
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Daniela Ionela Istrati | -- | 2889 | 2022-11-28 11:50:20 | | | |
| 2 | Jessie Wu | Meta information modification | 2889 | 2022-11-29 03:47:48 | | | | |
| 3 | Jessie Wu | -6 word(s) | 2883 | 2022-11-29 03:52:29 | | |
Video Upload Options
Anthocyanins are water-soluble pigments characterized by various intense colors found in fruits and vegetables. The extraction and separation of anthocyanins from plants is important, especially due to the instability of plant anthocyanins, selecting and optimizing. Anthocyanins are prone to degradation by several factors, including pH, temperature, oxygen, water activity, co-pigments and enzymes. Unwanted compounds, such as sugars, proteins, lipids, acids and other flavonoids, can also be removed from plant material by appropriate extraction methods. The most used method for anthocyanin extraction is the conventional one, solid–liquid extraction, also known as solvent extraction, during which anthocyanins can be dissolved in polar solvents (methanol/glycolic acid and acetone), followed by their quantification, achieved by using spectrophotometry, the differential pH method, which is a rapid and convenient quantitative assay. Starting from this point, it has developed and there are many anthocyanin-extraction methods, such as conventional solvent extraction (CSE), enzyme-assisted extraction (EAE), fermentation extraction (FE), supercritical fluid extraction (SFE) (CO2) extraction, microwave-assisted extraction (MAE), ultrasonic-assisted extraction (UAE), high-hydrostatic-pressure extraction (HHPE) and pressurized-liquid extraction (PLE). One of the most frequent techniques for obtaining anthocyanins from plants is conventional solvent extraction. In order to meet the demands of safety and environmental sustainability, new extraction technologies with shorter extraction periods and higher yields have been developed (e.g., PLE, EFS, UAE, MAE, EAE, etc.).
1. Preliminary Treatments
2. Extraction
2.1. Procedures for the Extraction of Anthocyanins from Fruits
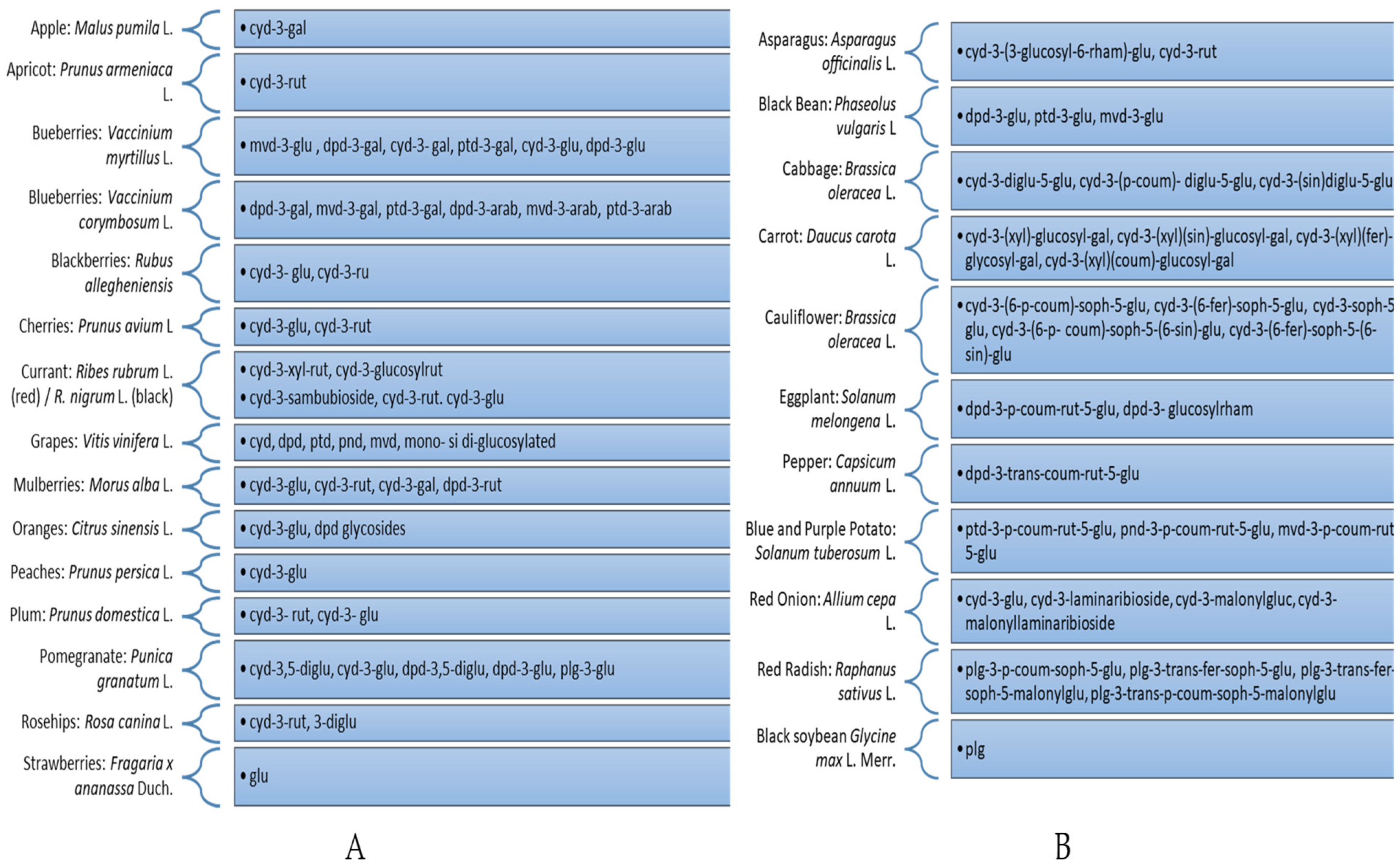
2.2. Procedures for the Extraction of Anthocyanins from Vegetables
2.3. Procedures for the Extraction of Anthocyanins from Cereals
3. Purification
References
- Silva, S.; Costa, E.M.; Calhau, C.; Morais, R.M.; Pintado, M.E. Anthocyanin extraction from plant tissues: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3072–3083.
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N. Natural colorants: Pigment stability and extraction yield enhancement via utilization of appropriate pretreatment and extraction methods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3243–3259.
- Li, B.; Wang, L.; Bai, W.; Chen, W.; Chen, F.; Shu, C. Anthocyanins: Chemistry, Processing & Bioactivity; Springer Nature: London, UK, 2021; p. 451.
- Deng, L.Z.; Mujumdar, A.S.; Zhang, Q.; Yang, X.H.; Wang, J.; Zheng, Z.A.; Gao, Z.J.; Xiao, H.W. Chemical and physical pretreatments of fruits and vegetables: Effects on drying characteristics and quality attributes—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1408–1432.
- Mazza, G.; Cacace, J.E.; Kay, C.D. Methods of analysis for anthocyanins in plants and biological fluids. J. AOAC Int. 2004, 87, 129–145.
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant Activity of Pomegranate Juice and Its Relationship with Phenolic Composition and Processing. J. Agric. Food Chem. 2000, 48, 4581–4589.
- Chaves, V.C.; Calvete, E.; Reginatto, F.H. Quality properties and antioxidant activity of seven strawberry (Fragaria x ananassa duch) cultivars. Sci. Hortic. 2017, 225, 293–298.
- Machado, A.P.D.F.; Pasquel-Reátegui, J.L.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from blackberry (Rubus fruticosus L.) residues: A comparison with conventional methods. Food Res. Int. 2015, 77, 675–683.
- Pop, A.; Fizeșan, I.; Vlase, L.; Rusu, M.E.; Cherfan, J.; Babota, M.; Gheldiu, A.M.; Tomuta, I.; Popa, D.S. Enhanced Recovery of Phenolic and Tocopherolic Compounds from Walnut (Juglans Regia L.) Male Flowers Based on Process Optimization of Ultrasonic Assisted-Extraction: Phytochemical Profile and Biological Activities. Antioxidants 2021, 10, 607.
- Heinonen, J.; Farahmandazad, H.; Vuorinen, A.; Kallio, H.; Yang, B.; Sainio, T. Extraction and purification of anthocyanins from purple-fleshed potato. Food Bioprod. Process. 2016, 99, 136–146.
- Jampani, C.; Naik, A.; Raghavarao, K.S.M.S. Purification of anthocyanins from jamun (Syzygium cumini L.) employing adsorption. Sep. Purif. Technol. 2014, 125, 170–178.
- Chandra Singh, M.; Probst, Y.; Price, W.E.; Kelso, C. Relative comparisons of extraction methods and solvent composition for Australian blueberry anthocyanins. J. Food Compos. Anal. 2021, 105, 104232.
- Fu, X.; Du, Y.; Zou, L.; Liu, X.; He, Y.; Xu, Y.; Li, L.; Luo, Z. Acidified glycerol as a one-step efficient green extraction and preservation strategy for anthocyanin from blueberry pomace: New insights into extraction and stability protection mechanism with molecular dynamic simulation. Food Chem. 2022, 390, 133226.
- Silva, D.T.D.; Pauletto, R.; Cavalheiro, S.D.S.; Bochi, V.C.; Rodrigues, E.; Weber, J.; Silva, C.d.B.D.; Morisso, F.D.P.; Barcia, M.T.; Emanuelli, T. Natural deep eutectic solvents as a biocompatible tool for the extraction of blueberry anthocyanins. J. Food Compos. Anal. 2020, 89, 103470.
- Wang, W.; Jung, J.; Tomasino, E.; Zhao, Y. Optimization of solvent and ultrasound-assisted extraction for different anthocyanin rich fruit and their effects on anthocyanin compositions. LWT–Food Sci. Technol. 2016, 72, 229–238.
- Zhang, J.; Celli, G.B.; Brooks, S.L. Natural sources of anthocyanins. In Anthocyanins from Natural Sources: Exploiting Targeted Delivery for Improved Health; Royal Society of Chemistry: London, UK, 2019; pp. 1–33.
- Blackhall, M.L.; Berry, R.; Davies, N.W.; Walls, J.T. Optimized extraction of anthocyanins from Reid Fruits’ Prunus avium ‘Lapins’ cherries. Food Chem. 2018, 256, 280–285.
- Grigoras, C.G.; Destandau, E.; Zubrzycki, S.; Elfakir, C. Sweet cherries anthocyanins: An environmental friendly extraction and purification method. Sep. Purif. Technol. 2012, 100, 51–58.
- Karaaslan, N.M.; Yaman, M. Determination of anthocyanins in cherry and cranberry by high-performance liquid chromatography–electrospray ionization–mass spectrometry. Eur. Food Res. Technol. 2016, 242, 127–135.
- Alrugaibah, M.; Yagiz, Y.; Gu, L. Use natural deep eutectic solvents as efficient green reagents to extract procyanidins and anthocyanins from cranberry pomace and predictive modeling by RSM and artificial neural networking. Sep. Purif. Technol. 2021, 255, 117720.
- Saldaña, M.D.A.; Martinez, E.R.; Sekhon, J.K.; Vo, H. The effect of different pressurized fluids on the extraction of anthocyanins and total phenolics from cranberry pomace. J. Supercrit. Fluids 2021, 175, 105279.
- Ji, M.; Li, C.; Li, Q. Rapid separation and identification of phenolics in crude red grape skin extracts by high performance liquid chromatography coupled to diode array detection and tandem mass spectrometry. J. Chromatogr. A 2015, 1414, 138–146.
- Loarce, L.; Oliver-Simancas, R.; Marchante, L.; Díaz-Maroto, M.C.; Alañón, M.E. Modifiers based on natural deep eutectic mixtures to enhance anthocyanins isolation from grape pomace by pressurized hot water extraction. LWT 2021, 149, 111889.
- Fang, Z.; Lin-Wang, K.; Jiang, C.; Zhou, D.; Lin, Y.; Pan, S.; Espley, R.V.; Ye, X. Postharvest temperature and light treatments induce anthocyanin accumulation in peel of ‘Akihime’ plum (Prunus salicina Lindl.) via transcription factor PsMYB10.1. Postharvest Biol. Technol. 2021, 179, 111592.
- Wiczkowski, W.; Topolska, J.; Honke, J. Anthocyanins profile and antioxidant capacity of red cabbages are influenced by genotype and vegetation period. J. Funct. Foods 2014, 7, 201–211.
- Strauch, R.C.; Mengist, M.F.; Pan, K.; Yousef, G.G.; Iorizzo, M.; Brown, A.F.; Lila, M.A. Variation in anthocyanin profiles of 27 genotypes of red cabbage over two growing seasons. Food Chem. 2019, 301, 125289.
- Yigit, U.; Turabi Yolacaner, E.; Hamzalioglu, A.; Gokmen, V. Optimization of microwave-assisted extraction of anthocyanins in red cabbage by response surface methodology. J. Food Process. Preserv. 2022, 46, 16120.
- Su, X.; Griffin, J.; Xu, J.; Ouyang, P.; Zhao, Z.; Wang, W. Identification and quantification of anthocyanins in purple-fleshed sweet potato leaves. Heliyon 2019, 5, e01964.
- Xu, J.; Su, X.; Lim, S.; Griffin, J.; Carey, E.; Katz, B.; Tomich, J.; Smith, J.S.; Wang, W. Characterisation and stability of anthocyanins in purple-fleshed sweet potato-P40. Food Chem. 2015, 186, 90–96.
- Lao, F.; Cheng, H.; Wang, Q.; Wang, X.; Liao, X.; Xu, Z. Enhanced water extraction with high-pressure carbon dioxide on purple sweet potato pigments: Comparison to traditional aqueous and ethanolic extraction. J. CO2 Util. 2020, 40, 101188.
- Blando, F.; Berland, H.; Maiorano, G.; Durante, M.; Mazzucato, A.; Picarella, M.E.; Nicoletti, I.; Gerardi, C.; Mita, G.; Andersen, Ø.M. Nutraceutical Characterization of Anthocyanin-Rich Fruits Produced by “Sun Black” Tomato Line. Front. Nutr. 2019, 6, 133.
- Wang, H.; Sun, S.; Zhou, Z.; Qiu, Z.; Cui, X. Rapid analysis of anthocyanin and its structural modifications in fresh tomato fruit. Food Chem. 2020, 333, 127439.
- Zhang, Y.; Yin, L.; Huang, L.; Tekliye, M.; Xia, X.; Li, J.; Dong, M. Composition, antioxidant activity, and neuroprotective effects of anthocyanin-rich extract from purple highland barley bran and its promotion on autophagy. Food Chem. 2021, 339, 127849.
- Catena, S.; Turrini, F.; Boggia, R.; Borriello, M.; Gardella, M.; Zunin, P. Effects of different cooking conditions on the anthocyanin content of a black rice (Oryza sativa L. ‘Violet Nori’). Eur. Food Res. Technol. 2019, 245, 2303–2310.
- Turrini, F.; Boggia, R.; Leardi, R.; Borriello, M.; Zunin, P. Optimization of the Ultrasonic-Assisted Extraction of Phenolic Compounds from Oryza Sativa L. ‘Violet Nori’ and Determination of the Antioxidant Properties of its Caryopses and Leaves. Molecules 2018, 23, 844.
- Yi, J.; Qiu, M.; Zhu, Z.; Dong, X.; Andrew Decker, E.; McClements, D.J. Robust and recyclable magnetic nanobiocatalysts for extraction of anthocyanin from black rice. Food Chem. 2021, 364, 130447.
- Constantin, O.; Skrt, M.; Ulrih, N.; Rapeanu, G. Anthocyanins profile, total phenolics and antioxidant activity of two Romanian red grape varieties: Feteasca neagra and Babeasca neagra (Vitis vinifera). Chem. Pap. 2015, 69, 1573–1581.
- Ferreiro-González, M.; Carrera, C.; Ruiz-Rodríguez, A.; Barbero, G.F.; Ayuso, J.; Palma, M.; Barroso, C.G. A New Solid Phase Extraction for the Determination of Anthocyanins in Grapes. Molecules 2014, 19, 21398–21410.
- Kähkönen, M.P.; Hopia, A.I.; Heinonen, M. Berry Phenolics and Their Antioxidant Activity. J. Agric. Food Chem. 2001, 49, 4076–4082.
- Chandrasekhar, J.; Madhusudhan, M.C.; Raghavarao, K.S.M.S. Extraction of anthocyanins from red cabbage and purification using adsorption. Food Bioprod. Process. 2012, 90, 615–623.





