Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fatima Ali | -- | 2284 | 2022-12-01 10:55:23 | | | |
| 2 | Sirius Huang | Meta information modification | 2284 | 2022-12-02 02:45:25 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Afzal, O.; Altamimi, A.S.A.; Mubeen, B.; Alzarea, S.I.; Almalki, W.H.; Al-Qahtani, S.D.; Atiya, E.M.; Al-Abbasi, F.A.; Ali, F.; Ullah, I.; et al. mTOR in the Treatment of Microbial Infections. Encyclopedia. Available online: https://encyclopedia.pub/entry/37653 (accessed on 08 February 2026).
Afzal O, Altamimi ASA, Mubeen B, Alzarea SI, Almalki WH, Al-Qahtani SD, et al. mTOR in the Treatment of Microbial Infections. Encyclopedia. Available at: https://encyclopedia.pub/entry/37653. Accessed February 08, 2026.
Afzal, Obaid, Abdulmalik S. A. Altamimi, Bismillah Mubeen, Sami I. Alzarea, Waleed Hassan Almalki, Salwa D. Al-Qahtani, Eman M. Atiya, Fahad A. Al-Abbasi, Fatima Ali, Inam Ullah, et al. "mTOR in the Treatment of Microbial Infections" Encyclopedia, https://encyclopedia.pub/entry/37653 (accessed February 08, 2026).
Afzal, O., Altamimi, A.S.A., Mubeen, B., Alzarea, S.I., Almalki, W.H., Al-Qahtani, S.D., Atiya, E.M., Al-Abbasi, F.A., Ali, F., Ullah, I., Nadeem, M.S., & Kazmi, I. (2022, December 01). mTOR in the Treatment of Microbial Infections. In Encyclopedia. https://encyclopedia.pub/entry/37653
Afzal, Obaid, et al. "mTOR in the Treatment of Microbial Infections." Encyclopedia. Web. 01 December, 2022.
Copy Citation
The mammalian target of rapamycin (mTOR) is the major controller of a number of important cellular activities, including protein synthesis, cell expansion, multiplication, autophagy, lysosomal function, and cellular metabolism. The mTOR signaling system regulates gene transcription and protein manufacturing to control proliferation of cell, differentiation of immune cell, and tumor metabolism. Due to its vital role in case of microbial infections, inflammations and cancer development and progression, mTOR has been considered as a key therapeutic target for the development of targeted medication.
mammalian target of rapamycin (mTOR)
infection
1. mTOR Inhibitors
mTOR inhibitors can be utilized to treat disorders in addition to the traditional therapeutic indication of organ transplantation. Recent research has suggested that mTOR inhibitors could be used to treat a variety of human malignancies. Rapamycin can, in fact, limit the proliferation of a variety of human and murin cell lines in a concentration dependent manner [1][2]. The therapeutical features of mTOR inhibitors appear to vanish at high drug concentrations, according to a recent experimental investigation, which was validated by a clinical case report, posing a medical difficulty in the treatment of high dosages of rapamycin as well as its derivatives [3]. In light of these assumptions, inhibitors of the pathway of mTOR can be divided into three groups: first, rapalogs, these got their name from rapamycin, second inhibitors with a more heterogeneous mode of inhibition [4]. The second generation of mTOR inhibitors can be classified into two subclasses: (a) mTOR/PI3K dual inhibitors (TPdIs), and (b) mTORC1/mTORC2 dual inhibitors (TORCdIs). This classification is based on the difference in mechanism of action [5]. Rodrik-Outmezguine et al. 2016 linked the 1st and 2nd generation of mTOR inhibitors to create rapalink-1, a third-generation mTOR inhibitor that can aim two targets on the mTOR enzyme at the same time [6][7].
The first historical inhibitor, rapamycin, inspired the name mTOR. This medication, also known as sirolimus, was first found as a product of the bacteria Streptomyces hygroscopicus in a soil sample on the island of Rapa Nui (Easter Island). Rapamycin was first created as an antifungal medication. However, later it was determined that it had significant immunosuppressive and anti-proliferative characteristics [8]. Indeed, mTOR inhibitors are a novel family of medications that are commonly employed in cancers, microbial infections, and immunosuppressive treatments. Their current usage as a viable therapeutic alternative is justified by their good efficacy and low toxicity [9] (Figure 1).
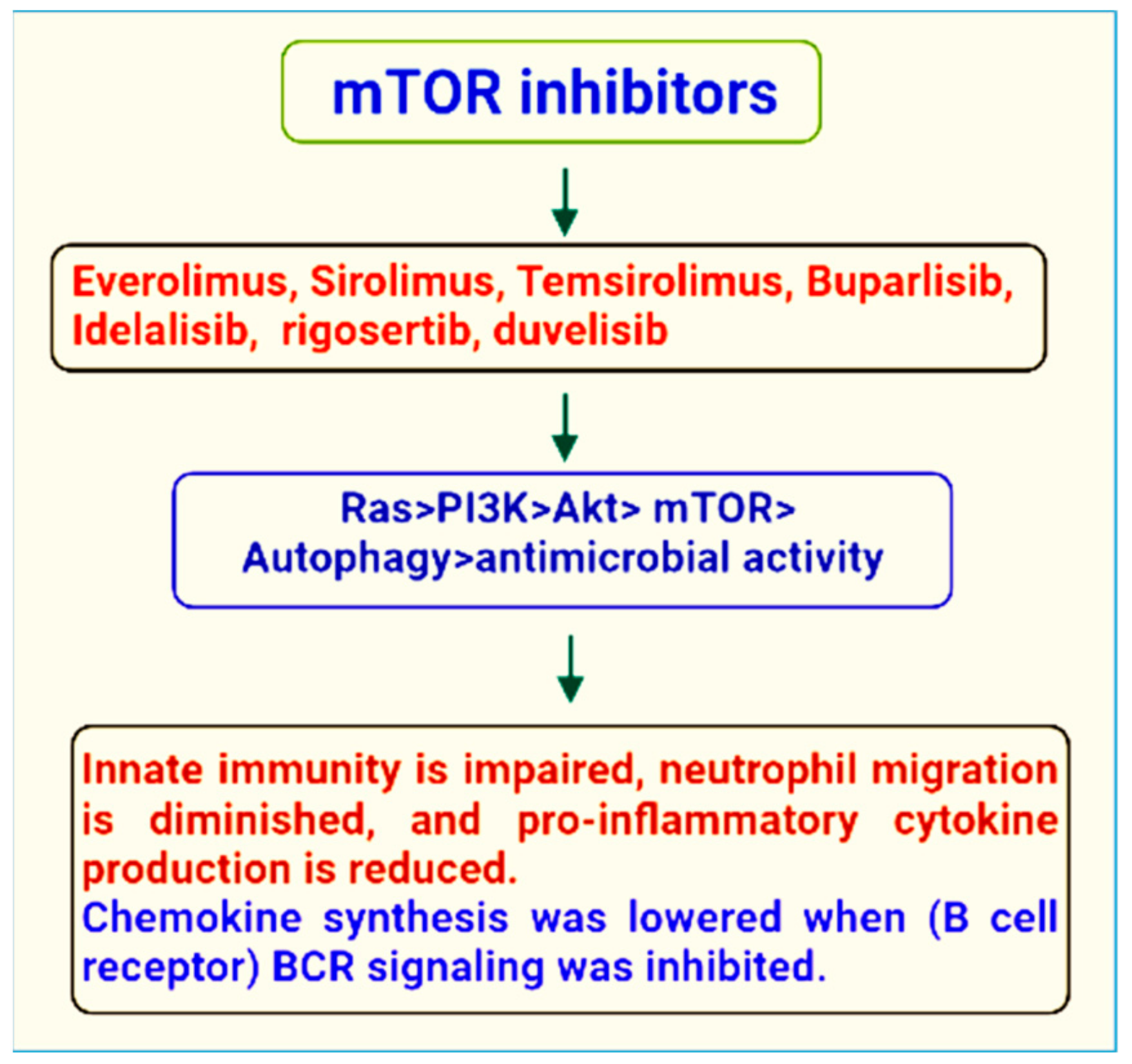
Figure 1. mTOR inhibitors of therapeutic importance.
Rapalink-1 is the third generation inhibitor, it lowers the size of the tumor resistant to 1st and 2nd generation inhibitors owing to its significant anticancer action. This strategy offers a novel concept and model for developing new anticancer, antiviral, and antibacterial medications. A variety of medicines have been discovered that suppress mTOR action, in addition to the usual therapeutic mTOR inhibitors [7] (Table 1).
Table 1. mTOR inhibitors and their therapeutic importance.
| Sr. No. | Name of mTOR Inhibitor | Therapeutic Importance | Reference |
|---|---|---|---|
| 1 | Rapamycin (Sirolimus) |
|
[10] [11] [12] [13] |
| 2 | Temsirolimus | Treat advanced Renal cancer | [7] |
| 3 | Everolimus |
|
[14] [15] [16] |
| 4 | Ridaforolimus/Deforolimus |
|
[17] [18] |
| 5 | Zotarolimus | Antitumor activity | [19] |
| 6 | Torin 1 | Suppress colon cancer cells | [20] |
| 7 | Torin 2 | Antitumor activity | [21] |
| 8 | MLN0128 | Advanced solid tumors | [22] |
| 9 | AZD2014 (Vistusertib) | Metastatic clear cell renal cancer | [22] |
| 10 | Voxtalisib (SAR24540; XL765) | non-Hodgkin lymphoma or chronic lymphocytic lymphoma that has relapsed or is refractory | [22] |
| 11 | Gedatolisib (PKI-587 PF05212384) | Recurrent endometrial cancer | [22] |
| 12 | Rapalink-1 |
|
[7] |
| 13 | Halitulin analog ICSN3250 | It has the ability to compete with and interchange phospholipids acid in the mTOR FRB domain | [23] |
| 14 | LY3023414 |
|
[24] |
| 15 | O SU-53 |
|
[25] |
| 16 | OSI-027 | Anticancer | [26] |
| 17 | C C-223 | Anticancer | [27] |
| 18 | PKI-587 | Gastroenteropancreatic Neuroendocrine tumor disease | [28] |
| 19 | INK-128 | Inhibit angiogenesis and tumor growth in
|
[29] |
| 20 | GSK2126458 | Robust activity in cancer models | [30] |
| 21 | XL765 | Glioblastoma development is inhibited by triggering ER stress-dependent apoptosis. | [31] |
| 22 | NVP-BEZ235 | Cancer cell proliferation is inhibited by this compound | [32] |
| 23 | P529 | Stops cancer cells from multiplying | [33] |
| 24 | JR-AB2-011 | Anti-glioblastoma multiforme properties | [34] |
2. Therapeutic Role of mTOR Signaling in the Treatment of Microbial Infections
mTOR has a crucial function in and against microbial infection, in addition to its eminent role in cell proliferation. The regulation of autophagy, a crucial mechanism in all myeloid and lymphoid cells in all myeloid cells is a key component in the cellular control of mTOR. Pathogen infection-induced stimuli can increase autophagy above basal levels to eliminate intracellular pathogens while also enhancing microbial antigen presentation on cell surfaces to drive the immune response. Infection with the bacteria Shigella flexneri, for example, induces amino acid deficiency and consequent mTOR downregulation, resulting in autophagy [35]. The immune system’s ability to recognize and respond to intracellular infections has prompted some viruses to evolve ways to avoid autophagy induction. In order to circumvent immune defense mechanisms, HSV-1 (herpes simplex virus type 1) and HSV-2 (herpes simplex virus type 2) impede autophagy induction [36]. Likewise, Listeria monocytogenes and Salmonella try to thwart autophagy induction by reactivating mTOR and suppressing the immune response [37]. As a result, these viruses hijack and sustain low levels of autophagy in order to take advantage of the host’s energy and nutrients for their own replication. In such instances, inhibiting mTOR and inducing autophagy above basal levels is advantageous for host defense mechanisms [38]. A cohesive perspective of mTOR-regulated lymphokine expression and surface molecule expression on APCs (antigen-presenting cells) and T cells during infection should assist to better comprehend the immunological and metabolic enigma of mTOR. On a molecular, immunological, and biochemical level, it is yet unknown whether targeting the mTORC1 and mTORC2 complexes with pathogen-derived molecules such as glycoproteins might swing the balance of pro-inflammatory and anti-inflammatory T-cell responses in favor of pathogen survival. Given the mTOR action in T cells and APCs has seemingly different immunological consequences [39], developing medicines that modulate the mTOR signaling axis to combat infectious diseases becomes even more difficult. The principal function of the metalloprotease Gp63 blocking mTOR in APCs in leishmaniosis is to limit translation by activating the translational repressor 4E-BPI. Inhibition of mTOR in T cells, on the other hand, may push them to develop into Treg (regulatory T cells)/Th2 cells (T helper cells), which would likely prolong the infection. As a result, it could be a targeted strategy that combines inhibiting 4E-BP1 in APCs while simultaneously activating mTOR in CD4 T cells to boost the host’s protective Th1 response in the first stages of infection. Because an increase in the quality and quantity of memory T cells is a characteristic of a long-lasting anti-leishmanial immune response, it is possible that pathogen specific recall responses could be improved by targeting mTOR inhibitors to memory cells in vivo in order to speed up their growth. Foxp3 expression can be reduced and tolerance broken by Treg-specific activation of mTOR by blocking upstream inhibitors of mTOR signaling such as TSC1/TSC2 or PTEN. During the later phases of infection, targeting memory cells and Treg cells can be extremely effective. By extension, using the metalloprotease Gp63 alone or in conjunction with other medications might open the door to sensible therapeutic treatments for autoimmune diseases such as multiple sclerosis, where type I interferons exacerbate the condition [40] (Figure 2).
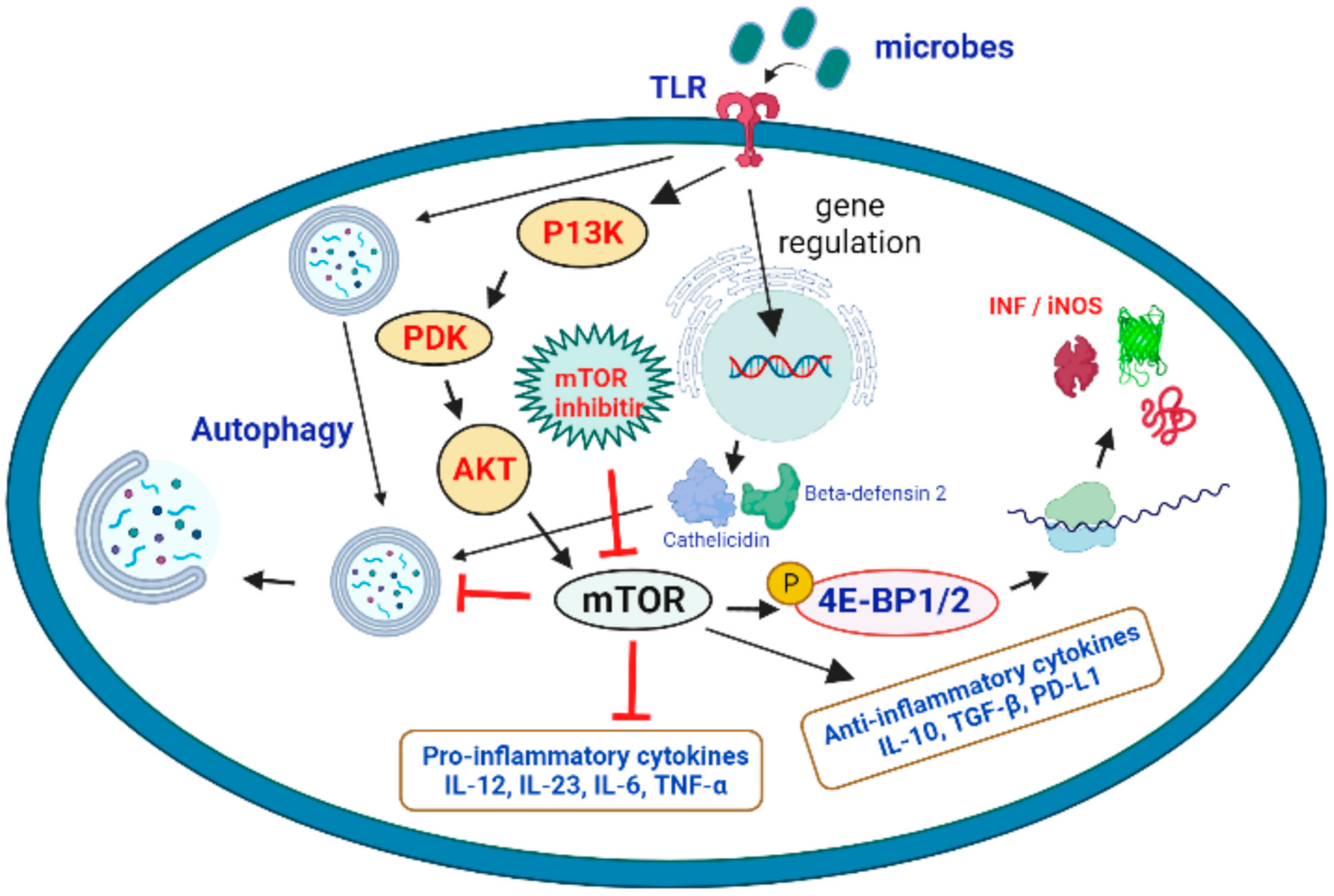
Figure 2. Activation of the mTOR pathway and its potential role in the targeting of microbial infections. Microbial interaction with TLRs activates the P13K > PDK > AKT > mTOR cascade, which results in mTOR activation. It promotes protein translation and the production of iNOS and interferons by phosphorylating 4E-BP1/2 (eukaryotic translation initiation factor 4E binding protein 1 and 2). Microbial detection also promotes autophagy by causing the expression of beta-defensin 2 and cathelicidin. Because mTOR inhibitor is an autophagy inhibitor, it can promote autophagy and fight microbial infections.
It is worth noting that mTORC1-mediated actions can have both immunosuppressive and immune-activating effects. T-cell energy is increased, Treg expansion is induced, and DC maturation is inhibited by the mTORC1 complex [41]. A recent overview of the role of the mTOR in B-cell growth and function was published [42]. It presents the idea that patients taking mTOR inhibitors may have a hindered innate immune response [43]. mTOR, as well as the generation of proinflammatory cytokines [44][45], is required for neutrophil migration to sites of inflammation [46]. The effect of mTOR inhibition on stromal cells may exacerbate the deficits in innate immunity, resulting in poor wound healing [47]. Stomatitis and pneumonitis occur in a significant number of mTOR inhibitor-treated patients, which may serve as an entrance point for pathogenic bacteria [48]. In a process involving the hypoxia-induced factor 1a (HIF1a) pathway, the mTOR pathway has also been involved in neutrophil activity, including the development of extracellular traps that capture and kill bacteria [49].
It is critical to determine if bacterial pathogens have developed mechanisms that disrupt mTOR-dependent regulation of innate and adaptive immune responses in their hosts. In addition, it would be intriguing to look at how common medications that are used to treat metabolic diseases alter immune responses. For instance, metformin is used to regulate blood sugar levels while simultaneously blocking mTORC1 and possibly inducing inflammation. Given that these drugs impact mTOR activity to improve the host’s innate immune response, it would be intriguing to explore if they can also be used to protect against bacterial infections, or if they can override pathogens’ capacity to modify mTOR activity, such as Salmonella [50][51]. Among microorganisms, viruses cannot replicate on their own and must rely on the transcription and translation machinery of the host to duplicate their genome and related proteins. To aid this process, viruses co-opt and modify normal cellular mechanism [52][53]. The regulation of mTOR molecule and its pathways by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) life cycle is of special interest [54]. As mTOR is a serine/threonine kinase that regulates the cellular development, mTOR signaling is also critical for viral translation [55]. In this context, metformin, everolimus, and rapamycin are just a few of the FDA-approved mTOR inhibitors marketed currently. Both DNA viruses such as adenoviruses, cytomegaloviruses, and herpesviruses and RNA viruses such as influenza, HIV, West Nile virus (WNV), and Zika virus (ZIKV) have been shown to affect the mTOR pathway by activating PI3, Akt, or mTOR itself. There are many confirmed evidence that mTOR inhibition reduces viral protein synthesis as well as interferes with virus-mediated transcription activities. WNV, for example, promotes the mTOR pathway via PI3K, prompting downstream protein synthesis activators. WNV-induced mTOR activation was also inhibited by inducible deletion of mTOR cofactors [56].
By modification in the autophagy response, HCV and ZIKV have been presented to increase their own viral reproduction. ZIKV is thought to stimulate autophagy by suppressing the Akt-mTOR pathway, and subsequently hijack the autophagic apparatus to support its own reproduction. In vitro, metformin treatment was reported to suppress ZIKV [56]. HCV can also modify mTOR at several places along the route, uses the autophagic mechanism to generate an infection, which subsequently leads to an increase in hepatocyte formation, allowing for a long-term infection. Metformin decreases the development of infected cells by HCV and inhibits replication of HCV through mTOR pathway, according to multiple studies [57][58]. The influenza A virus has been shown to hijack the Akt/mTOR signaling pathways in order to boost viral replication, with mTOR inhibition greatly restricting viral replication [59]. Buformin (an mTOR inhibitor) has been associated with an improved survival in influenza animal models [60]. In a clinical trial involving 200 patients with H3N2 influenza, treatment with the biguanides phenformin and buformin was found to decrease the incidence of influenza by 5.4% compared to the control group (24%). In randomized controlled studies, mTOR inhibition has also been shown to improve clinical outcomes in H1N1 influenza patients who required mechanical breathing [44][61][62]. mTOR inhibition using guanidine (a metformin derivative) has been associated to poliovirus cytopathic effects in vitro and in primate models [45][63]. Guanidine has also been demonstrated to suppress enterovirus [64][65]. Modulation of the mTOR pathway has been seen in viruses that are more closely linked to SARS-CoV-2. MERS-CoV infection revealed mTOR pathway modification, according to a kinome study. In vitro MERS-CoV investigations have shown that PI3K/Akt/mTOR signaling pathways are significant in viral pathogenesis. Pre-infection suppression of the mTOR pathway resulted in a 60% decrease of MERS-CoV infection in vitro [66]. So, mTOR–PI3K–AKT pathway proved as a critical signaling route in SARS-CoV-2 infection. The scientists tested three mTOR inhibitors in vitro and found that nanomolar medication doses of each agent significantly inhibited SARS-CoV-2 virus [67].
Multiple pathways demonstrated in vitro modification during the time of virus progression in infection, according to a proteo-transcriptomics investigation of infected cells by SARS-CoV-2. All of the linked pathways, in particular, converge on mTOR signaling, although it is unknown how each of them contributes to the viral life cycle or if they help with viral replication and growth [68]. If SARS-CoV-2 cycle and manipulation of cellular pathway is comparable to that of MERS-CoV and other RNA viruses, this could be a promising target for new or repurposed therapeutics. Targeting viral transcription, translation, or both, according to biophysical modelling of SARS-CoV-2, is a great sensitive target for therapeutic suppression and is expected to result in viral replication inhibition. A human protein–protein interaction map for SARS-CoV-2 was created recently in order to identify the possible therapeutic targets. In the cellular response to SARS-CoV-2, the PI3K/Akt/mTOR pathway is crucial. More biochemical research and clinical studies are urgently needed to better understand the specific function of mTOR inhibitors and modulators in the treatment of COVID-19 [69][70][71].
References
- Pieri, M.; Miraglia, N.; Polichetti, G.; Tarantino, G.; Acampora, A.; Capone, D. Analytical and Pharmacological Aspects of Therapeutic Drug Monitoring of mTOR Inhibitors. Curr. Drug Metab. 2011, 12, 253–267.
- Bullock, K.E.; Petros, W.P.; Younis, I.; Uronis, H.E.; Morse, M.A.; Blobe, G.C.; Zafar, S.Y.; Gockerman, J.P.; Lager, J.J.; Truax, R.; et al. A phase I study of bevacizumab (B) in combination with everolimus (E) and erlotinib (E) in advanced cancer (BEE). Cancer Chemother. Pharmacol. 2011, 67, 465–474.
- Capone, D.; Palmiero, G.; Gentile, A.; Basile, V.; Federico, S.; Sabbatini, M.; Potenza, M.; Perfetti, A.; Pieri, M.; Tarantino, G. A Pharmacokinetic Interaction Between Clarithromycin and Sirolimus in Kidney Transplant Recipient. Curr. Drug Metab. 2007, 8, 379–381.
- Palavra, F.; Robalo, C.; Reis, F. Recent Advances and Challenges of mTOR Inhibitors Use in the Treatment of Patients with Tuberous Sclerosis Complex. Oxidative Med. Cell. Longev. 2017, 2017, 1–11.
- Zhou, H.-Y.; Huang, S.-L. Current development of the second generation of mTOR inhibitors as anticancer agents. Chin. J. Cancer 2013, 32, 8–18.
- Popova, N.V.; Jücker, M. The Role of mTOR Signaling as a Therapeutic Target in Cancer. Int. J. Mol. Sci. 2021, 22, 1743.
- Rodrik-Outmezguine, V.S.; Okaniwa, M.; Yao, Z.; Novotny, C.J.; McWhirter, C.; Banaji, A.; Won, H.; Wong, W.; Berger, M.; De Stanchina, E.; et al. Overcoming mTOR resistance mutations with a new-generation mTOR inhibitor. Nature 2016, 534, 272–276.
- Powers, R.W.; Kaeberlein, M.; Caldwell, S.D.; Kennedy, B.K.; Fields, S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006, 20, 174–184.
- Granata, S.; Gassa, A.D.; Carraro, A.; Brunelli, M.; Stallone, G.; Lupo, A.; Zaza, G. Sirolimus and Everolimus Pathway: Reviewing Candidate Genes Influencing Their Intracellular Effects. Int. J. Mol. Sci. 2016, 17, 735.
- Pal, S.K.; Quinn, D.I. Differentiating mTOR inhibitors in renal cell carcinoma. Cancer Treat. Rev. 2013, 39, 709–719.
- Malagelada, C.; Jin, Z.H.; Jackson-Lewis, V.; Przedborski, S.; Greene, L.A. Rapamycin Protects against Neuron Death in In Vitro andIn Vivo Models of Parkinson’s Disease. J. Neurosci. 2010, 30, 1166–1175.
- Ma, K.L.; Ruan, X.Z.; Powis, S.H.; Moorhead, J.F.; Varghese, Z. Anti-atherosclerotic effects of sirolimus on human vascular smooth muscle cells. Am. J. Physiol. Circ. Physiol. 2007, 292, H2721–H2728.
- Goudar, R.K.; Shi, Q.; Hjelmeland, M.D.; Keir, S.T.; McLendon, R.E.; Wikstrand, C.J.; Reese, E.D.; Conrad, C.A.; Traxler, P.; Lane, H.A.; et al. Combination therapy of inhibitors of epidermal growth factor receptor/vascular endothelial growth factor receptor 2 (AEE788) and the mammalian target of rapamycin (RAD001) offers improved glioblastoma tumor growth inhibition. Mol. Cancer Ther. 2005, 4, 101–112.
- Kirchner, G.I.; Meier-Wiedenbach, I.; Manns, M.P. Clinical Pharmacokinetics of Everolimus. Clin. Pharmacokinet. 2004, 43, 83–95.
- Kovarik, M.; Everolimus, A. Proliferation signal inhibitor targeting primary causes of allograft dysfunction. Drugs Today 2004, 40, 101–109.
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; de Vries, E.G.; et al. Everolimus for Advanced Pancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2011, 364, 514–523.
- Sessa, C.; Tosi, D.; Viganò, L.; Albanell, J.; Hess, D.; Maur, M.; Cresta, S.; Locatelli, A.; Angst, R.; Rojo, F.; et al. Phase Ib study of weekly mammalian target of rapamycin inhibitor ridaforolimus (AP23573; MK-8669) with weekly paclitaxel. Ann. Oncol. 2009, 21, 1315–1322.
- Chawla, S.P.; Staddon, A.P.; Baker, L.H.; Schuetze, S.M.; Tolcher, A.W.; D’Amato, G.Z.; Blay, J.-Y.; Mita, M.M.; Sankhala, K.K.; Berk, L.; et al. Phase II Study of the Mammalian Target of Rapamycin Inhibitor Ridaforolimus in Patients with Advanced Bone and Soft Tissue Sarcomas. J. Clin. Oncol. 2020, 30, 78–84, Coriggendum in 2017, 35, 2722.
- Serruys, P.W.; Silber, S.; Garg, S.; van Geuns, R.J.; Richardt, G.; Buszman, P.E.; Kelbæk, H.; van Boven, A.J.; Hofma, S.H.; Linke, A.; et al. Comparison of Zotarolimus-Eluting and Everolimus-Eluting Coronary Stents. N. Engl. J. Med. 2010, 363, 136–146.
- Francipane, M.G.; Lagasse, E. mTOR pathway in colorectal cancer: An update. Oncotarget 2014, 5, 49–66.
- Liu, Q.; Wang, J.; Kang, S.A.; Thoreen, C.C.; Hur, W.; Ahmed, T.; Sabatini, D.M.; Gray, N.S. Discovery of 9-(6-Aminopyridin-3-yl)-1-(3-(trifluoromethyl)phenyl)benzo1,6naphthyridin-2(1H)-one (Torin2) as a Potent, Selective, and Orally Available Mammalian Target of Rapamycin (mTOR) Inhibitor for Treatment of Cancer. J. Med. Chem. 2011, 54, 1473–1480.
- Hua, H.; Kong, Q.; Zhang, H.; Wang, J.; Luo, T.; Jiang, Y. Targeting mTOR for cancer therapy. J. Hematol. Oncol. 2019, 12, 71.
- Nguyen, T.-L.; Nokin, M.-J.; Egorov, M.; Tomé, M.; Bodineau, C.; Di Primo, C.; Minder, L.; Wdzieczak-Bakala, J.; Garcia-Alvarez, M.C.; Bignon, J.; et al. mTOR Inhibition via Displacement of Phosphatidic Acid Induces Enhanced Cytotoxicity Specifically in Cancer Cells. Cancer Res. 2018, 78, 5384–5397.
- Bendell, J.C.; Varghese, A.M.; Hyman, D.M.; Bauer, T.M.; Pant, S.; Callies, S.; Lin, J.; Martinez, R.; Wickremsinhe, E.R.; Fink, A.; et al. A First-in-Human Phase 1 Study of LY3023414, an Oral PI3K/mTOR Dual Inhibitor, in Patients with Advanced Cancer. Clin. Cancer Res. 2018, 24, 3253–3262.
- Plews, R.L.; Yusof, A.M.; Wang, C.; Saji, M.; Zhang, X.; Chen, C.-S.; Ringel, M.D.; Phay, J.E. A Novel Dual AMPK Activator/mTOR Inhibitor Inhibits Thyroid Cancer Cell Growth. J. Clin. Endocrinol. Metab. 2015, 100, E748–E756.
- Lee, D.-F.; Kuo, H.-P.; Chen, C.-T.; Hsu, J.-M.; Chou, C.-K.; Wei, Y.; Sun, H.-L.; Li, L.-Y.; Ping, B.; Huang, W.-C.; et al. IKKβ Suppression of TSC1 Links Inflammation and Tumor Angiogenesis via the mTOR Pathway. Cell 2007, 130, 440–455.
- von Manteuffel, S.R.; Gingras, A.C.; Ming, X.F.; Sonenberg, N.; Thomas, G. 4E-BP1 phosphorylation is mediated by the FRAP-p70s6k pathway and is independent of mitogen-activated protein kinase. Proc. Natl. Acad. Sci. USA 1996, 93, 4076–4080.
- Yip, C.K.; Murata, K.; Walz, T.; Sabatini, D.M.; Kang, S.A. Structure of the Human mTOR Complex I and Its Implications for Rapamycin Inhibition. Mol. Cell 2010, 38, 768–774.
- Saucedo, L.J.; Gao, X.; Chiarelli, D.A.; Li, L.; Pan, D.; Edgar, B.A. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat. Cell Biol. 2003, 5, 566–571.
- Ma, L.; Chen, Z.; Erdjument-Bromage, H.; Tempst, P.; Pandolfi, P.P. Phosphorylation and Functional Inactivation of TSC2 by Erk: Implications for Tuberous Sclerosisand Cancer Pathogenesis. Cell 2005, 121, 179–193.
- Inoki, K.; Ouyang, H.; Zhu, T.; Lindvall, C.; Wang, Y.; Zhang, X.; Yang, Q.; Bennett, C.; Harada, Y.; Stankunas, K.; et al. TSC2 Integrates Wnt and Energy Signals via a Coordinated Phosphorylation by AMPK and GSK3 to Regulate Cell Growth. Cell 2006, 126, 955–968.
- Hardie, D.G. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 2007, 8, 774–785.
- Shahbazian, D.; Roux, P.; Mieulet, V.; Cohen, M.S.; Raught, B.; Taunton, J.; Hershey, J.W.B.; Blenis, J.; Pende, M.; Sonenberg, N. The mTOR/PI3K and MAPK pathways converge on eIF4B to control its phosphorylation and activity. EMBO J. 2006, 25, 2781–2791.
- Lenz, G.; Avruch, J. Glutamatergic Regulation of the p70S6 Kinase in Primary Mouse Neurons. J. Biol. Chem. 2005, 280, 38121–38124.
- Keating, R.; McGargill, M.A. mTOR regulation of lymphoid cells in immunity to pathogens. Front. Immunol. 2016, 7, 180.
- Parate, S.; Kumar, V.; Lee, G.; Rampogu, S.; Hong, J.; Lee, K. Marine-Derived Natural Products as ATP-Competitive mTOR Kinase Inhibitors for Cancer Therapeutics. Pharmaceuticals 2021, 14, 282.
- Yordy, B.; Iwasaki, A. Autophagy in the control and pathogenesis of viral infection. Curr. Opin. Virol. 2011, 1, 196–203.
- Siqueira, M.d.S.; Ribeiro, R.d.M.; Travassos, L.H. Autophagy and its interaction with intracellular bacterial pathogens. Front. Immunol. 2018, 9, 935.
- Bahia, D.; Satoskar, A.R.; Dussurget, O. Cell signaling in host–pathogen interactions: The host point of view. Front. Immunol. 2018, 9, 221.
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Schumm, L.P.; Sharma, Y.; Anderson, C.A.; et al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491, 119–124.
- Axtell, R.C.; De Jong, B.A.; Boniface, K.; Van Der Voort, L.F.; Bhat, R.; De Sarno, P.; Naves, R.; Han, M.; Zhong, F.; Castellanos, J.G.; et al. T helper type 1 and 17 cells determine efficacy of interferon-β in multiple sclerosis and experimental encephalomyelitis. Nat. Med. 2010, 16, 406–412.
- Pollizzi, K.N.; Powell, J.D. Regulation of T cells by mTOR: The known knowns and the known unknowns. Trends Immunol. 2014, 36, 13–20.
- Limon, J.J.; Fruman, D.A. Akt and mTOR in B Cell Activation and Differentiation. Front. Immunol. 2012, 3, 228.
- Babiński, S.; Giermaziak, H. Influenza epidemic in 1971 in diabetics treated with 1-butyl-biguanidine hydrochloride (Silubin retard) and 1-phenylethyl-biguanidine hydrochloride (Phenformin). Polski Tyg. Lek. 1973, 28, 1815–1817.
- Chuang, Y.-C.; Ruan, S.-Y. Compelling Results of Adjuvant Therapy with Sirolimus for Severe H1N1 Pneumonia. Crit. Care Med. 2014, 42, e687–e688.
- Liu, L.; Das, S.; Losert, W.; Parent, C.A. mTORC2 regulates neutrophil chemotaxis in a cAMP-and RhoA-dependent fashion. Dev. Cell 2010, 19, 845–857.
- Yang, H.; Wang, X.; Zhang, Y.; Liu, H.; Liao, J.; Shao, K.; Chu, Y.; Liu, G. Modulation of TSC-mTOR signaling on immune cells in immunity and autoimmunity. J. Cell. Physiol. 2014, 229, 17–26.
- Squarize, C.; Castilho, R.M.; Bugge, T.H.; Gutkind, J.S. Accelerated Wound Healing by mTOR Activation in Genetically Defined Mouse Models. PLoS ONE 2010, 5, e10643.
- Katholnig, K.; Linke, M.; Pham, H.; Hengstschläger, M.; Weichhart, T. Immune responses of macrophages and dendritic cells regulated by mTOR signalling. Biochem. Soc. Trans. 2013, 41, 927–933.
- Kufer, T.A.; Banks, D.J.; Philpott, D.J. Innate Immune Sensing of Microbes by Nod Proteins. Ann. N. Y. Acad. Sci. 2006, 1072, 19–27.
- Abdel-Nour, M.; Tsalikis, J.; Kleinman, D.; Girardin, S.E. The emerging role of mTOR signalling in antibacterial immunity. Immunol. Cell Biol. 2014, 92, 346–353.
- Rampersad, S.; Tennant, P. Replication and Expression Strategies of Viruses. Viruses 2018, 55–82.
- Le Sage, V.; Cinti, A.; Amorim, R.; Mouland, A.J. Adapting the Stress Response: Viral Subversion of the mTOR Signaling Pathway. Viruses 2016, 8, 152.
- Terrazzano, G.; Rubino, V.; Palatucci, A.T.; Giovazzino, A.; Carriero, F.; Ruggiero, G. An open question: Is it rational to inhibit the mTor-dependent pathway as COVID-19 therapy? Front. Pharmacol. 2020, 11, 856.
- Castle, B.T.; Dock, C.; Hemmat, M.; Kline, S.; Tignanelli, C.; Rajasingham, R.; Masopust, D.; Provenzano, P.; Langlois, R.; Schacker, T.; et al. Biophysical modeling of the SARS-CoV-2 viral cycle reveals ideal antiviral targets. Biorxiv 2020.
- Beatman, E.; Oyer, R.; Shives, K.D.; Hedman, K.; Brault, A.C.; Tyler, K.L.; Beckham, J.D. West Nile virus growth is independent of autophagy activation. Virology 2012, 433, 262–272.
- Cheng, F.; da Silva, S.R.; Huang, I.-C.; Jung, J.U.; Gao, S.-J. Suppression of Zika Virus Infection and Replication in Endothelial Cells and Astrocytes by PKA Inhibitor PKI 14-22. J. Virol. 2018, 92, e02019-17.
- Nakashima, K.; Takeuchi, K.; Chihara, K.; Hotta, H.; Sada, K. Inhibition of hepatitis C virus replication through adenosine monophosphate-activated protein kinase-dependent and-independent pathways. Microbiol. Immunol. 2011, 55, 774–782.
- Del Campo, J.A.; Garcia-Valdecasas, M.; Gil-Gomez, A.; Rojas, A.; Gallego, P.; Ampuero, J.; Gallego-Durán, R.; Pastor, H.; Grande, L.; Padillo, F.J.; et al. Simvastatin and metformin inhibit cell growth in hepatitis C virus infected cells via mTOR increasing PTEN and autophagy. PLoS ONE 2018, 13, e0191805.
- Proud, C.G. Phosphorylation and Signal Transduction Pathways in Translational Control. Cold Spring Harb. Perspect. Biol. 2019, 11, a033050.
- Wang, C.-H.; Chung, F.-T.; Lin, S.-M.; Huang, S.-Y.; Chou, C.-L.; Lee, K.-Y.; Lin, T.-Y.; Kuo, H.-P. Adjuvant Treatment With a Mammalian Target of Rapamycin Inhibitor, Sirolimus, and Steroids Improves Outcomes in Patients With Severe H1N1 Pneumonia and Acute Respiratory Failure*. Crit. Care Med. 2014, 42, 313–321.
- Denys, A.; Bocian, J. Effect of Silubin-retard (1-butyl-biguanide hydrochloride) on the course of influenza-virus infection in mice. Polski Tyg. Lek. 1970, 25, 332–334.
- Rightsel, W.A.; Dice, J.R.; McAlpine, R.J.; Timm, E.A.; McLean, I.W.; Dixon, G.J.; Schabel, F.M. Antiviral Effect of Guanidine. Science 1961, 134, 558–559.
- Ueda, T.; Toyoshima, S.; Tsuji, T.; Seto, Y.; Nomoto, J. Antiviral effect of guanidine and its derivatives part 1. the inhibitory effect of guanidine on the multiplication of poliomyelitis virus in tissue culture. Keio J. Med. 1961, 10, 257–265.
- Loddo, B. Inhibition of the multiplication in vitro of poliomyelitis viruses by guanidine. VIII. Elective inhibitory activity of guanidine on the PCE of enteroviruses. Boll. Della Soc. Ital. Di Biol. Sper. 1962, 38, 8–10.
- Loddo, B.; Ferrari, W.; Brotzu, G.; Spanedda, A. Inhibitory action of guanidine on the multiplication of enteroviruses and especially of polioviruses. Boll. Dell’Istituto Sieroter. Milan. 1962, 41, 111–120.
- Kindrachuk, J.; Ork, B.; Hart, B.J.; Mazur, S.; Holbrook, M.R.; Frieman, M.B.; Traynor, D.; Johnson, R.F.; Dyall, J.; Kuhn, J.H.; et al. Antiviral Potential of ERK/MAPK and PI3K/AKT/mTOR Signaling Modulation for Middle East Respiratory Syndrome Coronavirus Infection as Identified by Temporal Kinome Analysis. Antimicrob. Agents Chemother. 2015, 59, 1088–1099.
- Garcia, G., Jr.; Sharma, A.; Ramaiah, A.; Sen, C.; Kohn, D.; Gomperts, B.; Svendsen, C.N.; Damoiseaux, R.D.; Arumugaswami, V. Antiviral drug screen of kinase inhibitors identifies cellular signaling pathways critical for SARS-CoV-2 replication. BioRxiv 2020.
- Betz, C.; Hall, M.N. Where is mTOR and what is it doing there? J. Cell Biol. 2013, 203, 563–574.
- Appelberg, S.; Gupta, S.; Svensson Akusjärvi, S.; Ambikan, A.T.; Mikaeloff, F.; Saccon, E.; Végvári, Á.; Benfeitas, R.; Sperk, M.; Ståhlberg, M. Dysregulation in Akt/mTOR/HIF-1 signaling identified by proteo-transcriptomics of SARS-CoV-2 infected cells. Emerg. Microbes Infect. 2020, 9, 1748–1760.
- Luo, P.; Qiu, L.; Liu, Y.; Liu, X.-L.; Zheng, J.-L.; Xue, H.-Y.; Liu, W.-H.; Liu, D.; Li, J. Metformin Treatment Was Associated with Decreased Mortality in COVID-19 Patients with Diabetes in a Retrospective Analysis. Am. J. Trop. Med. Hyg. 2020, 103, 69–72.
More
Information
Subjects:
Cell & Tissue Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
2 times
(View History)
Update Date:
02 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




