
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yanjie Liu | -- | 1565 | 2022-11-29 06:06:03 | | | |
| 2 | Rita Xu | Meta information modification | 1565 | 2022-11-29 06:50:22 | | |
Video Upload Options
As important pollinators, bees play a critical role in maintaining the balance of the ecosystem and improving the yield and quality of crops. However, in recent years, the bee population has significantly declined due to various pathogens and environmental stressors including viruses, bacteria, parasites, and increased pesticide application. The above threats trigger or suppress the innate immunity of bees, their only immune defense system, which is essential to maintaining individual health and that of the colony. In addition, bees can be divided into solitary and eusocial bees based on their life traits, and eusocial bees possess special social immunities, such as grooming behavior, which cooperate with innate immunity to maintain the health of the colony.
1. Introduction
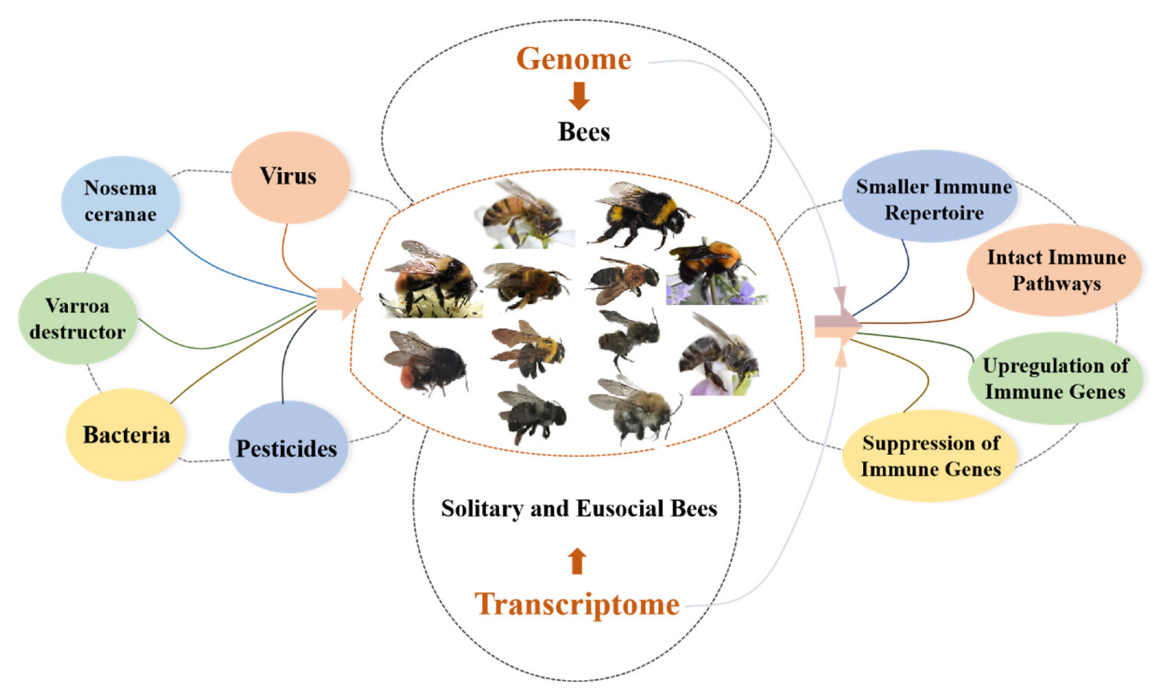
2. Genomic Perspective of Innate Bee Immunity
3. Transcriptomic Perspective of Innate Bee Immune Response
3.1. Immune Responses to Viruses
3.2. Immune Response to Parasites
References
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957.
- Hristov, P.; Shumkova, R.; Palova, N.; Neov, B. Factors Associated with Honey Bee Colony Losses: A Mini-Review. Vet. Sci. 2020, 7, 166.
- Sánchez-Bayo, F.; Goulson, D.; Pennacchio, F.; Nazzi, F.; Goka, K.; Desneux, N. Are bee diseases linked to pesticides?—A brief review. Environ. Int. 2016, 89–90, 7–11.
- Cameron, S.A.; Sadd, B.M. Global Trends in Bumble Bee Health. Annu. Rev. Entomol. 2020, 65, 209–232.
- Kluser, S.; Peduzzi, P. Global Pollinator Decline: A Literature Review; UNEP/GRIDEurope: Geneva, Switzerland, 2007; Volume 8, 10p.
- LeBuhn, G.; Vargas Luna, J. Pollinator decline: What do we know about the drivers of solitary bee declines? Curr. Opin. Insect Sci. 2021, 46, 106–111.
- Pareek, C.S.; Smoczynski, R.; Tretyn, A. Sequencing technologies and genome sequencing. J. Appl. Genet. 2011, 52, 413–435.
- Martin, J.A.; Wang, Z. Next-generation transcriptome assembly. Nat. Rev. Genet. 2011, 12, 671–682.
- Branstetter, M.G.; Childers, A.K.; Cox-Foster, D.; Hopper, K.R.; Kapheim, K.M.; Toth, A.L.; Worley, K.C. Genomes of the Hymenoptera. Curr. Opin. Insect Sci. 2018, 25, 65–75.
- Grozinger, C.M.; Robinson, G.E. The power and promise of applying genomics to honey bee health. Curr. Opin. Insect Sci. 2015, 10, 124–132.
- Consortium, H.G.S. Insights into social insects from the genome of the honeybee Apis mellifera. Nature 2006, 443, 931–949.
- Elsik, C.G.; Worley, K.C.; Bennett, A.K.; Beye, M.; Camara, F.; Childers, C.P.; de Graaf, D.C.; Debyser, G.; Deng, J.; Devreese, B. Finding the missing honey bee genes: Lessons learned from a genome upgrade. BMC Genom. 2014, 15, 86.
- McAfee, A.; Harpur, B.A.; Michaud, S.; Beavis, R.C.; Kent, C.F.; Zayed, A.; Foster, L.J. Toward an Upgraded Honey Bee (Apis mellifera L.) Genome Annotation Using Proteogenomics. J. Proteome Res. 2016, 15, 411–421.
- Evans, J.; Aronstein, K.; Chen, Y.P.; Hetru, C.; Imler, J.L.; Jiang, H.; Kanost, M.; Thompson, G.; Zou, Z.; Hultmark, D. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol. Biol. 2006, 15, 645–656.
- Park, D.; Jung, J.W.; Choi, B.-S.; Jayakodi, M.; Lee, J.; Lim, J.; Yu, Y.; Choi, Y.-S.; Lee, M.-L.; Park, Y. Uncovering the novel characteristics of Asian honey bee, Apis cerana, by whole genome sequencing. BMC Genom. 2015, 16, 1.
- Diao, Q.; Sun, L.; Zheng, H.; Zeng, Z.; Wang, S.; Xu, S.; Zheng, H.; Chen, Y.; Shi, Y.; Wang, Y.; et al. Genomic and transcriptomic analysis of the Asian honeybee Apis cerana provides novel insights into honeybee biology. Sci. Rep. 2018, 8, 822.
- Lan, L.; Shi, P.; Song, H.; Tang, X.; Zhou, J.; Yang, J.; Yang, M.; Xu, J. De Novo Genome Assembly of Chinese Plateau Honeybee Unravels Intraspecies Genetic Diversity in the Eastern Honeybee, Apis cerana. Insects 2021, 12, 891.
- Sun, C.; Huang, J.; Wang, Y.; Zhao, X.; Su, L.; Thomas, G.W.; Zhao, M.; Zhang, X.; Jungreis, I.; Kellis, M. Genus-wide characterization of bumblebee genomes provides insights into their evolution and variation in ecological and behavioral traits. Mol. Biol. Evol. 2021, 38, 486–501.
- Sadd, B.M.; Barribeau, S.M.; Bloch, G.; De Graaf, D.C.; Dearden, P.; Elsik, C.G.; Gadau, J.; Grimmelikhuijzen, C.J.; Hasselmann, M.; Lozier, J.D. The genomes of two key bumblebee species with primitive eusocial organization. Genome Biol. 2015, 16, 76.
- Oppenheim, S.; Cao, X.; Rueppel, O.; Krongdang, S.; Phokasem, P.; DeSalle, R.; Goodwin, S.; Xing, J.; Chantawannakul, P.; Rosenfeld, J.A. Whole genome sequencing and assembly of the Asian honey bee Apis dorsata. Genome Biol. Evol. 2020, 12, 3677–3683.
- Cremer, S.; Armitage, S.A.O.; Schmid-Hempel, P. Social Immunity. Curr. Biol. 2007, 17, R693–R702.
- Xu, J.; James, R. Genes related to immunity, as expressed in the alfalfa leafcutting bee, Megachile rotundata, during pathogen challenge. Insect Mol. Biol. 2009, 18, 785–794.
- Kapheim, K.M.; Pan, H.; Li, C.; Salzberg, S.L.; Puiu, D.; Magoc, T.; Robertson, H.M.; Hudson, M.E.; Venkat, A.; Fischman, B.J. Genomic signatures of evolutionary transitions from solitary to group living. Science 2015, 348, 1139–1143.
- Werren, J.H.; Richards, S.; Desjardins, C.A.; Niehuis, O.; Gadau, J.; Colbourne, J.K.; Group, N.G.W.; Beukeboom, L.W.; Desplan, C.; Elsik, C.G. Functional and evolutionary insights from the genomes of three parasitoid Nasonia species. Science 2010, 327, 343–348.
- Xiao, J.-H.; Yue, Z.; Jia, L.-Y.; Yang, X.-H.; Niu, L.-H.; Wang, Z.; Zhang, P.; Sun, B.-F.; He, S.-M.; Li, Z.; et al. Obligate mutualism within a host drives the extreme specialization of a fig wasp genome. Genome Biol. 2013, 14, R141.
- Barribeau, S.M.; Sadd, B.M.; du Plessis, L.; Brown, M.J.F.; Buechel, S.D.; Cappelle, K.; Carolan, J.C.; Christiaens, O.; Colgan, T.J.; Erler, S.; et al. A depauperate immune repertoire precedes evolution of sociality in bees. Genome Biol. 2015, 16, 83.
- Zhou, Q.-S.; Luo, A.; Zhang, F.; Niu, Z.-Q.; Wu, Q.-T.; Xiong, M.; Orr, M.C.; Zhu, C.-D. The First Draft Genome of the Plasterer Bee Colletes gigas (Hymenoptera: Colletidae: Colletes). Genome Biol. Evol. 2020, 12, 860–866.
- Wey, B.; Heavner, M.E.; Wittmeyer, K.T.; Briese, T.; Hopper, K.R.; Govind, S. Immune suppressive extracellular vesicle proteins of Leptopilina heterotoma are encoded in the wasp genome. G3 Genes Genomes Genet. 2020, 10, 1–12.
- Heraghty, S.D.; Sutton, J.M.; Pimsler, M.L.; Fierst, J.L.; Strange, J.P.; Lozier, J.D. De Novo Genome Assemblies for Three North American Bumble Bee Species: Bombus bifarius, Bombus vancouverensis, and Bombus vosnesenskii. G3 Genes Genomes Genet. 2020, 10, 2585–2592.
- Tvedte, E.S.; Walden, K.K.; McElroy, K.E.; Werren, J.H.; Forbes, A.A.; Hood, G.R.; Logsdon, J.M., Jr.; Feder, J.L.; Robertson, H.M. Genome of the parasitoid wasp Diachasma alloeum, an emerging model for ecological speciation and transitions to asexual reproduction. Genome Biol. Evol. 2019, 11, 2767–2773.
- Kapheim, K.M.; Pan, H.; Li, C.; Blatti, C., III; Harpur, B.A.; Ioannidis, P.; Jones, B.M.; Kent, C.F.; Ruzzante, L.; Sloofman, L. Draft genome assembly and population genetics of an agricultural pollinator, the solitary alkali bee (Halictidae: Nomia melanderi). G3 Genes Genomes Genet. 2019, 9, 625–634.
- Yin, C.; Li, M.; Hu, J.; Lang, K.; Chen, Q.; Liu, J.; Guo, D.; He, K.; Dong, Y.; Luo, J. The genomic features of parasitism, polyembryony and immune evasion in the endoparasitic wasp Macrocentrus cingulum. BMC Genom. 2018, 19, 420.
- Haddad, N.J.; Adjlane, N.; Saini, D.; Menon, A.; Krishnamurthy, V.; Jonklaas, D.; Tomkins, J.P.; Loucif-Ayad, W.; Horth, L. Whole-genome sequencing of north African honey bee Apis mellifera intermissa to assess its beneficial traits. Entomol. Res. 2018, 48, 174–186.
- Geib, S.M.; Liang, G.H.; Murphy, T.D.; Sim, S.B. Whole genome sequencing of the braconid parasitoid wasp Fopius arisanus, an important biocontrol agent of pest tepritid fruit flies. G3 Genes Genomes Genet. 2017, 7, 2407–2411.
- Brand, P.; Saleh, N.; Pan, H.; Li, C.; Kapheim, K.M.; Ramírez, S.R. The nuclear and mitochondrial genomes of the facultatively eusocial orchid bee Euglossa dilemma. G3 Genes Genomes Genet. 2017, 7, 2891–2898.
- Standage, D.S.; Berens, A.J.; Glastad, K.M.; Severin, A.J.; Brendel, V.P.; Toth, A.L. Genome, transcriptome and methylome sequencing of a primitively eusocial wasp reveal a greatly reduced DNA methylation system in a social insect. Mol. Ecol. 2016, 25, 1769–1784.
- Rehan, S.M.; Glastad, K.M.; Lawson, S.P.; Hunt, B.G. The genome and methylome of a subsocial small carpenter bee, Ceratina calcarata. Genome Biol. Evol. 2016, 8, 1401–1410.
- Haddad, N.J.; Loucif-Ayad, W.; Adjlane, N.; Saini, D.; Manchiganti, R.; Krishnamurthy, V.; AlShagoor, B.; Batainh, A.M.; Mugasimangalam, R. Draft genome sequence of the Algerian bee Apis mellifera intermissa. Genom. Data 2015, 4, 24–25.
- Kocher, S.D.; Li, C.; Yang, W.; Tan, H.; Yi, S.V.; Yang, X.; Hoekstra, H.E.; Zhang, G.; Pierce, N.E.; Yu, D.W. The draft genome of a socially polymorphic halictid bee, Lasioglossum albipes. Genome Biol. 2013, 14, R142.
- Hrdlickova, R.; Toloue, M.; Tian, B. RNA-Seq methods for transcriptome analysis. Wiley Interdiscip. Rev. RNA 2017, 8, e1364.
- DeGrandi-Hoffman, G.; Chen, Y. Nutrition, immunity and viral infections in honey bees. Curr. Opin. Insect Sci. 2015, 10, 170–176.
- Grozinger, C.M.; Flenniken, M.L. Bee Viruses: Ecology, Pathogenicity, and Impacts. Annu. Rev. Entomol. 2019, 64, 205–226.
- McMenamin, A.J.; Daughenbaugh, K.F.; Parekh, F.; Pizzorno, M.C.; Flenniken, M.L. Honey Bee and Bumble Bee Antiviral Defense. Viruses 2018, 10, 395.
- Wang, K.; Zhu, L.; Rao, L.; Zhao, L.; Wang, Y.; Wu, X.; Zheng, H.; Liao, X. Nano- and micro-polystyrene plastics disturb gut microbiota and intestinal immune system in honeybee. Sci. Total Environ. 2022, 842, 156819.
- McMenamin, A.J.; Flenniken, M.L. Recently identified bee viruses and their impact on bee pollinators. Curr. Opin. Insect Sci. 2018, 26, 120–129.
- de Miranda, J.R.; Gauthier, L.; Ribière, M.; Chen, Y.P. Honey bee viruses and their effect on bee and colony health. In Honey Bee Colony Health; CRC Press: Boca Raton, FL, USA, 2011; pp. 71–102.
- Yañez, O.; Piot, N.; Dalmon, A.; de Miranda, J.R.; Chantawannakul, P.; Panziera, D.; Amiri, E.; Smagghe, G.; Schroeder, D.; Chejanovsky, N. Bee Viruses: Routes of Infection in Hymenoptera. Front. Microbiol. 2020, 11, 943.
- Galbraith, D.A.; Yang, X.; Niño, E.L.; Yi, S.; Grozinger, C. Parallel Epigenomic and Transcriptomic Responses to Viral Infection in Honey Bees (Apis mellifera). PLoS Pathog. 2015, 11, e1004713.
- Li-Byarlay, H.; Boncristiani, H.; Howell, G.; Herman, J.; Clark, L.; Strand, M.K.; Tarpy, D.; Rueppell, O. Transcriptomic and Epigenomic Dynamics of Honey Bees in Response to Lethal Viral Infection. Front. Genet. 2020, 11, 566320.
- Doublet, V.; Paxton, R.J.; McDonnell, C.M.; Dubois, E.; Nidelet, S.; Moritz, R.F.A.; Alaux, C.; Le Conte, Y. Brain transcriptomes of honey bees (Apis mellifera) experimentally infected by two pathogens: Black queen cell virus and Nosema ceranae. Genom. Data 2016, 10, 79–82.
- Ryabov, E.V.; Fannon, J.M.; Moore, J.D.; Wood, G.R.; Evans, D.J. The Iflaviruses Sacbrood virus and Deformed wing virus evoke different transcriptional responses in the honeybee which may facilitate their horizontal or vertical transmission. PeerJ 2016, 4, e1591.
- Kim, W.J.; Lee, S.-H.; Kim, J.H.; Fang, Y.; Ha, K.B.; Park, D.H.; Choi, J.Y.; Je, Y.H. Differential gene expressions of innate immune related genes of the Asian honeybee, Apis cerana, latently infected with sacbrood virus. J. Asia-Pac. Entomol. 2017, 20, 17–21.
- Deng, Y.; Zhao, H.; Shen, S.; Yang, S.; Yang, D.; Deng, S.; Hou, C. Identification of Immune Response to Sacbrood Virus Infection in Apis cerana Under Natural Condition. Front. Genet. 2020, 11, 587509.
- Guo, Y.; Zhang, Z.; Zhuang, M.; Wang, L.; Li, K.; Yao, J.; Yang, H.; Huang, J.; Hao, Y.; Ying, F. Transcriptome profiling reveals a novel mechanism of antiviral immunity upon sacbrood virus infection in honey bee larvae (Apis cerana). Front. Microbiol. 2021, 12, 615893.
- Pizzorno, M.C.; Field, K.; Kobokovich, A.L.; Martin, P.L.; Gupta, R.A.; Mammone, R.; Rovnyak, D.; Capaldi, E.A. Transcriptomic responses of the honey bee brain to infection with deformed wing virus. Viruses 2021, 13, 287.
- Amiri, E.; Herman, J.J.; Strand, M.K.; Tarpy, D.R.; Rueppell, O. Egg transcriptome profile responds to maternal virus infection in honey bees, Apis mellifera. Infect. Genet. Evol. 2020, 85, 104558.
- Traynor, K.S.; Mondet, F.; de Miranda, J.R.; Techer, M.; Kowallik, V.; Oddie, M.A.Y.; Chantawannakul, P.; McAfee, A. Varroa destructor: A Complex Parasite, Crippling Honey Bees Worldwide. Trends Parasitol. 2020, 36, 592–606.
- Nazzi, F.; Brown, S.P.; Annoscia, D.; Del Piccolo, F.; Di Prisco, G.; Varricchio, P.; Della Vedova, G.; Cattonaro, F.; Caprio, E.; Pennacchio, F. Synergistic Parasite-Pathogen Interactions Mediated by Host Immunity Can Drive the Collapse of Honeybee Colonies. PLoS Pathog. 2012, 8, e1002735.
- Annoscia, D.; Brown, S.P.; Di Prisco, G.; De Paoli, E.; Del Fabbro, S.; Frizzera, D.; Zanni, V.; Galbraith, D.A.; Caprio, E.; Grozinger, C.M.; et al. Haemolymph removal by Varroa mite destabilizes the dynamical interaction between immune effectors and virus in bees, as predicted by Volterra’s model. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190331.
- Doublet, V.; Poeschl, Y.; Gogol-Döring, A.; Alaux, C.; Annoscia, D.; Aurori, C.; Barribeau, S.M.; Bedoya-Reina, O.C.; Brown, M.J.F.; Bull, J.C.; et al. Unity in defence: Honeybee workers exhibit conserved molecular responses to diverse pathogens. BMC Genom. 2017, 18, 207.
- Noël, A.; Le Conte, Y.; Mondet, F. Varroa destructor: How does it harm Apis mellifera honey bees and what can be done about it? Emerg. Top. Life Sci. 2020, 4, 45–57.
- Ryabov, E.V.; Wood, G.R.; Fannon, J.M.; Moore, J.D.; Bull, J.C.; Chandler, D.; Mead, A.; Burroughs, N.; Evans, D.J. A Virulent Strain of Deformed Wing Virus (DWV) of Honeybees (Apis mellifera) Prevails after Varroa destructor-Mediated, or In Vitro, Transmission. PLoS Pathog. 2014, 10, e1004230.
- Zanni, V.; Galbraith, D.A.; Annoscia, D.; Grozinger, C.M.; Nazzi, F. Transcriptional signatures of parasitization and markers of colony decline in Varroa-infested honey bees (Apis mellifera). Insect Biochem. Mol. Biol. 2017, 87, 1–13.
- Navajas, M.; Migeon, A.; Alaux, C.; Martin-Magniette, M.L.; Robinson, G.E.; Evans, J.D.; Cros-Arteil, S.; Crauser, D.; Le Conte, Y. Differential gene expression of the honey bee Apis mellifera associated with Varroa destructor infection. BMC Genom. 2008, 9, 301.
- Le Conte, Y.; Alaux, C.; Martin, J.F.; Harbo, J.R.; Harris, J.W.; Dantec, C.; Séverac, D.; Cros-Arteil, S.; Navajas, M. Social immunity in honeybees (Apis mellifera): Transcriptome analysis of varroa-hygienic behaviour. Insect Mol. Biol. 2011, 20, 399–408.
- Alaux, C.; Dantec, C.; Parrinello, H.; Le Conte, Y. Nutrigenomics in honey bees: Digital gene expression analysis of pollen’s nutritive effects on healthy and varroa-parasitized bees. BMC Genom. 2011, 12, 496.




