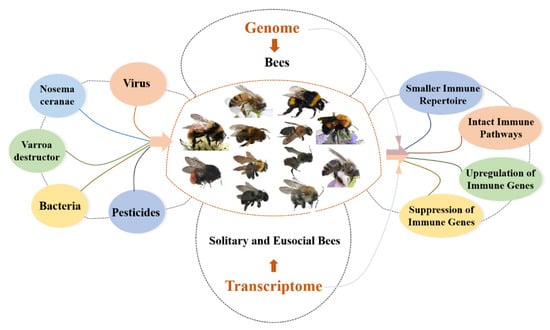
As important pollinators, bees play a critical role in maintaining the balance of the ecosystem and improving the yield and quality of crops. However, in recent years, the bee population has significantly declined due to various pathogens and environmental stressors including viruses, bacteria, parasites, and increased pesticide application. The above threats trigger or suppress the innate immunity of bees, their only immune defense system, which is essential to maintaining individual health and that of the colony. In addition, bees can be divided into solitary and eusocial bees based on their life traits, and eusocial bees possess special social immunities, such as grooming behavior, which cooperate with innate immunity to maintain the health of the colony.
1. Introduction
While the demand for crop pollination by insects has tripled over the past 50 years, the pollinator-bee population has drastically declined due to climate change, habitat loss, emerging parasites and pathogens, and increased pesticide application [
1,
2,
3,
4,
5,
6]. Due to these challenges and environmental stresses such as poor nutrition and pesticide residues, pollinator bees use their innate immune system, which is their only defense, to maintain their individual health and that of the colony. The emergence and development of genomic and transcriptomic technology provide an opportunity to understand the mysteries of life sciences [
7,
8]. Similarly, genomic and transcriptomic research on bees have helped identify and understand the genetic traits of immunity and the immune response to environmental stressors and pathogens, both primordial aspects of the colony’s health [
9,
10]. The genetic characteristics of innate immunity and immune response to pathogens and pesticides in bees from a genomic and transcriptomic perspective (
Figure 1).
Figure 1. Overview of the innate immunity of bees from the genome and transcriptome perspectives.
2. Genomic Perspective of Innate Bee Immunity
The first complete bee genome, the
Apis mellifera genome, was assembled and annotated in 2006 [
11], and it was updated in 2014 and 2016 [
12,
13]. Compared to known genomes of model organisms such as
Drosophila melanogaster and
Anopheles gambiae, the
A. mellifera genome encodes fewer immune proteins involved in the immune response process, starting from pathogen recognition to immune effectors. In fact, nearly two-thirds of the immune genes are reduced, but a small number of genes encode the components of the insect’s classical immune pathways, such as Toll, IMD, and JAK/STAT pathways [
11,
12,
14]. Based on the genomic and transcriptomic analyses,
Apis cerana, a species similar to
A. mellifera, also possesses a small amount of innate immune genes and similar classical immune pathways compared to those of flies and mosquitoes, and most of its immune genes are similar to those of
A. mellifera [
15,
16]. Recently, de novo genome assembly of Chinese plateau
A. cerana has shown that the gene number of this genome is different from that of known
A. cerana genomes [
17]. As a representative of primary eusocial bees, bumblebee genomes from 17 species show that the major immune repertoire and immune gene number are both similar to those of
A. mellifera, which is significantly lower compared with that of Dipteran models [
18,
19]. Moreover, another important Asian honeybee (
Apis dorsata) genome also exhibits an immune repertoire similar to that of known bee genomes [
20]. A reduced number of immune proteins might be seen as a result of the social immunity of social bees; eusocial and primary eusocial bees can cooperate to reduce disease transmission risk through their behavior, known as social immunity, which can be prophylactic or activated on demand [
21]. However, expressed sequence tag (EST) databases of healthy and pathogen-challenged alfalfa leaf-cutting bee larvae have identified 104 putative immunity-related genes, including innate immune response genes that are highly conserved with honey bee genes, such as those involved in pathogen recognition, phagocytosis, prophenoloxidase cascade, melanization, coagulation, and several signaling pathways [
22]. Similar smaller immune repertoires have been discovered in other available solitary or eusocial bee genomes, including those of
A. florea,
Bombus terrestris,
B. impatiens,
Eufriesea Mexicana,
Melipona quadrifasciata,
Habropoda laboriosa,
Megachile rotundata,
Lasioglossum albipes, and
Dufourea novaeangliae [
19,
23]. Additionally, genomes of three parasitoid
Nasonia species (
N. vitripennis,
N. giraulti,
and N. longicornis) show an immune repertoire similar to that of
A. mellifera but with a slightly higher gene count than that of the latter, although several immune genes are not yet identified [
24]. Furthermore, a fig wasp (
Ceratosolen solmsi) genome exhibits an immune repertoire and gene counts similar to those of
A. mellifera [
25]. Therefore, although different bee species possess slightly different immune gene counts, their innate immune system is characterized by integral immune pathways, and the reduced immune gene number is interestingly not related to the bees’ sociality [
26]. Thus, genomic analysis is a powerful tool for exploring the innate immune components of both solitary and eusocial bees. Until now, several genomes of different bees have been determined, but the immune repertoire of these bees has to be further analyzed [
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39].
3. Transcriptomic Perspective of Innate Bee Immune Response
Transcriptomic analysis in bees indicates gene expression changes under certain conditions. For instance, the expression profile of the immune genes is mainly influenced by invasion by pathogens, such as viruses, bacteria, and parasites, as well as exposure to pesticides and other hazardous substances, as well as poor nutrition [
40]. While nutrient status is key to an individual’s immune response, the relationship between nutrition and innate immunity is driven by energy consumption [
41]. Pathogens adversely affect the health of wild and managed bees [
42], and their infestation can trigger the innate immune response, thus blocking the infection and eliminating the pathogens [
43]. In contrast, pesticides inhibit the innate immune response and promote pathogen spread and virulence, contributing to bee colony loss [
3]. Hazardous substances such as nano- and micro-polystyrene plastics can disturb gut microbiota and inhibit intestinal immune response [
44]. Regardless of the suppression or triggering of immune gene expression, transcriptomic analysis can directly reveal gene expression profile changes in different tissues of managed and wild bees infected by various pathogens and exposed to pesticides.
3.1. Immune Responses to Viruses
Bees can be infected by more than 20 viruses worldwide, most commonly by deformed wing virus (DWV), black queen cell virus (BQCV), Israeli acute paralysis virus (IAPV), and Sacbrood virus (SBV) [
45,
46,
47]. Following an IAPV infection in
A. mellifera, two immune genes involved in RNAi pathways Ago2 and Dicer, as well as other immune genes, were identified to be implicated in Toll and JAK/STAT pathways, and these findings overlap with those on immune gene response following other viral infections based on transcriptome analysis [
48]. This analysis also demonstrated the dynamic changes in immune gene expression in the hours following an IAPV infection [
49], and it has shown that BQCV infection triggers significant upregulation of immune genes such as those encoding antimicrobial peptides (abaecin, apidaecin, and hymenoptaecin), peptidoglycan recognition protein S2 (PGRP-S2), Ago2, and Dicer, (the latter two both implicated in RNAi pathways in
A. mellifera brains [
50]). A transcriptomic analysis of larvae and pupae has revealed changes in immune genes involved in antimicrobial peptides (AMPs) and melanization pathways following DWV and SBV infection in
A. mellifera; both are positive-strand RNA viruses and members of the iflavirus group [
51]. In SBV-carrying
A. mellifera larvae, approximately 20 differentially expressed immune-related genes have been identified [
52]. In
A. cerana larvae naturally infected with CSBV, small interfering RNA-targeting serine proteases that are involved in the immune response are upregulated [
53]. Moreover, transcriptomic analysis has revealed that the sirtuin signaling pathway may be a novel mechanism of immune response to CSBV infection in honeybees [
54] and that the immune genes for AMPs, Ago2, and Dicer are involved in the innate immune response to DWV infection in
A. mellifera brains [
55]. The transcriptome profile of
A. mellifera eggs shows the trans-generational effects of SBV and DWV on several gene expression levels, indicating the different virulence of DWV and SBV during vertical transmission [
56]. Bee viruses can be transmitted by
Varroa destructor mites, which drives changes in virus distribution, prevalence, and virulence [
57]. Transcriptomic analysis shows that
varroa-induced viral replication is closely related to the expression of immune genes
PGRP-S2,
NimC2, and
Eater-like as well as serine protease levels in
A. mellifera adults [
58]. Furthermore, transcriptomic analysis revealed that the Varroa mite alone and the DWV coupled with the mite could induce upregulation of different immune genes involved in the Toll and JNK pathways, respectively [
59]. In addition, multiple transcriptome data have shown that
hymenoptaecin,
defensin-2,
PGRP-S1, and
B-gluc1 are common host immune genes that respond to the major pathogens and parasites such as RNA viruses,
V. destructor,
N. apis, and
N. ceranae in
A. mellifera [
60]. Meanwhile, despite the fact that some common genes are identified above, important differences in the transcription responses of honey bees to various pathogens were revealed [
60].
3.2. Immune Response to Parasites
Along with acting as a virus vector, the parasitic
Varroa destructor also reduces nutrient levels and suppresses individual immune function, and is an underestimated parasite threatening the health of bee colonies [
41,
61]. Transcriptomic analysis has shown that immune gene expression levels change as a response to the mite
V. destructor (e.g.,
PGRP-S3,
GNBP1, Toll receptors, and serine protease) [
62]. Updated transcriptomic analysis of newly emerged
A. mellifera has identified three immune genes encoding PGRP-2, hymenoptaecin, and glucan recognition protein, which could be good candidates as markers for immune response to
Varroa infestation [
63]. Moreover,
Varroa parasitism could also cause downregulation of autophagic-specific gene 18 and poly (U) binding factor 68 Kd (pUf68), and Rab7 upregulation in
A. mellifera [
64]. A set of genes related to social immunity has been identified in
A. mellifera by analyzing the comparative transcriptome of
varroa-hygienic bees [
65]. Nutrigenomics shows that pollen and sugar supplements positively affect the production of some AMPs but cannot reverse the harmful effects of
varroa parasitism [
66].
This entry is adapted from the peer-reviewed paper 10.3390/ijms232214278