
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chien-Hao Huang | -- | 6543 | 2022-11-30 14:01:12 | | | |
| 2 | Lindsay Dong | -4221 word(s) | 2322 | 2022-12-01 09:25:57 | | |
Video Upload Options
Spontaneous bacterial peritonitis (SBP) is defined as a bacterial infection of the ascitic fluid without a surgically treatable intra-abdominal infection source. SBP is a common, severe complication in cirrhosis patients with ascites, and if left untreated, in-hospital mortality may exceed 90%. However, the incidence of SBP has been lowered to approx. 20% through early diagnosis and antibiotic therapy. There are three types of SBP. Bacterial translocation from the gastrointestinal tract is the most common source of SBP. Distinguishing SBP from secondary bacterial peritonitis is essential because the conditions require different therapeutic strategies. The standard treatment for SBP is prompt broad-spectrum antibiotic administration and should be tailored according to community-acquired SBP, healthcare-associated or nosocomial SBP infections, and local resistance profile. Albumin supplementation, especially in patients with renal impairment, is also beneficial. Selective intestinal decontamination is associated with a reduced risk of bacterial infection and mortality in the high-risk group.
1. Introduction
| Ascites Fluid | Classic SBP | CNNA 1 | MNB 2 |
|---|---|---|---|
| PMN count (cells/mm3) | ≥250 | ≥250 | <250 |
| Ascites culture | positive | negative | positive |
2. Pathogenesis
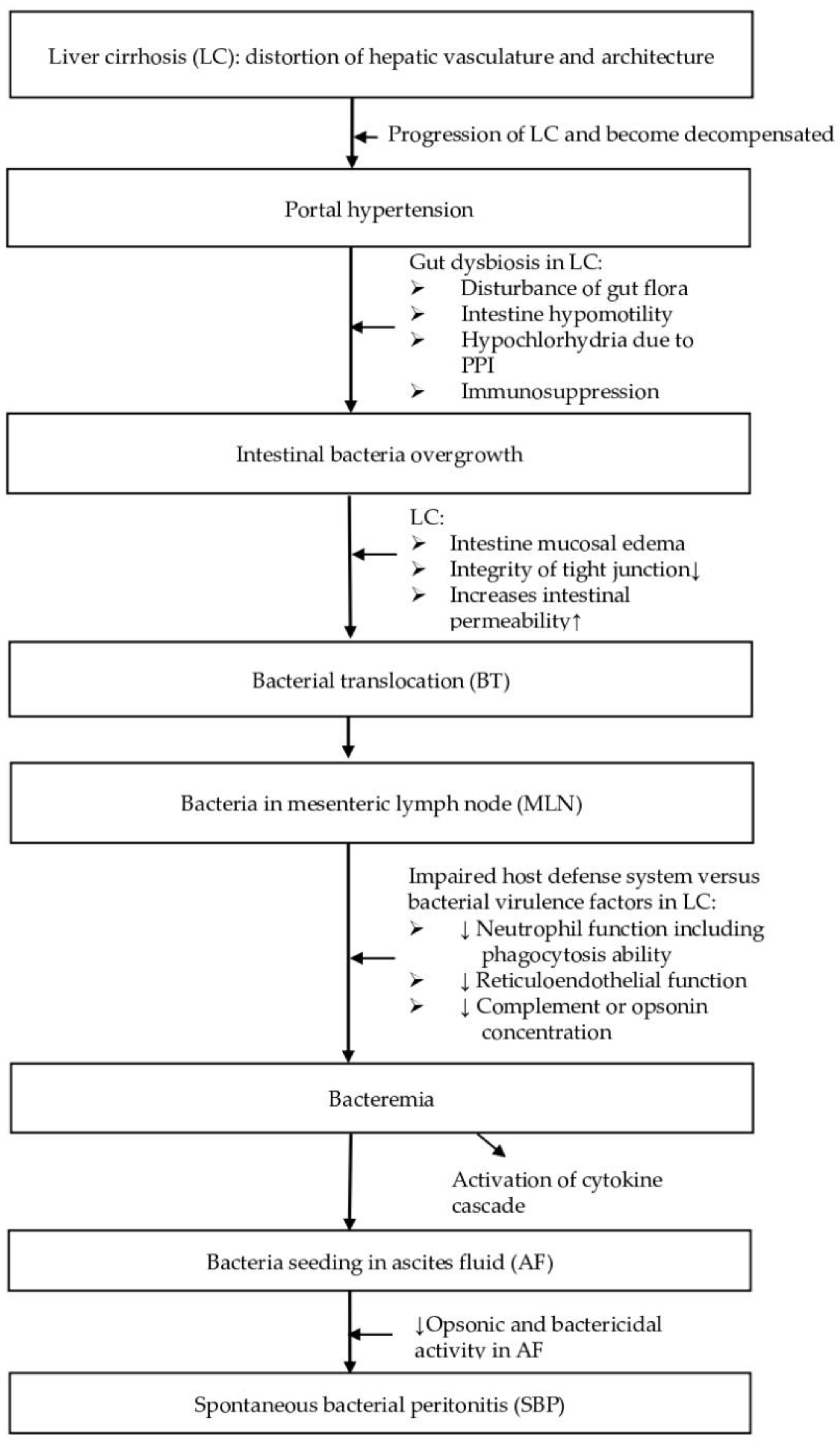
2.1. Gut Dysbiosis
2.2. Bacterial Translocation
2.3. Impaired Host Defense System
3. Bacteriology
4. Diagnosis
Spontaneous bacterial peritonitis (SBP) should be suspected in patients with cirrhosis who develop signs or symptoms, such as fever (69%), abdominal pain (59%), altered mental status (54%), abdominal tenderness (49%), diarrhea (32%), ileus (30%), hypotension/shock (21%), or hypothermia (17%) [29]. However, 10% of cases show no signs or symptoms, partly because a large volume of ascites prevents contact of the visceral and parietal peritoneal surfaces to elicit the spinal reflux that cause abdominal rigidity [29].
A diagnostic paracentesis should be performed in all patients with cirrhosis and ascites who require emergency room care or hospitalization, who demonstrate or report signs/symptoms mentioned above in the clinical presentations, or who present gastrointestinal bleeding, in order to confirm evidence of SBP [31]. However, low clinical suspicion for SBP does not preclude the necessity for paracentesis, since 10% of cases have no signs or symptoms [29].
Ascitic fluid tests should include cell count with a differential, Gram stain, culture, total protein, and albumin to calculate the serum-ascites albumin gradient (SAAG), if not already known [27]. When the diagnosis of cirrhosis is not definite, an ascites SAAG greater than or equal to (≧) 1.1 g/dl is ascribed to portal hypertension with approximately 97% accuracy [31]. Total ascitic fluid protein concentration should be measured to assess the risk of SBP since patients suffering from ascites with a total protein concentration lower than (<) 1.5 g/dL are at increased risk of SBP [7].
A diagnosis of (1) classic SBP is made if PMN count in the ascitic fluid is ≥250 cells/mm3, culture results are positive, and secondary causes of peritonitis are excluded [7][31]. A potential source of error in PMN count is hemorrhage into the ascitic fluid, such as with traumatic paracentesis, which can cause both red and white blood cells to enter the ascites. A corrected PMN count should be calculated if there are bloody ascites by subtracting one PMN from the absolute PMN count for every 250 red cells/mm3 [32]. Distinguishing SBP from secondary bacterial peritonitis is essential because the conditions require different therapeutic strategies. Mortality from SBP can be as high as 85% if a patient undergoes an unnecessary exploratory laparotomy [33], while mortality of secondary bacterial peritonitis can exceed 80% if treatment consists of antibiotics without surgical intervention [9].
5. Treatment
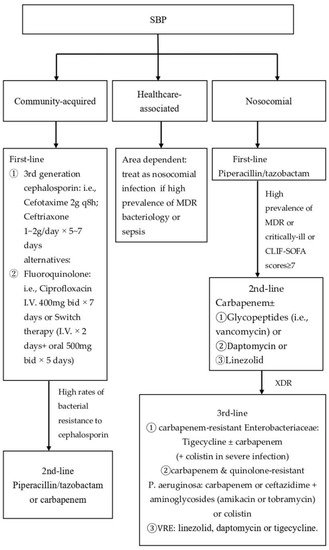
5.1. Antibiotic Therapy
5.2. Albumin Supplement in Patients with Renal Impairment
5.3. Discontinue NSBB in Patients with SBP
5.4. Other Novel Therapeutic Strategies
6. Conclusions
References
- Wasmuth, H.E.; Kunz, D.; Yagmur, E.; Timmer-Stranghöner, A.; Vidacek, D.; Siewert, E.; Bach, J.; Geier, A.; Purucker, E.A.; Gressner, A.M. Patients with acute on chronic liver failure display ‘sepsis-like’immune paralysis. J. Hepatol. 2005, 42, 195–201.
- Wiest, R.; Garcia-Tsao, G. Bacterial translocation (BT) in cirrhosis. Hepatology 2005, 41, 422–433.
- Fernández, J.; Navasa, M.; Gómez, J.; Colmenero, J.; Vila, J.; Arroyo, V.; Rodés, J. Bacterial infections in cirrhosis: Epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology 2002, 35, 140–148.
- Huang, C.H.; Lin, C.Y.; Sheen, I.S.; Chen, W.T.; Lin, T.N.; Ho, Y.P.; Chiu, C.T. Recurrence of spontaneous bacterial peritonitis in cirrhotic patients non-prophylactically treated with norfloxacin: Serum albumin as an easy but reliable predictive factor. Liver Int. 2011, 31, 184–191.
- Aithal, G.P.; Palaniyappan, N.; China, L.; Harmala, S.; Macken, L.; Ryan, J.M.; Wilkes, E.A.; Moore, K.; Leithead, J.A.; Hayes, P.C.; et al. Guidelines on the management of ascites in cirrhosis. Gut 2021, 70, 9–29.
- Garcia-Tsao, G. Current management of the complications of cirrhosis and portal hypertension: Variceal hemorrhage, ascites, and spontaneous bacterial peritonitis. Gastroenterology 2001, 120, 726–748.
- Rimola, A.; García-Tsao, G.; Navasa, M.; Piddock, L.J.; Planas, R.; Bernard, B.; Inadomi, J.M. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: A consensus document. J. Hepatol. 2000, 32, 142–153.
- Runyon, B.A.; AASLD. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology 2013, 57, 1651–1653.
- Akriviadis, E.A.; Runyon, B.A. Utility of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology 1990, 98, 127–133.
- Runyon, B.A.; Committee, A.P.G. Management of adult patients with ascites due to cirrhosis: An update. Hepatology 2009, 49, 2087–2107.
- Runyon, B.A.; Squier, S.; Borzio, M. Translocation of gut bacteria in rats with cirrhosis to mesenteric lymph nodes partially explains the pathogenesis of spontaneous bacterial peritonitis. J. Hepatol. 1994, 21, 792–796.
- Berg, R.D.; Garlington, A.W. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect. Immun. 1979, 23, 403–411.
- Vilela, E.G.; Thabut, D.; Rudler, M.; Bittencourt, P.L. Management of Complications of Portal Hypertension. Can. J. Gastroenterol. Hepatol. 2019, 2019, 6919284.
- Dunn, D.L.; Barke, R.A.; Knight, N.B.; Humphrey, E.W.; Simmons, R.L. Role of resident macrophages, peripheral neutrophils, and translymphatic absorption in bacterial clearance from the peritoneal cavity. Infect. Immun. 1985, 49, 257–264.
- Ruiz-Alcaraz, A.J.; Martinez-Banaclocha, H.; Marin-Sanchez, P.; Carmona-Martinez, V.; Iniesta-Albadalejo, M.A.; Tristan-Manzano, M.; Tapia-Abellan, A.; Garcia-Penarrubia, P.; Machado-Linde, F.; Pelegrin, P.; et al. Isolation of functional mature peritoneal macrophages from healthy humans. Immunol. Cell Biol. 2020, 98, 114–126.
- Charles, A.; Janeway, J.; Travers, P.; Walport, M.; Shlomchik, M.J. Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Publishing: New York, NY, USA, 2001.
- Huang, C.H.; Jeng, W.J.; Ho, Y.P.; Teng, W.; Hsieh, Y.C.; Chen, W.T.; Chen, Y.C.; Lin, H.H.; Sheen, I.S.; Lin, C.Y. Increased EMR2 expression on neutrophils correlates with disease severity and predicts overall mortality in cirrhotic patients. Sci. Rep. 2016, 6, 38250.
- Rimola, A.; Soto, R.; Bory, F.; Arroyo, V.; Piera, C.; Rodes, J. Reticuloendothelial system phagocytic activity in cirrhosis and its relation to bacterial infections and prognosis. Hepatology 1984, 4, 53–58.
- Runyon, B.A. Low-protein-concentration ascitic fluid is predisposed to spontaneous bacterial peritonitis. Gastroenterology 1986, 91, 1343–1346.
- Hoefs, J.C.; Runyon, B.A. Spontaneous bacterial peritonitis. Dis. Mon. 1985, 31, 1–48.
- Wiest, R.; Krag, A.; Gerbes, A. Spontaneous bacterial peritonitis: Recent guidelines and beyond. Gut 2012, 61, 297–310.
- Oladimeji, A.A.; Temi, A.P.; Adekunle, A.E.; Taiwo, R.H.; Ayokunle, D.S. Prevalence of spontaneous bacterial peritonitis in liver cirrhosis with ascites. Pan Afr. Med. J. 2013, 15, 128.
- Bhuva, M.; Ganger, D.; Jensen, D. Spontaneous bacterial peritonitis: An update on evaluation, management, and prevention. Am. J. Med. 1994, 97, 169–175.
- Runyon, B.A. Monomicrobial nonneutrocytic bacterascites: A variant of spontaneous bacterial peritonitis. Hepatology 1990, 12, 710–715.
- Alexopoulou, A.; Papadopoulos, N.; Eliopoulos, D.G.; Alexaki, A.; Tsiriga, A.; Toutouza, M.; Pectasides, D. Increasing frequency of gram-positive cocci and gram-negative multidrug-resistant bacteria in spontaneous bacterial peritonitis. Liver Int. 2013, 33, 975–981.
- Fernandez, J.; Acevedo, J.; Castro, M.; Garcia, O.; de Lope, C.R.; Roca, D.; Pavesi, M.; Sola, E.; Moreira, L.; Silva, A.; et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: A prospective study. Hepatology 2012, 55, 1551–1561.
- Dever, J.B.; Sheikh, M.Y. Review article: Spontaneous bacterial peritonitis--bacteriology, diagnosis, treatment, risk factors and prevention. Aliment. Pharmacol. Ther. 2015, 41, 1116–1131.
- Reuken, P.A.; Pletz, M.W.; Baier, M.; Pfister, W.; Stallmach, A.; Bruns, T. Emergence of spontaneous bacterial peritonitis due to enterococci—Risk factors and outcome in a 12-year retrospective study. Aliment Pharm. 2012, 35, 1199–1208.
- Such, J.; Runyon, B.A. Spontaneous bacterial peritonitis. Clin. Infect. Dis. 1998, 27, 669–674, quiz 675–666.
- Sheckman, P.; Onderdonk, A.B.; Bartlett, J.G. Anaerobes in spontaneous peritonitis. Lancet 1977, 2, 1223.
- European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J. Hepatol. 2010, 53, 397–417.
- Hoefs, J.C. Increase in ascites white blood cell and protein concentrations during diuresis in patients with chronic liver disease. Hepatology 1981, 1, 249–254.
- Garrison, R.N.; Cryer, H.M.; Howard, D.A.; Polk, H.C., Jr. Clarification of risk factors for abdominal operations in patients with hepatic cirrhosis. Ann. Surg. 1984, 199, 648–655.
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018, 69, 406–460.
- Cabrera, J.; Arroyo, V.; Ballesta, A.M.; Rimola, A.; Gual, J.; Elena, M.; Rodes, J. Aminoglycoside nephrotoxicity in cirrhosis. Value of urinary beta 2-microglobulin to discriminate functional renal failure from acute tubular damage. Gastroenterology 1982, 82, 97–105.
- Umgelter, A.; Reindl, W.; Miedaner, M.; Schmid, R.M.; Huber, W. Failure of current antibiotic first-line regimens and mortality in hospitalized patients with spontaneous bacterial peritonitis. Infection 2009, 37, 2–8.
- Sort, P.; Navasa, M.; Arroyo, V.; Aldeguer, X.; Planas, R.; Ruiz-del-Arbol, L.; Castells, L.; Vargas, V.; Soriano, G.; Guevara, M.; et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N. Engl. J. Med. 1999, 341, 403–409.
- Sigal, S.H.; Stanca, C.M.; Fernandez, J.; Arroyo, V.; Navasa, M. Restricted use of albumin for spontaneous bacterial peritonitis. Gut 2007, 56, 597–599.
- Salerno, F.; Navickis, R.J.; Wilkes, M.M. Albumin infusion improves outcomes of patients with spontaneous bacterial peritonitis: A meta-analysis of randomized trials. Clin. Gastroenterol. Hepatol. 2013, 11, 123–130.e1.
- Mandorfer, M.; Bota, S.; Schwabl, P.; Bucsics, T.; Pfisterer, N.; Kruzik, M.; Hagmann, M.; Blacky, A.; Ferlitsch, A.; Sieghart, W.; et al. Nonselective beta blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology 2014, 146, 1680–1690.e1.
- Pampalone, M.; Vitale, G.; Gruttadauria, S.; Amico, G.; Iannolo, G.; Douradinha, B.; Mularoni, A.; Conaldi, P.G.; Pietrosi, G. Human Amnion-Derived Mesenchymal Stromal Cells: A New Potential Treatment for Carbapenem-Resistant Enterobacterales in Decompensated Cirrhosis. Int. J. Mol. Sci. 2022, 23, 857.
- Huang, C.H.; Tseng, H.J.; Amodio, P.; Chen, Y.L.; Wang, S.F.; Chang, S.H.; Hsieh, S.Y.; Lin, C.Y. Hepatic Encephalopathy and Spontaneous Bacterial Peritonitis Improve Cirrhosis Outcome Prediction: A Modified Seven-Stage Model as a Clinical Alternative to MELD. J. Pers. Med. 2020, 10, 186.
- Hung, T.H.; Tsai, C.C.; Hsieh, Y.H.; Tsai, C.C. The long-term mortality of spontaneous bacterial peritonitis in cirrhotic patients: A 3-year nationwide cohort study. Turk. J. Gastroenterol. 2015, 26, 159–162.
- Andreu, M.; Sola, R.; Sitges-Serra, A.; Alia, C.; Gallen, M.; Vila, M.C.; Coll, S.; Oliver, M.I. Risk factors for spontaneous bacterial peritonitis in cirrhotic patients with ascites. Gastroenterology 1993, 104, 1133–1138.
- Bac, D.J. Spontaneous bacterial peritonitis: An indication for liver transplantation? Scand. J. Gastroenterol. Suppl. 1996, 218, 38–42.
- Gines, P.; Rimola, A.; Planas, R.; Vargas, V.; Marco, F.; Almela, M.; Forne, M.; Miranda, M.L.; Llach, J.; Salmeron, J.M.; et al. Norfloxacin prevents spontaneous bacterial peritonitis recurrence in cirrhosis: Results of a double-blind, placebo-controlled trial. Hepatology 1990, 12, 716–724.
- Soriano, G.; Guarner, C.; Teixido, M.; Such, J.; Barrios, J.; Enriquez, J.; Vilardell, F. Selective intestinal decontamination prevents spontaneous bacterial peritonitis. Gastroenterology 1991, 100, 477–481.
- Soriano, G.; Guarner, C.; Tomas, A.; Villanueva, C.; Torras, X.; Gonzalez, D.; Sainz, S.; Anguera, A.; Cusso, X.; Balanzo, J.; et al. Norfloxacin prevents bacterial infection in cirrhotics with gastrointestinal hemorrhage. Gastroenterology 1992, 103, 1267–1272.
- Grange, J.D.; Roulot, D.; Pelletier, G.; Pariente, E.A.; Denis, J.; Ink, O.; Blanc, P.; Richardet, J.P.; Vinel, J.P.; Delisle, F.; et al. Norfloxacin primary prophylaxis of bacterial infections in cirrhotic patients with ascites: A double-blind randomized trial. J. Hepatol. 1998, 29, 430–436.




