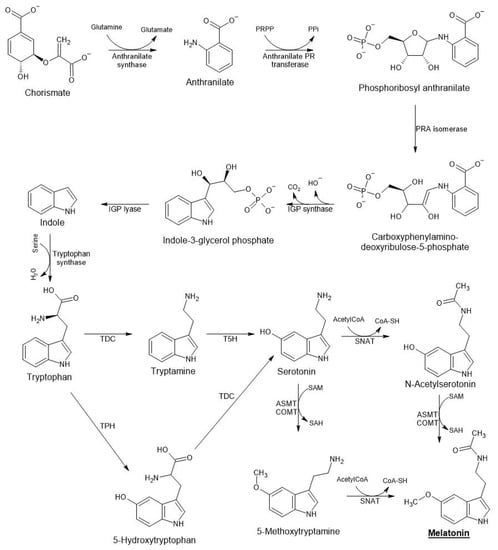
Figure 1.
Pathways of melatonin biosynthesis in plants.
3. Role of Melatonin in Nitrogen Metabolism
The role of melatonin on nitrogen metabolism has been studied under conditions of nitrogen excess and deficiency. Table 1 summarizes several representative examples of the effect of melatonin on nitrogen metabolism in different plant species and conditions. Under nitrogen-excess conditions, melatonin treatments induced a decrease in endogenous nitrogen levels in the form of nitrate and ammonium. In cucumber plants grown with excess nitrate, 100 µM melatonin increased nitrogen tolerance and growth, reorganizing NPK balance and lowering nitrogen damage by reducing nitrate and ammonium levels in seedlings. Furthermore, cucumber melatonin-treated seedlings increased enzyme activities, and nitrate reductase (NR), glutamine synthase (GS), glutamate synthase (GOGAT), and glutamate dehydrogenase (GDH) gene expression, reducing the negative effect of excess nitrate [45]. Also in nitrate excess, melatonin, in co-action with nitric oxide (NO), reversed the excess nitrogen inhibition of root growth, and also up/down-regulated several genes of IAA and ABA metabolism, including melatonin biosynthesis genes [46]. Previously Zhao et al. (2012), also investigating cucumber, demonstrated for the first time, that melatonin increased high-temperature tolerance, regulating nitrate and ammonium levels and nitrogen-related enzymes [47]. The role that melatonin plays in increasing nitrogen-excess tolerance was also studied in alfalfa plants (Table 1). In this case, melatonin increased nitrogen-excess tolerance through the up-regulation of NR, GS, GOGAT, and GDH enzymes. Furthermore, it reduced the total nitrogen levels (nitrate and ammonium content) and increased the biomass, length, width of leaves, and energy levels (P and ATP) and decreased the Na, K, and Ca mineral contents [48]. Similar results were obtained in soybean plants treated with melatonin in nitrogen-excess [49].
Table 1.
Effects on nitrogen metabolism by melatonin in different species.
| Plant Species | Nitrogen Nutrition/Stress | Melatonin Treatment (µM) | Observed Effects | Reference |
|---|---|---|---|---|
| Cucumber | Normal High temperature |
100 | ↑ temperature tolerance, NR, GS, GOGAT, GDH, nitrate, ammonium restrained | [47] |
| Nitrate: N-excess | 100 | ↑ tolerance, growth, NPK balance, Ca, NR, GS, GOGAT, GDH ↓ damage, nitrate, ammonium |
[45] | |
| Nitrate: N-excess | 2 | ↑ tolerance N excess, co-action with NO, lateral roots, root length, Ca, Mg, Fe, melatonin, NO, IAA, ABA, transcription levels of several genes of N metabolism, IAA, ABA and melatonin | [46] | |
| Apple | Normal (urea) Drought stress |
100 | ↑ drought tolerance, growth, photosynthesis, stomatal open, chls, RWC, NR, NiR, GS, GOGAT, N uptake genes (AMTs, NRTs), N, P, K, Ca, Mg, Cu, Zn, and B levels | [50] |
| Alfalfa | Nitrate: N-excess | 100 | ↑ tolerance N excess, shoot height, leaves (length, width, area), P, ATP, biomass, amino acids, energy charge, upregulates NR, GS, GOGAT, GDH ↓ total N, nitrate, ammonium, Na, K, Ca |
[48] |
| Wheat | Nitrate and ammonium: N-low | 1 | ↑ N and nitrate, N absorption, N metabolism, NR, GS, growth, yield, in shoots and roots | [51] |
| Maize | Normal | 100 | ↑ nitrite, nitrate, NR, NiR, GS, GOGAT, GDH ↓ ammonium |
[52] |
| Soybean | Normal Salt/drought stress |
50–100 | ↑ stress tolerance, growth, seed yield and fatty acid; up-regulates cell division, photosynthesis, carbohydrate, fatty acid, and ASC genes | [53] |
| Normal | 100 | ↑ number and size of nodules, fresh shoot biomass in 3 varieties | [54] | |
| Nitrate and ammonium: N-excess, N-normal and N-low | 100 | ↑ tolerance N-excess, N content in N-low, stem diameter, leaf area, nodule number, ATP, biomass in three-N conditions, antioxidant enzymes at N-excess, N-related genes | [49] | |
| Nitrate and ammonium: N-low | 100 | ↑ nodule number, total N fixed, tolerance to N deficiency, upregulating genes: NR2, NiR, GS1β, GOGAT, AAP6a, promoting enzyme activity: NR, GS, GOGAT, GDH, amino acids, protein, total N, chls, seed yield | [55] | |
| Normal Drought stress |
100 | ↑ N, NR, NiR, NRT, GS, GOGAT, GDH, protein, proline, ureides, N transport, growth, biomass | [56] | |
| Normal Drought stress |
100 | ↑ stress tolerance, growth, seed yield, amino acids, photosynthesis, antioxidants, regulates C/N ratio, and plant hormone levels | [57] |
↑ Increased content or action; ↓ Decreased content or action.
In nitrogen-normal conditions, melatonin-treated maize seedlings were found to increase nitrogen content (nitrate and nitrite) and decrease ammonium content with respect to the control plants. Furthermore, NR, NiR GS, GOGAT, and GDH activities and gene expression were up-regulated in melatonin treatments [52]. Unexpectedly, melatonin is also able to favorably regulate nitrogen levels under deficit conditions. Winter wheat grown in a nitrogen-deficit medium was capable of increasing nitrogen uptake and nitrate contents in melatonin-treated seedlings, increasing shoot and root growth, as well as yield, and possibly improving nitrogen metabolism [51]. Also in soybean plants, the role of melatonin in nitrogen-low conditions has been studied. In nitrate and ammonium deficiency, melatonin improved plant tolerance, increasing the total number of nodules and fixed nitrogen, and up-regulating several nitrogen-related gene expressions (see Table 1), with the result of an increase in the levels of amino acids, proteins, chlorophyll, and also an increase in seed yield [49][55][49,55].
Moreover, in different stress conditions, such as drought stress, melatonin improved stress tolerance and nitrogen uptake. It also improved the contents of amino acid, protein, proline, and ureides, whilst up-regulating NR, NiR, NRT, GS, GOGAT, and GDH gene expression, consequently improving the growth and total biomass of soybean plants [56]. The authors suggested that melatonin regulated the assimilation, metabolism and transport of nitrogen, thereby maintaining the carbon/nitrogen balance [48][50][58][59][48,50,58,59].
