Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Prawej Ansari | -- | 2351 | 2022-12-01 12:43:19 | | | |
| 2 | Dean Liu | Meta information modification | 2351 | 2022-12-02 03:20:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ansari, P.; Akther, S.; Khan, J.T.; Islam, S.S.; Masud, M.S.R.; Rahman, A.; Seidel, V.; Abdel-Wahab, Y.H.A. Diabetic Foot Complications. Encyclopedia. Available online: https://encyclopedia.pub/entry/37666 (accessed on 13 January 2026).
Ansari P, Akther S, Khan JT, Islam SS, Masud MSR, Rahman A, et al. Diabetic Foot Complications. Encyclopedia. Available at: https://encyclopedia.pub/entry/37666. Accessed January 13, 2026.
Ansari, Prawej, Samia Akther, Joyeeta T. Khan, Sara S. Islam, Md. Samim R. Masud, Anisur Rahman, Veronique Seidel, Yasser H. A. Abdel-Wahab. "Diabetic Foot Complications" Encyclopedia, https://encyclopedia.pub/entry/37666 (accessed January 13, 2026).
Ansari, P., Akther, S., Khan, J.T., Islam, S.S., Masud, M.S.R., Rahman, A., Seidel, V., & Abdel-Wahab, Y.H.A. (2022, December 01). Diabetic Foot Complications. In Encyclopedia. https://encyclopedia.pub/entry/37666
Ansari, Prawej, et al. "Diabetic Foot Complications." Encyclopedia. Web. 01 December, 2022.
Copy Citation
Diabetes mellitus, a major cause of mortality around the globe, can result in several secondary complications, including diabetic foot syndrome, which is brought on by diabetic neuropathy and ischemia. Approximately 15% of diabetic patients suffer from diabetic foot complications, and among them 25% are at risk of lower limb amputations. Diabetic foot ulcers are characterized as skin lesions, gangrene, or necrosis, and may develop due to several reasons, including hyperglycemia and slower wound healing in diabetic patients.
hyperglycemia
diabetic foot
infection
glucose
1. Introduction
Diabetes mellitus (DM) is considered a worldwide epidemic, being one of the top ten diseases causing mortality globally and affecting more than 10.5% of the adult population [1][2]. It is a chronic metabolic disorder manifested by persistent high blood glucose levels occurring due to deficiencies in insulin production, insulin resistance, or both [3]. According to its etiology and pathogenesis, diabetes mellitus can be broadly classified into two major types: type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) [4]. T1DM is a result of the autoimmune destruction of the pancreatic β-cells of the islets of Langerhans, leading to significantly diminished insulin secretion, whereas T2DM, often associated with obesity and an unhealthy lifestyle, is the outcome of either impaired insulin secretion, decreased insulin sensitivity to cells, or both [5][6]. The long-term secondary complications of diabetes include coronary heart disease, peripheral artery disease, cardiomyopathy, stroke, and cerebrovascular disease, as well as diabetic retinopathy (which causes visual impairment), diabetic nephropathy (which leads to kidney failure), and diabetic neuropathy (which increases the risk of foot ulcers) [7][8][9]. Individuals with long-term DM are prone to these complications, which may lead to increased morbidity and mortality [10].
Diabetic foot is a major complication of DM that affects approximately 15% of diabetic patients, with 25% of them facing the possibility of lower limb amputation [11][12][13]. Diabetic patients are often hospitalized due to the consequences of foot-related conditions such as infections, ulcers, and gangrene or foot necrosis [14]. The ulceration is the starting point for a severe stage of the disease, and if left untreated, this can lead to amputations. Diabetic neuropathy, neuro-ischemia, and infections all have an impact on whether lesions heal or deteriorate, and amputations in diabetic patients are often precipitated by a foot ulcer that progresses to serious gangrene or infection [12].
To accelerate the healing process of diabetic foot ulcerations, various interventions such as wound cleansing, revascularization, antibiotic therapy, dead tissue removal, and in extreme situations surgery have been implemented [15]. The conventional therapy for treating diabetic foot is often impracticable, particularly in underdeveloped countries, because of the high costs, adverse side effects, and the unavailability of vascular surgeons. In recent years, there has been a surge of interest in the search for alternative medications derived from natural resources, especially medicinal plants, for the prevention and treatment of diabetic foot [16][17].
Since ancient times, herbal remedies have been used as effective therapies to treat a wide range of ailments, including diabetic foot [16]. Recent findings have revealed that biologically-active phytomolecules present in plants demonstrate various pharmacological effects that help to prevent different forms of cell damage, including chronic wounds [17]. Numerous medicinal plants, including Aloe vera, Annona squamosa, Azadirachta indica, Carica papaya, Catharanthus roseus, Centella asiatica, Curcuma longa, Hylocereus undatus, and Punica granatum, have exhibited potential wound healing, anti-inflammatory, antioxidative, antibacterial, and other antidiabetic properties with little to no side effects, and might be considered as possible effective treatments for diabetic injuries [18][19][20]. Several bioactive phytomolecules such as quercetin, kaempferol, isoquercetrin, apigenin, tangeretin, naringenin, luteolin, catechin, gallic acid, methyl gallate, and rutin present in medicinal plants including Allium cepa, Beta vulgaris, Citrus sinensis, Schinus polygamus, and Sorghum bicolor exert their antidiabetic action via different mechanistic pathways to improve β-cell function and insulin secretion; enhance insulin sensitivity; increase GLUT-4 expression; decrease gluconeogenesis; and inhibit α-amylase, α-glucosidase, and DPP-IV activity and the formation of advanced glycation end products. Such phytochemicals not only ameliorate diabetic foot wounds but also contribute to the overall antidiabetic action of the medicinal plants to prevent and manage other diabetes-related complications [21][22]. Thus, due to the abundance of bioactive compounds in plants, researchers have directed their focus toward examining the significance of medicinal plants and isolating their phytoconstituents to assess their prospective wound-healing properties [18].
2. Types of Diabetic Foot Complications
Diabetic foot ulcers can be classified depending on the degree of tissue loss, size, perfusion, infection site, depth, area, and sensation [23]. The changes in these parameters are based on an individual’s age, sex, medical conditions, and existing comorbidities, including a loss of peripheral sensation (LOPS) and peripheral arterial diseases (PAD), which ultimately categorize diabetic foot ulcers into different grades [24].
According to Meggitt and Wagner, diabetic foot ulcers can be classified into 6 grades (Figure 1):
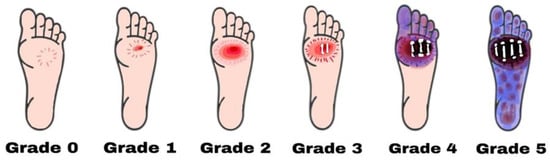
Figure 1. Diabetic foot ulcers are categorized using the Wagner–Meggitt classification. Grade 0 means no break in the skin. Grade 1 indicates a superficial ulcer. Grade 2 indicates a deep ulcer. Grade 3 shows the presence of osteomyelitis. Grade 4 is identified as forefoot gangrene. Grade 5 is recognized as complete foot exposure to gangrene.
-
Grade zero: No lesion on the skin, potential bone deformity or hyperkeratotic lesion, pain;
-
Grade one: Superficial viable or necrotic ulcers; subcutaneous tissue loss; potential bone deformity; no penetration into the deeper layers of the skin;
-
Grade two: Deeper penetrates including bones, tendons, ligaments, or deep fascia; bone deformity prominent in some aspects; absence of abscess or osteomyelitis;
-
Grade Three: Presence of osteomyelitis, deep abscess, or tendinitis; severe infection symptoms (e.g., redness, heat, and swelling);
-
Grade four: Gangrene (dry, wet, infected, or non-infected) in toe or forefoot; surgical ablation of the foot required with minimal blood supply for below-knee amputations;
Another scientist, named Brodsky, later discovered that the grade 4 and grade 5 foot ulcers in Wagner–Meggitt’s classification were ischemic, and revised the classification in the following manner (Figure 2):
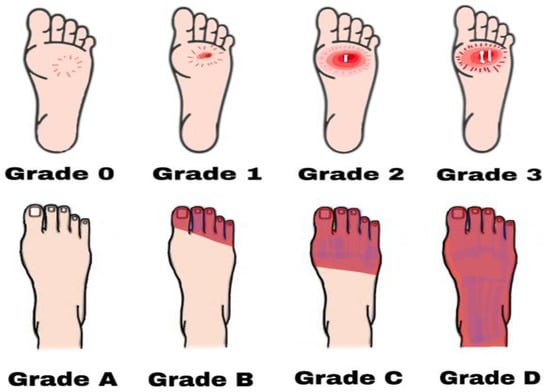
Figure 2. Diabetic foot ulcers based on Brodsky’s classification. Grade 0 has no sign of ulceration but persistent pain is present. Grade 1 is a superficial ulcer. Grade 2 indicates deep ulceration towards the bones. Grade 3 means exposure to severe infection (osteomyelitis). Grade A has no ischemia. Grade B indicates ischemia but no gangrene. Grades C and D are partial and complete gangrene infections with ischemia, respectively.
-
Grade 0: Intact skin; no sign of ulceration but the foot is at risk;
-
Grade 1: Superficial ulcer; no sign of osteomyelitis or exposed bones; no deep ulceration;
-
Grade 2: Deep ulceration; deeper penetration towards bones; bone deformity may be present to some extent;
-
Grade 3: Presence of osteomyelitis or abscess; severe infection and redness; no gangrene exposure;
-
Grade A: Not gangrenous; no ischemia;
-
Grade B: Presence of ischemia but no gangrene;
-
Grade C: Presence of ischemia and partial foot gangrene;
-
Grade D: Presence of ischemia and complete foot gangrene [28].
3. Pathophysiology
Diabetic foot ulcers generally develop in three stages. Initially, neuropathy induces skin inflammation and forms a callus. Then, the involvement of the motor and autonomic nervous system causes bone deformation, trauma, and abnormal skin conditions. Finally, a subcutaneous hemorrhage mediated by frequent trauma erodes and forms the ulcer [29]. In addition, atherosclerosis in the microcirculation causes neovascularization and inflammation, contributing to the delayed healing of wounds, necrosis, and gangrene [25]. The stages of DFU formation and impaired wound healing are, however, associated with certain dysfunctional mechanisms in diabetic individuals [30].
The neuropathic foot is generally warm, dry, numb, and lacks pain sensations, involving neuropathic ulcers, the Charcot joint, and neuropathic edema. On the contrary, the ischemic foot is usually cold and has localized pressure necrosis and gangrene with the absence of pulses [30]. The key features of diabetes mellitus (i.e., hyperglycemia, insulin resistance, hyperlipidemia, and increased oxidative stress) are considered the main causes of endothelial dysfunction, cellular defense disorders, and other diabetes-related complications. In hyperglycemia, the endothelial nitric oxide production is suppressed by the inhibition of nitric oxide synthase, and as a result the high level of reactive oxygen species (ROS), particularly superoxide radicals, increases the hydrogen peroxide levels. This, in turn, causes the formation of highly reactive hydroxyl radicals and damages the cells. Nitric oxide and superoxide together produce peroxynitrite, which affects the endothelial vasodilation and mediates lipid peroxidation. The concentrations of low-density lipoproteins are increased, followed by atherosclerosis in the microcirculation, increased inflammation, abnormal intimal growth, platelet aggregation, and thrombosis [31][32]. Moreover, the impairment of the autonomic nervous system is linked to degeneration of the postganglionic unmyelinated sudomotor axons, reducing sweat production. This, in turn, triggers dry skin, thick plaques, and callus formation [30]. Thus, diabetic neuropathy can be regarded as one of the key contributing factors of DFU, which proceed from a foot deformity and callus formation to increased local pressure and repeated injury, tissue necrosis, and finally ulceration [33][34].
Neuropathy, defined as a long-term painless degenerative arthropathy or Charcot neuroarthropathy, is also characterized by disrupted a sensory innervation of the foot joint. The affected autonomic nervous system increases the local and resting blood flow, causing massive calcium dissolution, leading to subluxation, joint dislocation, bone deformation, osteolysis, and soft tissue edema [35]. Studies have also reported that elevated inflammatory cytokines such as tumor necrosis factor-α and interleukin-1β activate the nuclear factor NF-κb, leading to osteoclast maturation, ultimately contributing to bone deformities [36].
In peripheral arteries, the endothelial dysfunction elevates the vasoconstrictor thromboxane A2, which can aggregate platelets and increase the risk of plasma hypercoagulation. This can lead to ischemia in the lower limb microcirculation and eventually results in ulceration. The ulcer may become exposed and develop into gangrene and infection [37][38]. Moreover, the inhibition of nitric oxide, which is known as an anticoagulant, also contributes to the propensity of atherosclerosis, constricting the blood vessels in the microcirculation and ultimately leading to ischemia. Clinical reports suggest that DFU patients with ischemia and vascular insufficiency experience intense pain, limb hair loss, thinner skin, and a lack of peripheral pulses [36][37].
DFU gets worse over time due to the impaired healing ability existing in diabetic patients. When skin tissues, blood vessels, nerves, and other associated tissues become damaged, even controlled blood glucose levels fail to improve the condition. The slow wound-healing process deteriorates the condition and leads to life-threatening infections including cellulitis, osteomyelitis, abscesses, gangrene, and sepsis [38][39]. Additionally, the immune system of diabetic patients becomes weaker than normal and the hyperglycemic state increases the number of pro-inflammatory cytokines, affecting cell defenses such as phagocytosis, intracellular killing, chemotaxis, and leukocyte activity. The loss of leukocyte activity, decreased chemotaxis, negative nitrogen balance, and increased gluconeogenesis, as well as the impaired synthesis of fibroblasts, protein, and collagen, alter the normal wound-healing process and lead to a prolonged inflammatory state [37][40][41]. In summary, hyperglycemia, neuropathy, vascular insufficiency, arterial diseases, neuroarthropathy, and impaired immunology all contribute to the pathophysiology of diabetic foot ulcers (Figure 3) [36].
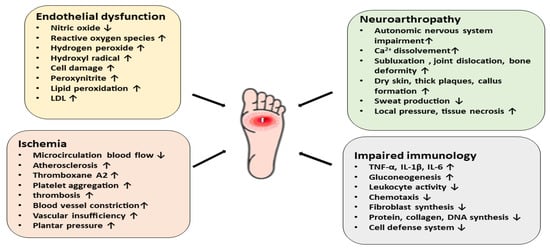
Figure 3. A schematic diagram representing the pathophysiology of diabetic foot ulcers via different mechanistic pathways. Endothelial dysfunction, ischemia, neuroarthropathy, and impaired immunology contribute to the pathophysiology of DFU.
4. Current Therapy for Diabetic Foot Complications
In order to treat and manage the diabetic foot, it is essential to properly diagnose its stage and severity. Alongside the maintenance of blood sugar levels, current therapeutic strategies involve targeting wound healing, controlling the spread of infection, relieving pressure, and improving blood flow [42]. Analgesic medications such as tapentadol, pregabalin, tramadol, duloxetine, acetaminophen, and some opioids (e.g., oxycodone) are employed to alleviate diabetic peripheral neuropathy (DPN)-associated pain. Although these drugs are effective in reducing mild to moderate pain, their frequent use causes nausea, constipation, drowsiness, and confusion [42]. Many antibiotics including nafcillin, flucloxacillin, dicloxacillin, ceftazidime, cefazolin, ceftriaxone, dalbavancin, oritavancin, telavancin, doxycycline, sulfamethoxazole, or trimethoprim have been effectively used to treat diabetic foot infections, even though no prospective comparative trials have been conducted [43]. It should be noted that several factors such as wound age, the host’s immunological state, polymicrobial infection, sanitary conditions, and former antimicrobial therapy may contribute to the development of antimicrobial resistance [44]. Furthermore, the cost of antimicrobial agents recommended for the treatment of infected diabetic foot burdens a large number of diabetic patients, particularly in underdeveloped nations. This results in advanced grades of diabetic foot ulcers that are untreatable in a resource-constrained healthcare system [43][44]. The initial and most crucial step to heal wounds and lower the likelihood of amputations in patients with DFU is debridement. This involves the removal of necrotic and senescent tissues along with foreign and infectious materials from the wound. Debridement tends to lower the bacterial count while increasing the generation of local growth factors, as well as relieving pressure and facilitating wound drainage [45]. The primary purpose of surgical debridement is to transform a chronic ulcer into an acute ulcer. This has been demonstrated to be more successful in treating DFU than mechanical, biological, enzymatic, or autolytic debridement methods [45]. The use of appropriate wound dressing, revascularization for ischemia, pressure relief, laser therapy, and surgery also play an important role in the treatment and control of diabetic foot [42][43][45]. More research is needed, however, to assess the patient demographics for whom these treatments are promising, as well as their cost-to-benefit ratios. [42]. Table 1 summarizes some of the available conventional therapies and their advantages and disadvantages in the management of diabetic foot.
Table 1. Current therapeutic strategies for the treatment of diabetic foot.
| Conventional Therapies for Diabetic Foot Management | Examples | Advantages | Disadvantages | References |
|---|---|---|---|---|
|
Tapentadol, pregabalin, tramadol, duloxetine, acetaminophen, oxycodone | Alleviates diabetic peripheral neuropathy-induced pain | Constipation, nausea, drowsiness, confusion, drug abuse (opioids) | [42] |
|
Nafcillin, ceftazidime, cefazolin, clindamycin, dalbavancin, sulfamethoxazole/trimethoprim | The most common treatment for diabetic foot infections | Antibiotic resistance, high cost, unavailability | [42][44] |
|
Surgical, autolytic, mechanical, enzymatic, maggot debridement, polysaccharide beads/paste | Aids in a complete assessment of the wound, enhances breakdown of necrotic tissue, speeds up ulcer healing | High cost, time-consuming, patient reluctance, surgical debridement may increase wound size | [46][47][48] |
|
Hydrogels, hydrocolloids | Prevent infections, easy to use, effective, provides thermal insulation and mechanical protection | Costly, unavailability | [42][46][48] |
|
- | Heals diabetic foot ulcers | Costly | [49] |
|
Half-shoes, rigid-soled post-operative shoes, total contact casts, accommodative dressings | Heals diabetic foot ulcers, significantly reduces pressure, high acceptability | Costly, patient compliance, ineffective against inflammation | [42][50] |
|
Low-level laser therapy | Effective in healing wounds, reduces inflammatory phase | High cost, discontinuous in efficacy | [42] |
|
- | Heals amputation or non-healing ulcers, one of the most effective methods to treat foot ulcers | Costly, lack of skilled surgeons | [51] |
References
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45.
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119.
- Alam, U.; Asghar, O.; Azmi, S.; Malik, R.A. General aspects of diabetes mellitus. Handb. Clin. Neurol. 2014, 126, 211–222.
- Mayfield, J.A. Diagnosis and classification of diabetes mellitus: New criteria. Am. Fam. Physician 1998, 58, 1355.
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, Å. Type 1 diabetes mellitus. Nat. Rev. Dis. Prim. 2017, 3, 17016.
- Ohlson, L.-O.; Larsson, B.; Björntorp, P.; Eriksson, H.; Svärdsudd, K.; Welin, L.; Tibblin, G.; Wilhelmsen, L. Risk factors for type 2 (non-insulin-dependent) diabetes mellitus. Thirteen and one-half years of follow-up of the participants in a study of Swedish men born in 1913. Diabetologia 1988, 31, 798–805.
- Viigimaa, M.; Sachinidis, A.; Toumpourleka, M.; Koutsampasopoulos, K.; Alliksoo, S.; Titma, T. Macrovascular complications of type 2 diabetes mellitus. Curr. Vasc. Pharmacol. 2020, 18, 110–116.
- Fowler, M.J. Microvascular and macrovascular complications of diabetes. Clin. Diabetes 2008, 26, 77–82.
- Giurato, L.; Uccioli, L. The diabetic foot: Charcot joint and osteomyelitis. Nucl. Med. Commun. 2006, 27, 745–749.
- Nathan, D.M. Long-term complications of diabetes mellitus. N. Engl. J. Med. 1993, 328, 1676–1685.
- Volmer-Thole, M.; Lobmann, R. Neuropathy and diabetic foot syndrome. Int. J. Mol. Sci. 2016, 17, 917.
- Brocco, E.; Ninkovic, S.; Marin, M.; Whisstock, C.; Bruseghin, M.; Boschetti, G.; Viti, R.; Forlini, W.; Volpe, A. Diabetic foot management: Multidisciplinary approach for advanced lesion rescue. J. Cardiovasc. Surg. 2018, 59, 670–684.
- Markakis, K.; Bowling, F.; Boulton, A. The diabetic foot in 2015: An overview. Diabetes/Metab. Res. Rev. 2016, 32, 169–178.
- Bandyk, D.F. The diabetic foot: Pathophysiology, evaluation, and treatment. Semin. Vasc. Surg. 2018, 31, 43–48.
- Bekele, F.; Chelkeba, L. Amputation rate of diabetic foot ulcer and associated factors in diabetes mellitus patients admitted to Nekemte referral hospital, western Ethiopia: Prospective observational study. J. Foot Ankle Res. 2020, 13, 65.
- Ansari, P.; Akther, S.; Hannan, J.M.A.; Seidel, V.; Nujat, N.J.; Abdel-Wahab, Y.H.A. Pharmacologically Active Phytomolecules Isolated from Traditional Antidiabetic Plants and Their Therapeutic Role for the Management of Diabetes Mellitus. Molecules 2022, 27, 4278.
- Ahmadian, R.; Bahramsoltani, R.; Marques, A.M.; Rahimi, R.; Farzaei, M.H. Medicinal Plants as Efficacious Agents for Diabetic Foot Ulcers: A Systematic Review of Clinical Studies. Wounds Compend. Clin. Res. Pract. 2021, 33, 207–218.
- Oguntibeju, O.O. Medicinal plants and their effects on diabetic wound healing. Vet. World 2019, 12, 653.
- Nagori, B.P.; Solanki, R. Role of medicinal plants in wound healing. Res. J. Med. Plant 2011, 5, 392–405.
- Sharma, A.; Khanna, S.; Kaur, G.; Singh, I. Medicinal plants and their components for wound healing applications. Future J. Pharm. Sci. 2021, 7, 53.
- El-Nashar, H.A.; Mostafa, N.M.; El-Shazly, M.; Eldahshan, O.A. The role of plant-derived compounds in managing diabetes mellitus: A review of literature from 2014 to 2019. Curr. Med. Chem. 2021, 28, 4694–4730.
- Abdelghffar, E.A.; Mostafa, N.M.; El-Nashar, H.A.; Eldahshan, O.A.; Singab, A.N.B. Chilean pepper (Schinus polygamus) ameliorates the adverse effects of hyperglycaemia/dyslipidaemia in high fat diet/streptozotocin-induced type 2 diabetic rat model. Ind. Crops Prod. 2022, 183, 114953.
- Schaper, N. Diabetic foot ulcer classification system for research purposes: A progress report on criteria for including patients in research studies. Diabetes/Metab. Res. Rev. 2004, 20, S90–S95.
- Jeffcoate, W.J.; Bus, S.A.; Game, F.L.; Hinchliffe, R.J.; Price, P.E.; Schaper, N.C. International Working Group on the Diabetic Foot and the European Wound Management Association. Reporting standards of studies and papers on the prevention and management of foot ulcers in diabetes: Required details and markers of good quality. Lancet Diabetes Endocrinol. 2016, 4, 781–788.
- Oliver, T.I.; Mutluoglu, M. Diabetic foot ulcer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019.
- Wagner, F.W., Jr. The dysvascular foot: A system for diagnosis and treatment. Foot Ankle 1981, 2, 64–122.
- Abid, A.; Hosseinzadeh, S. Foot Ulcer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020.
- Robinson, A.; Pasapula, C.; Brodsky, J. Surgical aspects of the diabetic foot. J. Bone Jt. Surgery. Br. Vol. 2009, 91, 1–7.
- Armstrong, D.G.; Boulton, A.J.; Bus, S.A. Diabetic foot ulcers and their recurrence. N. Engl. J. Med. 2017, 376, 2367–2375.
- Edmonds, M. The diabetic foot: Pathophysiology and treatment. Clin. Endocrinol. Metab. 1986, 15, 889–916.
- Apelqvist, J.; Ragnarson-Tennvall, G.; Larsson, J.; Persson, U. Diabetic foot ulcers in a multidisciplinary setting an economic analysis of primary healing and healing with amputation. J. Intern. Med. 1994, 235, 463–471.
- Eslami, M.H.; Zayaruzny, M.; Fitzgerald, G.A. The adverse effects of race, insurance status, and low income on the rate of amputation in patients presenting with lower extremity ischemia. J. Vasc. Surg. 2007, 45, 55–59.
- Alavi, A.; Sibbald, R.G.; Mayer, D.; Goodman, L.; Botros, M.; Armstrong, D.G.; Woo, K.; Boeni, T.; Ayello, E.A.; Kirsner, R.S. Diabetic foot ulcers: Part I. Pathophysiology and prevention. J. Am. Acad. Dermatol. 2014, 70, e1.
- Galkowska, H.; Wojewodzka, U.; Olszewski, W.L. Low recruitment of immune cells with increased expression of endothelial adhesion molecules in margins of the chronic diabetic foot ulcers. Wound Repair Regen. 2005, 13, 248–254.
- Schaper, N.; Van Netten, J.; Apelqvist, J.; Lipsky, B.; Bakker, K.; Foot, I.W.G.o.t.D. Prevention and management of foot problems in diabetes: A Summary Guidance for Daily Practice 2015, based on the IWGDF Guidance Documents. Diabetes/Metab. Res. Rev. 2016, 32, 7–15.
- Noor, S.; Zubair, M.; Ahmad, J. Diabetic foot ulcer—A review on pathophysiology, classification and microbial etiology. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 192–199.
- Clayton, W., Jr.; Elasy, T.A. A review of the pathophysiology, classification, and treatment of foot ulcers in diabetic patients. Clin. Diabetes 2009, 27, 52–58.
- Wild, T.; Rahbarnia, A.; Kellner, M.; Sobotka, L.; Eberlein, T. Basics in nutrition and wound healing. Nutrition 2010, 26, 862–866.
- Sharp, A.; Clark, J. Diabetes and its effects on wound healing. Nurs. Stand. 2011, 25, 41–47.
- Singh, S.; Pai, D.R.; Yuhhui, C. Diabetic foot ulcer–diagnosis and management. Clin. Res. Foot Ankle 2013, 1, 120.
- Syafril, S. Pathophysiology diabetic foot ulcer. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Medan, Indonesia, 15–18 November 2017; IOP Publishing: Bristol, UK, 2018; p. 012161.
- Perez-Favila, A.; Martinez-Fierro, M.L.; Rodriguez-Lazalde, J.G.; Cid-Baez, M.A.; Zamudio-Osuna, M.D.J.; Martinez-Blanco, M.D.R.; Mollinedo-Montaño, F.E.; Rodriguez-Sanchez, I.P.; Castañeda-Miranda, R.; Garza-Veloz, I. Current therapeutic strategies in diabetic foot ulcers. Medicina 2019, 55, 714.
- Lipsky, B.A.; Aragón-Sánchez, J.; Diggle, M.; Embil, J.; Kono, S.; Lavery, L.; Senneville, É.; Urbančič-Rovan, V.; Van Asten, S.; Peters, E.J. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes/Metab. Res. Rev. 2016, 32, 45–74.
- Singh, S.; Gupta, B. Choices and challenges of antibiotics therapy in diabetic foot infection. Indian J. Endocrinol. Metab. 2017, 21, 647.
- Yazdanpanah, L.; Nasiri, M.; Adarvishi, S. Literature review on the management of diabetic foot ulcer. World J. Diabetes 2015, 6, 37.
- Ramirez-Acuña, J.M.; Cardenas-Cadena, S.A.; Marquez-Salas, P.A.; Garza-Veloz, I.; Perez-Favila, A.; Cid-Baez, M.A.; Flores-Morales, V.; Martinez-Fierro, M.L. Diabetic foot ulcers: Current advances in antimicrobial therapies and emerging treatments. Antibiotics 2019, 8, 193.
- Edwards, J.; Stapley, S. Debridement of diabetic foot ulcers. Cochrane Database Syst. Rev. 2010, 2010, CD003556.
- Kavitha, K.V.; Tiwari, S.; Purandare, V.B.; Khedkar, S.; Bhosale, S.S.; Unnikrishnan, A.G. Choice of wound care in diabetic foot ulcer: A practical approach. World J. Diabetes 2014, 5, 546.
- Hinchliffe, R.; Andros, G.; Apelqvist, J.; Bakker, K.; Fiedrichs, S.; Lammer, J.; Lepantalo, M.; Mills, J.; Reekers, J.; Shearman, C. A systematic review of the effectiveness of revascularization of the ulcerated foot in patients with diabetes and peripheral arterial disease. Diabetes/Metab. Res. Rev. 2012, 28, 179–217.
- Mulder, G.; Alfieri, D. The diabetic foot: Considerations for pressure reduction and off-loading. Prim. Intent. Aust. J. Wound Manag. 2007, 15, 58–65.
- Adams, C.A., Jr.; Deitch, E.A. Diabetic foot infections. In Surgical Treatment: Evidence-Based and Problem-Oriented; Holzheimer, R.G., Mannick, J.A., Eds.; Zuckschwerdt: Munich, Germany, 2001.
More
Information
Subjects:
Integrative & Complementary Medicine
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
02 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




