| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Samuel Legeay | + 1931 word(s) | 1931 | 2020-03-12 09:42:10 | | | |
| 2 | Rita Xu | -190 word(s) | 1741 | 2020-03-13 03:08:57 | | | | |
| 3 | Rita Xu | -109 word(s) | 1632 | 2020-10-26 04:40:03 | | |
Video Upload Options
Polyphenols consumption has been associated to a lower risk of cardiovascular diseases (CVDs) notably through nitric oxide (NO)- and estrogen receptor α (ERα)-dependent pathways. Among polyphenolic compounds, chalcones have been suggested to prevent endothelial dysfunction and hypertension. However, the involvement of both the NO and the ERα pathways for the beneficial vascular effects of chalcones has never been demonstrated. In this study, we aimed to identify chalcones with high vasorelaxation potential and to characterize the signaling pathways in relation with ERα signaling and NO involvement. The evaluation of vasorelaxation potential was performed by myography on wild-type (WT) and ERα knock-out (ERα-KO) mice aorta in presence or in absence of the eNOS inhibitor Nω-nitro-L-arginine methyl ester (L-NAME). Among the set of chalcones that were synthesized, four exhibited a strong vasorelaxant effect (more than 80% vasorelaxation) while five compounds have shown a 60% relief of the pre-contraction and four compounds led to a lower vasorelaxation. We were able to demonstrate that the vasorelaxant effect of two highly active chalcones was either ERα-dependent and NO-independent or ERα-independent and NO-dependent.
1. Chemistry and cLogP
Synthetic polyhydroxylated chalcones 3, 6, 8 and 10 were synthesized through a two-step procedure starting from the corresponding methoxymethyl (MOM)-protected acetophenones and benzaldehydes. Although this strategy required an extra deprotection step compared to Claisen-Schmidt condensation of phenolic starting materials, it has led to a better overall yield as observed for the synthesis of chalcone 8 compared to literature data [1][2]. Due to reactivity and efficiency issues in basic medium, chalcones 11, 13 and 17 bearing a hydroxyl (OH) group in the C-4 position have been prepared through an acid-catalyzed coupling mechanism using thionyl chloride (SOCl2) in ethanol (EtOH). Thus it avoided the need for a synthesis strategy with a MOM protection-deprotection approach [3]. Nevertheless, in situ generated hydrochloric acid (HCl) catalysis was unsuccessful when applied to the synthesis of diphenolic chalcone 18. Thus, it has been prepared under microwave (MW) irradiations in the presence of piperidine [4]. In this work two other synthetic chalcones 12 and 14, bearing a phenol function on the C-2’ position, have been prepared through selective demethylation of the corresponding 2’-methoxychalcones 13 and 15 in the presence of aluminum chloride (AlCl3) [5]. It is worth mentioning that the demethylation of hexamethoxychalcone 16 led only to 17 (52% yield) without any trace of 18 (Scheme 1). Finally, the calculated octanol-water partition coefficient (cLogP) were similar, ranging from 1.80 for synthetic chalcone 6 to 3.33 for synthetic chalcone 14 (Table 1).
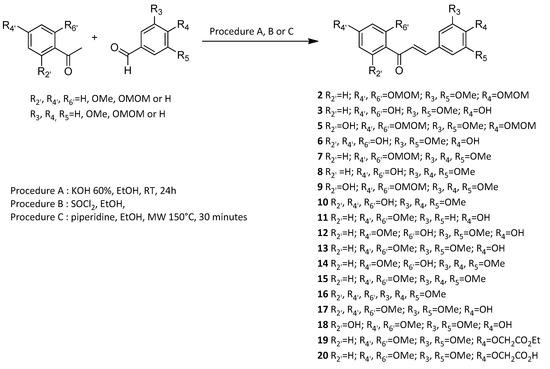
Scheme 1. General scheme for the synthesis of chalcones. Synthetic chalcones were synthesized through the coupling of the corresponding either MOM-protected or phenolic acetophenones and benzaldehydes. Hydrolysis of MOM groups or selective demethylation in the presence of AlCl3 were achieved. These procedures led to thirteen synthetic polyoxygenated chalcones whose vasorelaxant effect was evaluated.
Table 1. Synthesized chalcones and their related cLogP.
|
Compound |
R2’ |
R4’ |
R6’ |
R3 |
R4 |
R5 |
cLogP |
|
3 |
OH |
OH |
H |
OCH3 |
OH |
OCH3 |
2.42 |
|
6 |
OH |
OH |
OH |
OCH3 |
OH |
OCH3 |
1.80 |
|
8 |
OH |
OH |
H |
OCH3 |
OCH3 |
OCH3 |
2.75 |
|
10 |
OH |
OH |
OH |
OCH3 |
OCH3 |
OCH3 |
2.13 |
|
11 |
OCH3 |
OCH3 |
H |
H |
OH |
H |
3.20 |
|
12 |
OH |
OCH3 |
H |
OCH3 |
OH |
OCH3 |
2.99 |
|
13 |
OCH3 |
OCH3 |
H |
OCH3 |
OH |
OCH3 |
2.84 |
|
14 |
OH |
OCH3 |
H |
OCH3 |
OCH3 |
OCH3 |
3.33 |
|
15 |
OCH3 |
OCH3 |
H |
OCH3 |
OCH3 |
OCH3 |
3.17 |
|
16 |
OCH3 |
OCH3 |
OCH3 |
OCH3 |
OCH3 |
OCH3 |
3.13 |
|
17 |
OCH3 |
OCH3 |
OCH3 |
OCH3 |
OH |
OCH3 |
2.79 |
|
18 |
OH |
OCH3 |
OCH3 |
OCH3 |
OH |
OCH3 |
2.97 |
|
20 |
OCH3 |
OCH3 |
H |
OCH3 |
OCH2COOH |
OCH3 |
2.46 |
2. Evaluation of Vasorelaxant Activity
Vasorelaxation induced by each synthetic chalcone was first evaluated on WT mice thoracic aorta rings. Compounds 3, 8, 13 and 15 with identical substituents at C-2’ and C-4’ positions, absence of substituent at the C-6’ position and presence of OH and/or OCH3 groups at C-3, C-4 and C-5 positions induced a high vasorelaxation reaching more than 80%. Compounds 6, 10, 11, 16 and 17 with OH or OCH3 group at the C-6’ position or unsubstituted at C-3 and C-5 positions induced an average vasorelaxation between 50% to 80%. Finally, compounds 12, 14, 18 and 20 with both OH and OCH3 groups at C-2’, C-4’ and/or C-6’ positions or a bulkier OCH2COOH group at C-3 position exerted a low vasorelaxation effect below 50% (Figure 1 and Table 2).
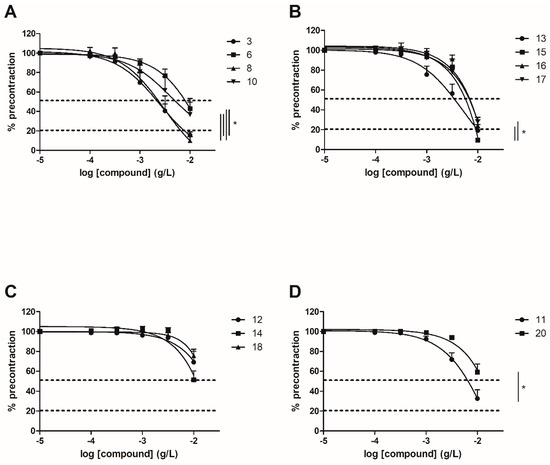
Figure 1. Concentration-response curves for the vasorelaxation effect on WT mice thoracic aorta of synthetic polyoxygenated chalcones. A: vasorelaxation effect of chalcones with R2’ = R4’ = OH and R6’ = H or OH; B: vasorelaxation effect of chalcones with R2’ = R4’ = OCH3 and R6’ = H or OCH3; C: vasorelaxation effect of chalcones with R2’ = OH, R4’ = OCH3 and R6’ = H or OCH3; D: vasorelaxation effect of chalcones with R2’ = R4’ = OCH3 and R6’ = H. Mice were ovariectomized 7 days before the experiment. Vessels were pre-contracted with U46619, phenylephrine, and serotonin, at 80 % of their maximal response. The presence of functional endothelium was assessed by determining the ability of acetylcholine (10 µM) to induce more than 50% relaxation of pre-contracted rings. Results are expressed in percentage of precontraction (y-axis) following the concentration (x-axis). Compounds 3, 8, 13 and 15 induced higher vasorelaxation, over 80%. Compounds 6, 10, 11, 16 and 17 induced an average vasorelaxation between 50% to 80%. Compounds 12, 14, 18 and 20 exerted a low vasorelaxation effect below 50%. N = 4–7, * p < 0.05 (Mann-Whitney).
Table 2. Summary of the potency and efficacy of chalcones.
|
Compound |
Potency (EC50, g/L) WT |
Efficacy (Emax, %) |
|||
|
WT |
WT with L-NAME |
ERα KO |
ERα KO with L-NAME |
||
|
3 |
2.44 × 10-3 |
84.28 ± 3.24a,d |
77.62 ± 4.92 |
56.23 ± 4.91£ |
43.22 ± 6.10$ |
|
6 |
2.12 × 10-3 |
57.10 ± 10.40c,d |
33.92 ± 12.66 |
60.69 ± 10.75 |
38.15 ± 10.91 |
|
8 |
2.99 × 10-3 |
89.96 ± 4.13b,c |
86.69 ± 3.47 |
79.73 ± 3.71 |
69.76 ± 7.99 |
|
10 |
4.08 × 10-3 |
63.29 ± 12.79a,b |
44.91 ± 15.07 |
52.83 ± 12.88 |
43.33 ± 10.37 |
|
11 |
1.93 × 10-2 |
67.35 ± 8.94g |
49.06 ± 9.47 |
74.81 ± 2.07 |
36.40 ± 5.81 |
|
12 |
NA |
30.95 ± 13.23 |
NA |
NA |
NA |
|
13 |
5.06 × 10-2 |
80.86 ± 6.11 |
19.85 ± 7.81* |
81.96 ± 2.46 |
26.96 ± 6.72 |
|
14 |
NA |
48.38 ± 7.75 |
NA |
4.64 ± 9.95 |
NA |
|
15 |
NA |
90.59 ± 1.56e,f |
76.07 ± 3.72 |
79.78 ± 5.51 |
76.74 ± 5.68 |
|
16 |
NA |
75.89 ± 5.99f |
NA |
74.91 ± 10.15 |
NA |
|
17 |
NA |
72.16 ± 4.59e |
NA |
86.88 ± 4.40 |
NA |
|
18 |
NA |
24.34 ± 4.60 |
NA |
NA |
NA |
|
20 |
NA |
40.77 ± 8.22g |
NA |
NA |
NA |
3. Involvement of ERα and NO Pathways
3.1. Evaluation of Vasorelaxant Activity on ERα KO Mice Aorta
To evaluate the involvement of the ERα pathway in the chalcones-induced vasorelaxation, experiments have been conducted on ERα KO mice aortas. To focus on the role of OH or OCH3 groups at C-6’ position with synthetic chalcones exhibiting a high vasorelaxant effect, compounds 3, 6, 8, 10, 13, 15, 16 and 17 have been tested on ERα KO mice thoracic aorta rings. Interestingly, only the synthetic chalcone 3 induced a significant lower vasorelaxation in ERα KO mice thoracic aorta rings compared to WT mice thoracic aorta rings (56.23 ± 4.91% vs. 84.28 ± 3.24%, respectively) while no significant differences were observed with synthetic chalcones 6, 8, 10, 13, 15, 16 and 17 (Figure 2 and Figure 3 and Table 2).
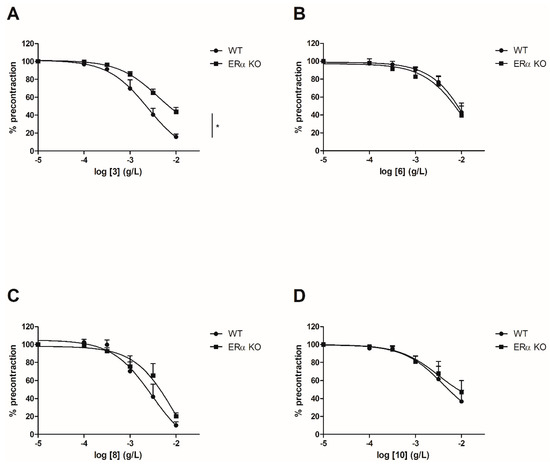
Figure 2. Concentration-response curves for the vasorelaxation effect on both WT and ERαKO mice thoracic aorta of synthetic chalcones 3 (A), 6 (B), 8 (C) and 10 (D). Mice were ovariectomized 7 days before the experiment. Vessels were pre-contracted with U46619, phenylephrine, and serotonin, at 80 % of their maximal response. The presence of functional endothelium was assessed by determining the ability of acetylcholine (10 µM) to induce more than 50% relaxation of pre-contracted rings. Results are expressed in percentage of precontraction (y-axis) following the concentration (x-axis). Only the synthetic chalcone 3 induced a significant lower vasorelaxation in ERα KO mice thoracic aorta rings compared to WT mice thoracic aorta rings (56.23 ± 4.91% vs. 84.28 ± 3.24% respectively) while no significant differences were observed with chalcones 6, 8 and 10. N = 4–7, * p < 0.05 (Mann-Whitney).
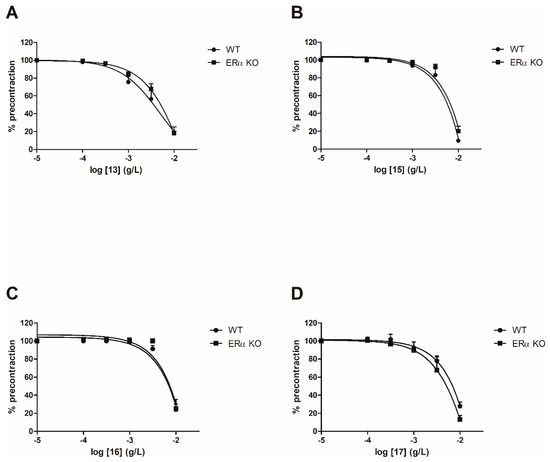
Figure 3. Concentration-response curves for the vasorelaxation effect on both WT and ERαKO mice thoracic aorta of synthetic chalcones 13 (A), 15 (B), 16 (C) and 17 (D). Mice were ovariectomized 7 days before the experiment. Vessels were pre-contracted with U46619, phenylephrine, and serotonin, at 80 % of their maximal response. The presence of functional endothelium was assessed by determining the ability of acetylcholine (10 µM) to induce more than 50% relaxation of pre-contracted rings. Results are expressed in percentage of precontraction (y-axis) following the concentration (x-axis). No significant differences were observed with synthetic chalcones 13, 15, 16 and 17. N = 4–7 (Mann-Whitney).
3.2. Evaluation of Vasorelaxant Activity in the Presence of Nω-nitro-L-arginine methyl ester (L-NAME)
In order to evaluate the involvement of the NO pathway, experiments have then been performed after incubation for 20 min with NOS inhibitor (10−4 M) on both WT and ERα KO mice thoracic aorta rings. To focus on the role of groups at C-2’, C-4’ and C-4 positions, synthetic chalcones 3, 8, 13 and 15 have been tested. Interestingly, L-NAME prevented the chalcone-induced vasorelaxation only for chalcone 13 in WT mice thoracic aorta rings at the highest concentration (10−2 g/L, Figure 4 and Table 2). It is noteworthy that L-NAME seemed to prevent the chalcone-induced vasorelaxation for compounds 3 and 8 at low concentrations and this effect is not recovered at 10−2 g/L. No difference was observed for the synthetic chalcone 15 (Figure 4A,B). Similarly, the presence of L-NAME on ERα KO mice thoracic aorta rings prevented the vasorelaxation only with the synthetic chalcone 13 (81.96 ± 2.46% vs. 26.96 ± 6.72%) while having no effect with synthetic chalcones 3, 8 and 15 (Figure 5 and Table 2).
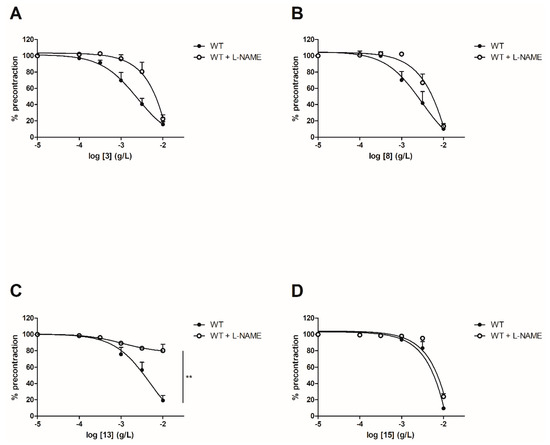
Figure 4. Concentration-response curves for the vasorelaxation effect on WT mice thoracic aorta of synthetic chalcones 3 (A), 8 (B), 13 (C) and 15 (D) in the presence or in absence of L-NAME, a selective eNOS inhibitor. Mice were ovariectomized 7 days before the experiment. Vessels were pre-contracted with U46619, phenylephrine, and serotonin, at 80 % of their maximal response. The presence of functional endothelium was assessed by determining the ability of acetylcholine (10 µM) to induce more than 50% relaxation of pre-contracted rings. Results are expressed in percentage of precontraction (y-axis) following the concentration (x-axis). L-NAME only prevented the chalcone-induced vasorelaxation for the synthetic chalcone 13 at the highest concentration (10−2 g/L) and seemed to prevent the chalcone-induced vasorelaxation for compounds 3 and 8 at low concentrations. No difference was observed for chalcone 15. N = 5–7, ** p < 0.01 (Mann-Whitney).
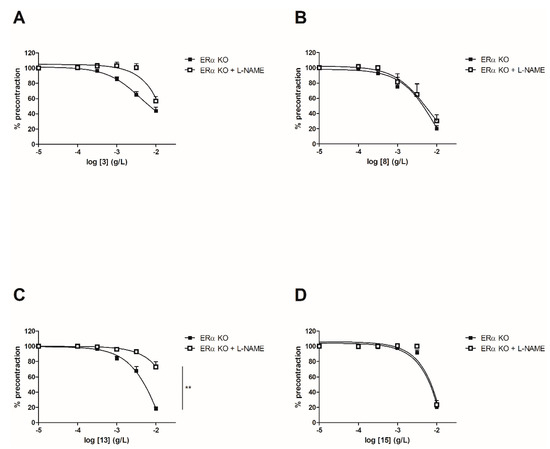
Figure 5. Concentration-response curves for the vasorelaxation effect on ERαKO mice thoracic aorta of synthetic chalcones 3 (A), 8 (B), 13 (C) and 15 (D). Mice were ovariectomized 7 days before the experiment. Vessels were pre-contracted with U46619, phenylephrine, and serotonin, at 80 % of their maximal response. The presence of functional endothelium was assessed by determining the ability of acetylcholine (10 µM) to induce more than 50% relaxation of pre-contracted rings. Results are expressed in percentage of precontraction (y-axis) following the concentration (x-axis). L-NAME only prevented the chalcone 13-induced vasorelaxation (81.96 ± 2.46% vs. 26.96 ± 6.72%) while having no effect with synthetic chalcones 3, 8 and 15. N = 4–7, ** p < 0.01 (Mann-Whitney).
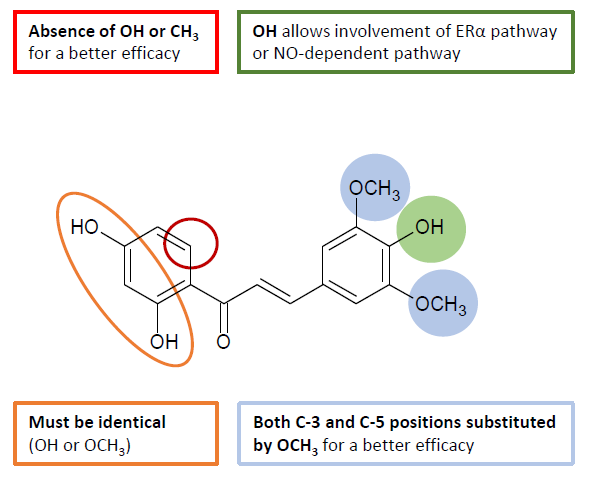
Structure-activity relationships (SAR) study allowed to identify the 3,5-dimethoxy-4-hydroxybenzene moiety as a pharmacophore for both a ERα- or NO-dependent vasorelaxation (Figure 6). Finally, the current study provides new promising data for the preventive and the therapeutic use of chalcones in the field of CVDs.
Figure 6. Structure-activity relationship of chalcones for vasodilation.
References
- Marta Perro Neves; Sara M. Cravo; Raquel T. Lima; M. Helena Vasconcelos; M. São José Nascimento; Artur Silva; Madalena Pinto; Honorina Cidade; Arlene Correa; Solid-phase synthesis of 2′-hydroxychalcones. Effects on cell growth inhibition, cell cycle and apoptosis of human tumor cell lines. Bioorganic & Medicinal Chemistry 2012, 20, 25-33, 10.1016/j.bmc.2011.11.042.
- Yesseny Vásquez-Martínez; Mauricio Osorio; Diego San Martín; Marcela Carvajal; Alejandra Vergara; Elizabeth Sanchez; Marcela Raimondi; Susana Zacchino; Carolina Mascayano; Claudia Torrent; Francisco Cabezas; Sophia Mejias; Margarita Montoya; Marcelo Cortez-San Martín; Antimicrobial, Anti-Inflammatory and Antioxidant Activities of Polyoxygenated Chalcones. Journal of the Brazilian Chemical Society 2018, null, , 10.21577/0103-5053.20180177.
- Ognyan Petrov; Yordanka Ivanova; Mariana Gerova; SOCl2/EtOH: Catalytic system for synthesis of chalcones. Catalysis Communications 2008, 9, 315-316, 10.1016/j.catcom.2007.06.013.
- Marina Roussaki; Belinda Hall; Sofia Costa Lima; Anabela Cordeiro Da Silva; Shane R. Wilkinson; Anastasia Detsi; ChemInform Abstract: Synthesis and anti-Parasitic Activity of a Novel Quinolinone-Chalcone Series.. ChemInform 2014, 45, , 10.1002/chin.201415168.
- Anupama Shirali; Madhavi Sriram; John J. Hall; Benson L. Nguyen; Rajsekhar Guddneppanavar; Mallinath B. Hadimani; J. Freeland Ackley; Rogelio Siles; Christopher J. Jelinek; Phyllis Arthasery; Rodney C. Brown; Victor Leon Murrell; Austin McMordie; Suman Sharma; David J. Chaplin; Kevin Pinney; Sanjay Sharma; Development of Synthetic Methodology Suitable for the Radiosynthesis of Combretastatin A-1 (CA1) and Its Corresponding Prodrug CA1P†. Journal of Natural Products 2009, 72, 414-421, 10.1021/np800661r.