Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Prawej Ansari and Version 2 by Dean Liu.
Diabetes mellitus, a major cause of mortality around the globe, can result in several secondary complications, including diabetic foot syndrome, which is brought on by diabetic neuropathy and ischemia. Approximately 15% of diabetic patients suffer from diabetic foot complications, and among them 25% are at risk of lower limb amputations. Diabetic foot ulcers are characterized as skin lesions, gangrene, or necrosis, and may develop due to several reasons, including hyperglycemia and slower wound healing in diabetic patients.
- hyperglycemia
- diabetic foot
- infection
- glucose
1. Introduction
Diabetes mellitus (DM) is considered a worldwide epidemic, being one of the top ten diseases causing mortality globally and affecting more than 10.5% of the adult population [1][2][1,2]. It is a chronic metabolic disorder manifested by persistent high blood glucose levels occurring due to deficiencies in insulin production, insulin resistance, or both [3]. According to its etiology and pathogenesis, diabetes mellitus can be broadly classified into two major types: type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) [4]. T1DM is a result of the autoimmune destruction of the pancreatic β-cells of the islets of Langerhans, leading to significantly diminished insulin secretion, whereas T2DM, often associated with obesity and an unhealthy lifestyle, is the outcome of either impaired insulin secretion, decreased insulin sensitivity to cells, or both [5][6][5,6]. The long-term secondary complications of diabetes include coronary heart disease, peripheral artery disease, cardiomyopathy, stroke, and cerebrovascular disease, as well as diabetic retinopathy (which causes visual impairment), diabetic nephropathy (which leads to kidney failure), and diabetic neuropathy (which increases the risk of foot ulcers) [7][8][9][7,8,9]. Individuals with long-term DM are prone to these complications, which may lead to increased morbidity and mortality [10].
Diabetic foot is a major complication of DM that affects approximately 15% of diabetic patients, with 25% of them facing the possibility of lower limb amputation [11][12][13][11,12,13]. Diabetic patients are often hospitalized due to the consequences of foot-related conditions such as infections, ulcers, and gangrene or foot necrosis [14]. The ulceration is the starting point for a severe stage of the disease, and if left untreated, this can lead to amputations. Diabetic neuropathy, neuro-ischemia, and infections all have an impact on whether lesions heal or deteriorate, and amputations in diabetic patients are often precipitated by a foot ulcer that progresses to serious gangrene or infection [12].
To accelerate the healing process of diabetic foot ulcerations, various interventions such as wound cleansing, revascularization, antibiotic therapy, dead tissue removal, and in extreme situations surgery have been implemented [15]. The conventional therapy for treating diabetic foot is often impracticable, particularly in underdeveloped countries, because of the high costs, adverse side effects, and the unavailability of vascular surgeons. In recent years, there has been a surge of interest in the search for alternative medications derived from natural resources, especially medicinal plants, for the prevention and treatment of diabetic foot [16][17][16,17].
Since ancient times, herbal remedies have been used as effective therapies to treat a wide range of ailments, including diabetic foot [16]. Recent findings have revealed that biologically-active phytomolecules present in plants demonstrate various pharmacological effects that help to prevent different forms of cell damage, including chronic wounds [17]. Numerous medicinal plants, including Aloe vera, Annona squamosa, Azadirachta indica, Carica papaya, Catharanthus roseus, Centella asiatica, Curcuma longa, Hylocereus undatus, and Punica granatum, have exhibited potential wound healing, anti-inflammatory, antioxidative, antibacterial, and other antidiabetic properties with little to no side effects, and might be considered as possible effective treatments for diabetic injuries [18][19][20][18,19,20]. Several bioactive phytomolecules such as quercetin, kaempferol, isoquercetrin, apigenin, tangeretin, naringenin, luteolin, catechin, gallic acid, methyl gallate, and rutin present in medicinal plants including Allium cepa, Beta vulgaris, Citrus sinensis, Schinus polygamus, and Sorghum bicolor exert their antidiabetic action via different mechanistic pathways to improve β-cell function and insulin secretion; enhance insulin sensitivity; increase GLUT-4 expression; decrease gluconeogenesis; and inhibit α-amylase, α-glucosidase, and DPP-IV activity and the formation of advanced glycation end products. Such phytochemicals not only ameliorate diabetic foot wounds but also contribute to the overall antidiabetic action of the medicinal plants to prevent and manage other diabetes-related complications [21][22][21,22]. Thus, due to the abundance of bioactive compounds in plants, researchers have directed their focus toward examining the significance of medicinal plants and isolating their phytoconstituents to assess their prospective wound-healing properties [18].
2. Types of Diabetic Foot Complications
Diabetic foot ulcers can be classified depending on the degree of tissue loss, size, perfusion, infection site, depth, area, and sensation [23][43]. The changes in these parameters are based on an individual’s age, sex, medical conditions, and existing comorbidities, including a loss of peripheral sensation (LOPS) and peripheral arterial diseases (PAD), which ultimately categorize diabetic foot ulcers into different grades [24][44]. According to Meggitt and Wagner, diabetic foot ulcers can be classified into 6 grades (Figure 1):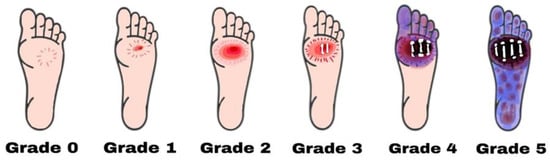
Figure 1. Diabetic foot ulcers are categorized using the Wagner–Meggitt classification. Grade 0 means no break in the skin. Grade 1 indicates a superficial ulcer. Grade 2 indicates a deep ulcer. Grade 3 shows the presence of osteomyelitis. Grade 4 is identified as forefoot gangrene. Grade 5 is recognized as complete foot exposure to gangrene.
-
Grade zero: No lesion on the skin, potential bone deformity or hyperkeratotic lesion, pain;
-
Grade one: Superficial viable or necrotic ulcers; subcutaneous tissue loss; potential bone deformity; no penetration into the deeper layers of the skin;
-
Grade two: Deeper penetrates including bones, tendons, ligaments, or deep fascia; bone deformity prominent in some aspects; absence of abscess or osteomyelitis;
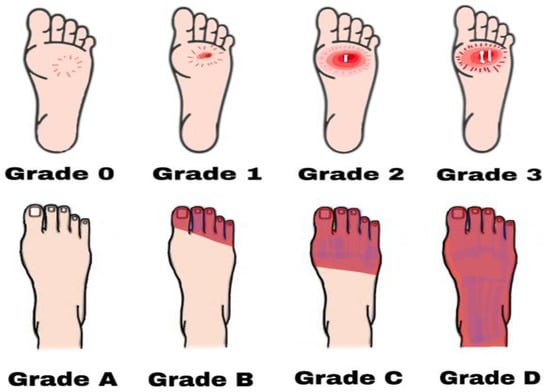
Figure 2. Diabetic foot ulcers based on Brodsky’s classification. Grade 0 has no sign of ulceration but persistent pain is present. Grade 1 is a superficial ulcer. Grade 2 indicates deep ulceration towards the bones. Grade 3 means exposure to severe infection (osteomyelitis). Grade A has no ischemia. Grade B indicates ischemia but no gangrene. Grades C and D are partial and complete gangrene infections with ischemia, respectively.
-
Grade 0: Intact skin; no sign of ulceration but the foot is at risk;
-
Grade 1:
- Grade Three: Presence of osteomyelitis, deep abscess, or tendinitis; severe infection symptoms (e.g., redness, heat, and swelling);
- Superficial ulcer; no sign of osteomyelitis or exposed bones; no deep ulceration;
-
Grade 2: Deep ulceration; deeper penetration towards bones; bone deformity may be present to some extent;
-
Grade 3: Presence of osteomyelitis or abscess; severe infection and redness; no gangrene exposure;Grade four: Gangrene (dry, wet, infected, or non-infected) in toe or forefoot; surgical ablation of the foot required with minimal blood supply for below-knee amputations;
-
Grade A: Not gangrenous; no ischemia;
- Presence of ischemia but no gangrene;
- Grade C:
- Presence of ischemia and partial foot gangrene;
3. Pathophysiology
Diabetic foot ulcers generally develop in three stages. Initially, neuropathy induces skin inflammation and forms a callus. Then, the involvement of the motor and autonomic nervous system causes bone deformation, trauma, and abnormal skin conditions. Finally, a subcutaneous hemorrhage mediated by frequent trauma erodes and forms the ulcer [29][48]. In addition, atherosclerosis in the microcirculation causes neovascularization and inflammation, contributing to the delayed healing of wounds, necrosis, and gangrene [25][28]. The stages of DFU formation and impaired wound healing are, however, associated with certain dysfunctional mechanisms in diabetic individuals [30][49]. The neuropathic foot is generally warm, dry, numb, and lacks pain sensations, involving neuropathic ulcers, the Charcot joint, and neuropathic edema. On the contrary, the ischemic foot is usually cold and has localized pressure necrosis and gangrene with the absence of pulses [30][49]. The key features of diabetes mellitus (i.e., hyperglycemia, insulin resistance, hyperlipidemia, and increased oxidative stress) are considered the main causes of endothelial dysfunction, cellular defense disorders, and other diabetes-related complications. In hyperglycemia, the endothelial nitric oxide production is suppressed by the inhibition of nitric oxide synthase, and as a result the high level of reactive oxygen species (ROS), particularly superoxide radicals, increases the hydrogen peroxide levels. This, in turn, causes the formation of highly reactive hydroxyl radicals and damages the cells. Nitric oxide and superoxide together produce peroxynitrite, which affects the endothelial vasodilation and mediates lipid peroxidation. The concentrations of low-density lipoproteins are increased, followed by atherosclerosis in the microcirculation, increased inflammation, abnormal intimal growth, platelet aggregation, and thrombosis [31][32][50,51]. Moreover, the impairment of the autonomic nervous system is linked to degeneration of the postganglionic unmyelinated sudomotor axons, reducing sweat production. This, in turn, triggers dry skin, thick plaques, and callus formation [30][49]. Thus, diabetic neuropathy can be regarded as one of the key contributing factors of DFU, which proceed from a foot deformity and callus formation to increased local pressure and repeated injury, tissue necrosis, and finally ulceration [33][34][31,52]. Neuropathy, defined as a long-term painless degenerative arthropathy or Charcot neuroarthropathy, is also characterized by disrupted a sensory innervation of the foot joint. The affected autonomic nervous system increases the local and resting blood flow, causing massive calcium dissolution, leading to subluxation, joint dislocation, bone deformation, osteolysis, and soft tissue edema [35][53]. Studies have also reported that elevated inflammatory cytokines such as tumor necrosis factor-α and interleukin-1β activate the nuclear factor NF-κb, leading to osteoclast maturation, ultimately contributing to bone deformities [36][54]. In peripheral arteries, the endothelial dysfunction elevates the vasoconstrictor thromboxane A2, which can aggregate platelets and increase the risk of plasma hypercoagulation. This can lead to ischemia in the lower limb microcirculation and eventually results in ulceration. The ulcer may become exposed and develop into gangrene and infection [37][38][55,56]. Moreover, the inhibition of nitric oxide, which is known as an anticoagulant, also contributes to the propensity of atherosclerosis, constricting the blood vessels in the microcirculation and ultimately leading to ischemia. Clinical reports suggest that DFU patients with ischemia and vascular insufficiency experience intense pain, limb hair loss, thinner skin, and a lack of peripheral pulses [36][37][54,55]. DFU gets worse over time due to the impaired healing ability existing in diabetic patients. When skin tissues, blood vessels, nerves, and other associated tissues become damaged, even controlled blood glucose levels fail to improve the condition. The slow wound-healing process deteriorates the condition and leads to life-threatening infections including cellulitis, osteomyelitis, abscesses, gangrene, and sepsis [38][39][56,57]. Additionally, the immune system of diabetic patients becomes weaker than normal and the hyperglycemic state increases the number of pro-inflammatory cytokines, affecting cell defenses such as phagocytosis, intracellular killing, chemotaxis, and leukocyte activity. The loss of leukocyte activity, decreased chemotaxis, negative nitrogen balance, and increased gluconeogenesis, as well as the impaired synthesis of fibroblasts, protein, and collagen, alter the normal wound-healing process and lead to a prolonged inflammatory state [37][40][41][55,58,59]. In summary, hyperglycemia, neuropathy, vascular insufficiency, arterial diseases, neuroarthropathy, and impaired immunology all contribute to the pathophysiology of diabetic foot ulcers (Figure 3) [36][54].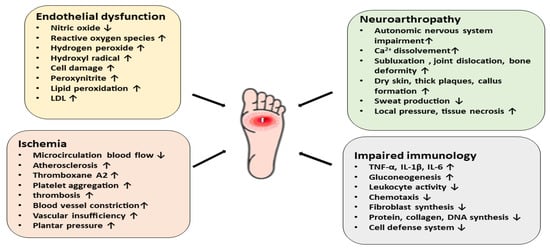
Figure 3. A schematic diagram representing the pathophysiology of diabetic foot ulcers via different mechanistic pathways. Endothelial dysfunction, ischemia, neuroarthropathy, and impaired immunology contribute to the pathophysiology of DFU.
4. Current Therapy for Diabetic Foot Complications
In order to treat and manage the diabetic foot, it is essential to properly diagnose its stage and severity. Alongside the maintenance of blood sugar levels, current therapeutic strategies involve targeting wound healing, controlling the spread of infection, relieving pressure, and improving blood flow [42][29]. Analgesic medications such as tapentadol, pregabalin, tramadol, duloxetine, acetaminophen, and some opioids (e.g., oxycodone) are employed to alleviate diabetic peripheral neuropathy (DPN)-associated pain. Although these drugs are effective in reducing mild to moderate pain, their frequent use causes nausea, constipation, drowsiness, and confusion [42][29]. Many antibiotics including nafcillin, flucloxacillin, dicloxacillin, ceftazidime, cefazolin, ceftriaxone, dalbavancin, oritavancin, telavancin, doxycycline, sulfamethoxazole, or trimethoprim have been effectively used to treat diabetic foot infections, even though no prospective comparative trials have been conducted [43][60]. It should be noted that several factors such as wound age, the host’s immunological state, polymicrobial infection, sanitary conditions, and former antimicrobial therapy may contribute to the development of antimicrobial resistance [44][61]. Furthermore, the cost of antimicrobial agents recommended for the treatment of infected diabetic foot burdens a large number of diabetic patients, particularly in underdeveloped nations. This results in advanced grades of diabetic foot ulcers that are untreatable in a resource-constrained healthcare system [43][44][60,61]. The initial and most crucial step to heal wounds and lower the likelihood of amputations in patients with DFU is debridement. This involves the removal of necrotic and senescent tissues along with foreign and infectious materials from the wound. Debridement tends to lower the bacterial count while increasing the generation of local growth factors, as well as relieving pressure and facilitating wound drainage [45][36]. The primary purpose of surgical debridement is to transform a chronic ulcer into an acute ulcer. This has been demonstrated to be more successful in treating DFU than mechanical, biological, enzymatic, or autolytic debridement methods [45][36]. The use of appropriate wound dressing, revascularization for ischemia, pressure relief, laser therapy, and surgery also play an important role in the treatment and control of diabetic foot [42][43][45][29,36,60]. More research is needed, however, to assess the patient demographics for whom these treatments are promising, as well as their cost-to-benefit ratios. [42][29]. Table 1 summarizes some of the available conventional therapies and their advantages and disadvantages in the management of diabetic foot.Table 1. Current therapeutic strategies for the treatment of diabetic foot.
| Conventional Therapies for Diabetic Foot Management | Examples | Advantages | Disadvantages | References |
|---|---|---|---|---|
|
Tapentadol, pregabalin, tramadol, duloxetine, acetaminophen, oxycodone | Alleviates diabetic peripheral neuropathy-induced pain | Constipation, nausea, drowsiness, confusion, drug abuse (opioids) | [42][29] |
|
Nafcillin, ceftazidime, cefazolin, clindamycin, dalbavancin, sulfamethoxazole/trimethoprim | The most common treatment for diabetic foot infections | Antibiotic resistance, high cost, unavailability | [42][44][29,61] |
|
Surgical, autolytic, mechanical, enzymatic, maggot debridement, polysaccharide beads/paste | Aids in a complete assessment of the wound, enhances breakdown of necrotic tissue, speeds up ulcer healing | High cost, time-consuming, patient reluctance, surgical debridement may increase wound size | [46]62[47][48][,63,64] |
|
Hydrogels, hydrocolloids | Prevent infections, easy to use, effective, provides thermal insulation and mechanical protection | Costly, unavailability | [42][46][48][29,62,64] |
|
- | Heals diabetic foot ulcers | Costly | [49][65] |
|
Half-shoes, rigid-soled post-operative shoes, total contact casts, accommodative dressings | Heals diabetic foot ulcers, significantly reduces pressure, high acceptability | Costly, patient compliance, ineffective against inflammation | [42][50][29,66] |
|
Low-level laser therapy | Effective in healing wounds, reduces inflammatory phase | High cost, discontinuous in efficacy | [42][29] |
|
- | Heals amputation or non-healing ulcers, one of the most effective methods to treat foot ulcers | Costly, lack of skilled surgeons | [51][67] |
