Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Liu, Y.; Ye, Q. Nucleic Acid Vaccines against SARS-CoV-2. Encyclopedia. Available online: https://encyclopedia.pub/entry/36642 (accessed on 08 February 2026).
Liu Y, Ye Q. Nucleic Acid Vaccines against SARS-CoV-2. Encyclopedia. Available at: https://encyclopedia.pub/entry/36642. Accessed February 08, 2026.
Liu, Ying, Qing Ye. "Nucleic Acid Vaccines against SARS-CoV-2" Encyclopedia, https://encyclopedia.pub/entry/36642 (accessed February 08, 2026).
Liu, Y., & Ye, Q. (2022, November 26). Nucleic Acid Vaccines against SARS-CoV-2. In Encyclopedia. https://encyclopedia.pub/entry/36642
Liu, Ying and Qing Ye. "Nucleic Acid Vaccines against SARS-CoV-2." Encyclopedia. Web. 26 November, 2022.
Copy Citation
The coronavirus disease 2019 (COVID-19) has spread worldwide and imposed a substantial burden on human health, the environment, and socioeconomic development, which has also accelerated the process of nucleic acid vaccine development and licensure. Nucleic acid vaccines are viral genetic sequence-based vaccines and third-generation vaccines after whole virus vaccines and recombinant subunit vaccines, including DNA vaccines and RNA vaccines.
nucleic acid vaccines
COVID-19
development process
advantages and shortcomings
optimization
1. Introduction
Coronaviruses (CoVs) are widespread in nature and can cause multisystem disorders in humans [1][2][3][4][5][6], including the respiratory and alimentary tract, nervous system, etc., and lead to immense financial loss at the same time [7]. To date, CoV infections, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), have resulted in three global pandemics [8][9][10][11]. Due to its greater ability to recognize receptors [12], SARS-CoV-2 spreads more efficiently from person to person [13][14]. Currently, drug development is side-by-side with vaccine upgrading. Vaccination is the most effective strategy for preventing and controlling infectious diseases [15][16][17][18]. Multiple types of SARS-CoV-2 vaccines have been developed at the same time [19], and some of them have successively been granted emergency use authorization by the World Health Organization (WHO), mainly including inactivated vaccines [20][21], adenovirus-vectored vaccines [22][23], and nucleic acid vaccines [24][25]. Due to well-established technology, vaccination with inactivated vaccines is the primary method used during our epidemic prevention and control mechanisms. However, vaccination with inactivated vaccines, in most cases, result in the generation of humoral, but not cell-mediated, immune responses. With the deepening research of virology and the gradual maturation of vaccine technology, substantial progress has been made in the development and application of nucleic acid vaccines, which, as an emerging platform, have become a hotspot in the vaccine research and development field.
Nucleic acid vaccines, as an emerging concept, were established in the early 1990s [26] and include DNA vaccines and RNA vaccines, which were also third-generation vaccines after whole virus vaccines and recombinant subunit vaccines. After introducing foreign target genes, they use the protein synthesis systems of host cells to express target proteins and then induce immune responses. Before the outbreak of coronavirus disease 2019 (COVID-19), nucleic acid vaccines were not yet available for human use on the market. The unprecedented pandemic scenario has accelerated the vaccine development and licensure process.
2. The Research and Development Process of Nucleic Acid Vaccines
The research and development procedure of nucleic acid vaccines involves two main phases: early design stage and clinical experiment stage. More details are shown in Figure 1. The early design stage generally consists of searching for immunogens, designing vaccine structures, and determining toxicological effects and immune effects in animal models. The clinical experiments targeting primarily practical application mainly aim to provide definitive evidence for the safety and efficacy of vaccines. According to regulations of special approval processes, on the premise of guaranteeing the security and stability of COVID-19 vaccines, it is admissible to reduce certain approval processes accordingly [27]. Additionally, the vaccine life cycle includes production, supply, available on the market, and post-marketing research in the real world. Currently, the ZyCoV-D vaccine developed by Cadila in Ahmedabad, Gujarat, India is the first DNA vaccine for people to be approved anywhere in the world [28]. INO-4800, developed by Inovio (the leading global development corporation of DNA vaccines), is the first DNA vaccine to advance to clinical trials and is currently undergoing phase three clinical trials, having the prospect of being commercially available within one year. BioNTech/Pfizer and Moderna are two leading research teams for COVID-19 RNA vaccines. BNT162b2 from BioNTech/Pfizer and mRNA 1273 from Moderna were granted emergency use authorization by the WHO on 14 January 2021 and on 3 February 2021, respectively. An illustration of the current COVID-19 nucleic acid vaccines is presented in Table 1.
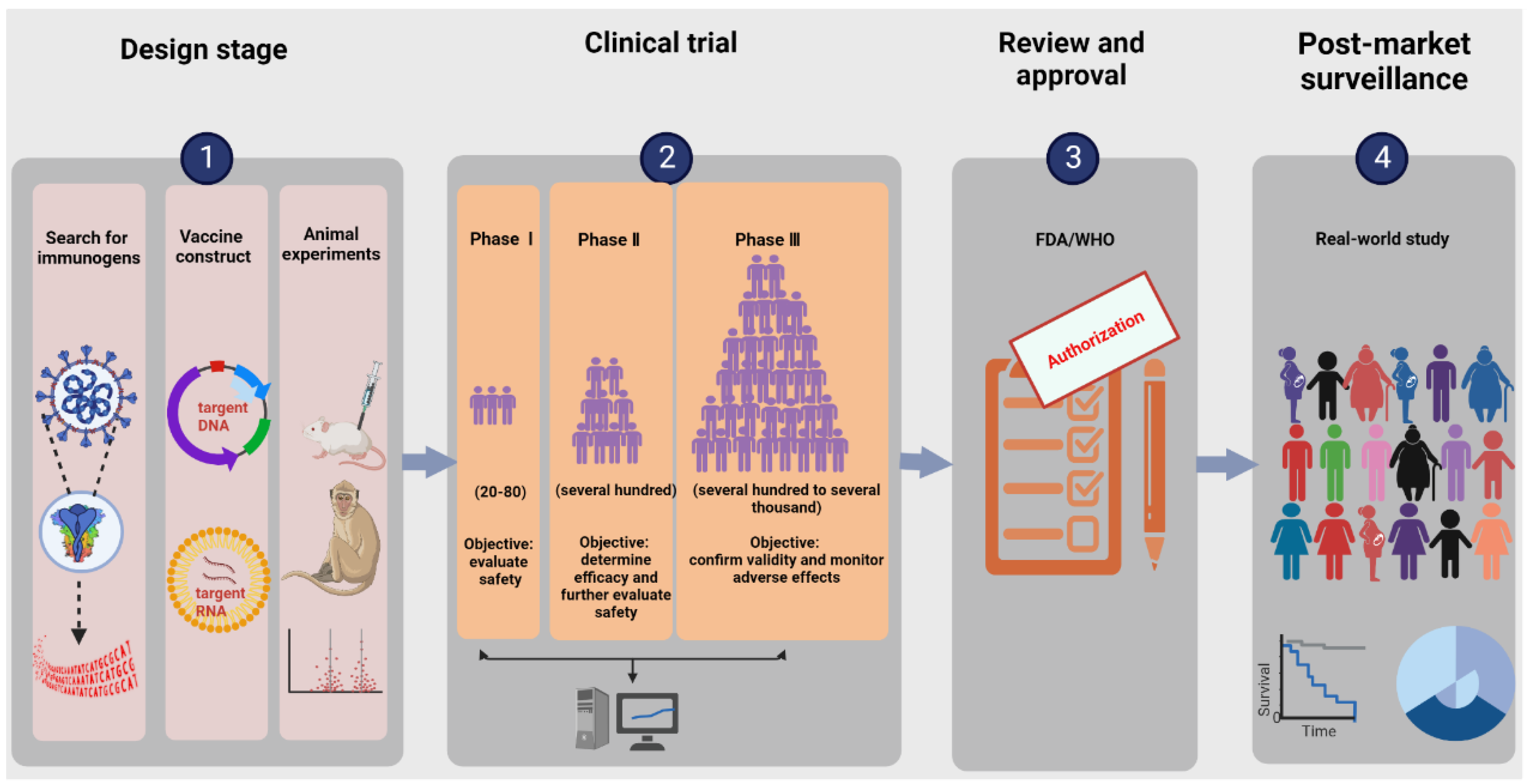
Figure 1. Illustration of the research and development process for nucleic acid vaccines. The procedure involves an early design stage, clinical trial stage, review and approval stage, and post-market surveillance. The early design stage generally consists of searching for immunogens, designing vaccine structures, and determining toxicological effects and immune effects in animal models. The clinical trials mainly include phases Ⅰ–Ⅲ, targeting primarily practical applications to provide definitive evidence for the safety and efficacy of vaccines.
Table 1. The current COVID-19 nucleic acid vaccines and details.
| Vaccine Name | Technology | Developer/Company | Immunization Protocol | Immunity | Effectiveness | Current Status |
|---|---|---|---|---|---|---|
| ZyCoV-D | DNA vaccine |
Zydus Cadila (Ahmedabad, India) | 3 doses (2.0 mg/dose), 4 weeks apart | humoral and cellular immunity | 66.6% | approved by India |
| INO-4800 | DNA vaccine |
Inovio (Plymouth Meeting, PA, USA) |
2 doses (2.0 mg/dose), 4 weeks apart | humoral and cellular immunity | unpublished results | phase Ⅱ/Ⅲ clinical trials |
| AG0302-COVID19 | DNA vaccine |
AnGes (Osaka, Japan) | 2 doses (2.0 mg/dose), 2/4 weeks apart | unpublished results | unpublished results | phase Ⅱ/Ⅲ clinical trials |
| GX-19N | DNA vaccine |
Genexine (Seoul, Korea) | unpublished results | unpublished results | unpublished results | phase Ⅱ/Ⅲ clinical trials |
| BNT162b2 | mRNA vaccine |
Pfizer (New York, NY, USA)/BioNTech (Mainz, Germany) | 2 doses (30 μg/0.3 mL/dose), 3 weeks apart | humoral and cellular immunity | 95.0% | approved by WHO |
| mRNA-1273 | mRNA vaccine |
Moderna (Cambridge, MA, USA) | 2 doses (100 μg/0.5 mL/dose), 28 days apart | humoral and cellular immunity | 94.1% | approved by WHO |
| ARCoV | mRNA vaccine |
WALVAX (Yunnan, China)/ABOGEN (Suzhou, China) | unpublished results | humoral and cellular immunity | unpublished results | phase Ⅱ/Ⅲ clinical trials |
3. The Pros and Cons of Nucleic Acid Vaccines
3.1. Comparison of Nucleic Acid Vaccines and Traditional Vaccines
Nucleic acid-based vaccines with theoretical advantages over conventional vaccines are attractive platforms with great opportunities and challenges. Compared to traditional vaccines, nucleic acid vaccines present several advantages. The major one is that the target genes can be anthropogenic modifications based on the dominant antigenic epitopes. Once new virus variants occur, targeted vaccines can be prepared rapidly and inexpensively on a large scale, which is essential for controlling an unexpected epidemic outbreak. Due to the singleness of antigen components, the phenomenon of antibody-dependent enhancement (ADE) is rare in nucleic acid-based vaccines [29]. As a consequence of containing partial, but not all, pathogen genome sequences, atavistic risk is absent. Moreover, nucleic acid vaccines can induce strong and long-lasting humoral and cellular immune responses simultaneously. It has been demonstrated that foreign plasmid DNA can still be detected by PCR in mice at 15 months after intramuscular injection [30].
Nucleic acid-based vaccines are highly promising. However, their development is nascent, and much remains to be further validated, such as safety. Synthetic raw materials and encrusting materials are likely to be toxic, presenting the risk of peripheral host cell damage. For example, it has been reported that the polyethylene glycol (PEG) used to conjugate lipids in mRNA vaccines is associated with anaphylaxis events [31]. The nucleic acid persisting in vivo contributes to the production of self-reactive antibodies and then induces autoimmune disease. Some patients with common autoimmune diseases are detected to have more than one anti-nucleic acid autoantibody, such as systemic lupus erythematosus [32], multiple sclerosis [33], rheumatoid arthritis [34], and polymyositis [35]. With rapid development, growing safety concerns are particularly apparent for DNA vaccines. This foreign DNA is likely to integrate randomly into the host chromosome, thereby leading to the activation of oncogenes, inactivation of tumor suppressor genes, or other chromosomal instability.
3.2. Comparison of DNA Vaccines and RNA Vaccines
Although similar in many ways, there are some important distinctions between DNA vaccines and RNA vaccines. First, the inoculation means are different in that DNA vaccines do not exert their function until they reach the nucleus, whereas RNA vaccines only need to enter the cytoplasm. Therefore, DNA vaccines struggle to induce potent immune responses in clinical trials after intramuscular vaccination, which is why research on their clinical application is progressing slowly. Many vaccination methods with gene guns [36] or electroporation apparatus [37] are now available; however, they are costly and experimentally more challenging. They all limit the application of DNA vaccines. Second, the risk of vaccination varies between DNA and RNA vaccines. RNA vaccines both preserve the advantages of intracellular expression of target antigens and overcome potential risks of integrating into the host DNA. In addition, their stability in vitro is different. DNA with a unique double-helix structure is strongly associated with good stability and an extended storage period, whereas mRNA is easily catabolized by host machinery under the physiologic conditions of ubiquitous ribonucleases. For example, the RNA vaccine of BNT162b2 requires storage at −70 °C, which introduces additional burdens for vaccine distribution and transportation.
References
- Han, X.; Ye, Q. Kidney involvement in COVID-19 and its treatments. J. Med. Virol. 2021, 93, 1387–1395.
- Tian, D.; Ye, Q. Hepatic complications of COVID-19 and its treatment. J. Med. Virol. 2020, 92, 1818–1824.
- Ye, Q.; Lai, E.Y.; Luft, F.C.; Persson, P.B.; Mao, J. SARS-CoV-2 effects on the renin-angiotensin-aldosterone system, therapeutic implications. Acta Physiol. 2021, 231, e13608.
- Ye, Q.; Lu, D.; Shang, S.; Fu, J.; Gong, F.; Shu, Q.; Mao, J. Crosstalk between coronavirus disease 2019 and cardiovascular disease and its treatment. ESC Heart. Fail. 2020, 7, 3464–3472.
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613.
- Ye, Q.; Wang, B.; Zhang, T.; Xu, J.; Shang, S. The mechanism and treatment of gastrointestinal symptoms in patients with COVID-19. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G245–G252.
- Cutler, D.M.; Summers, L.H. The COVID-19 Pandemic and the $16 Trillion Virus. JAMA 2020, 324, 1495–1496.
- Zhong, N.S.; Zheng, B.J.; Li, Y.M.; Poon; Xie, Z.H.; Chan, K.H.; Li, P.H.; Tan, S.Y.; Chang, Q.; Xie, J.P.; et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet 2003, 362, 1353–1358.
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820.
- Chen, Z.; Wang, B.; Mao, S.; Ye, Q. Assessment of global asymptomatic SARS-CoV-2 infection and management practices from China. Int. J. Biol. Sci. 2021, 17, 1119–1124.
- Ye, Q.; Wang, B.; Mao, J.; Fu, J.; Shang, S.; Shu, Q.; Zhang, T. Epidemiological analysis of COVID-19 and practical experience from China. J. Med. Virol. 2020, 92, 755–769.
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020, 94, e00127-20.
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269.
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733.
- Han, X.; Ye, Q. The variants of SARS-CoV-2 and the challenges of vaccines. J. Med. Virol. 2022, 94, 1366–1372.
- Meng, H.; Mao, J.; Ye, Q. Booster vaccination strategy: Necessity, immunization objectives, immunization strategy, and safety. J. Med. Virol. 2022, 94, 2369–2375.
- Meng, H.; Mao, J.; Ye, Q. Strategies and safety considerations of booster vaccination in COVID-19. Bosn. J. Basic Med. Sci. 2022, 22, 366–373.
- Tian, D.; Sun, Y.; Zhou, J.; Ye, Q. The global epidemic of SARS-CoV-2 variants and their mutational immune escape. J. Med. Virol. 2022, 94, 847–857.
- Thanh Le, T.; Andreadakis, Z.; Kumar, A.; Gómez Román, R.; Tollefsen, S.; Saville, M.; Mayhew, S. The COVID-19 Vaccine Development Landscape. Nat. Rev. Drug Discov. 2020, 19, 305–306.
- World Health Organization. Background Document on the Inactivated Vaccine Sinovac-CoronaVac against COVID-19. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-Sinovac-CoronaVac-background-2021.1 (accessed on 10 January 2022).
- World Health Organization. Background Document on the Inactivated COVID-19 Vaccine BIBP Developed by China National Biotec Group (CNBG), Sinopharm. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-BIBP-background-2021.1 (accessed on 10 January 2022).
- World Health Organization. Background Document on the AZD1222 Vaccine against COVID-19 Developed by Oxford University and AstraZeneca. Available online: https://www.who.int/publications/i/item/background-document-on-the-azd1222-vaccine-against-covid-19-developed-by-oxford-university-and-astrazeneca (accessed on 10 January 2022).
- World Health Organization. Background Document on the Janssen Ad26.COV2. S (COVID-19) Vaccine. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE-recommendation-Ad26.COV2.S-background-2021.1 (accessed on 10 January 2022).
- World Health Organization. Background Document on the mRNA-1273 Vaccine (Moderna) against COVID-19. Available online: https://www.who.int/publications/i/item/background-document-on-the-mrna-1273-vaccine-(moderna)-against-covid-19 (accessed on 10 January 2022).
- World Health Organization. Background Document on the mRNA Vaccine BNT162b2 (Pfizer-BioNTech) against COVID-19. Available online: https://www.who.int/publications/i/item/background-document-on-mrna-vaccine-bnt162b2-(pfizer-biontech)-against-covid-19 (accessed on 10 January 2022).
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468.
- Bennet, B.M.; Wolf, J.; Laureano, R.; Sellers, R.S. Review of Current Vaccine Development Strategies to Prevent Coronavirus Disease 2019 (COVID-19). Toxicol. Pathol. 2020, 48, 800–809.
- Mallapaty, S. India’s DNA COVID vaccine is a world first–more are coming. Nature 2021, 597, 161–162.
- Dejnirattisai, W.; Supasa, P.; Wongwiwat, W.; Rouvinski, A.; Barba-Spaeth, G.; Duangchinda, T.; Sakuntabhai, A.; Cao-Lormeau, V.M.; Malasit, P.; Rey, F.A.; et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat. Immunol. 2016, 17, 1102–1108.
- Yankauckas, M.A.; Morrow, J.E.; Parker, S.E.; Abai, A.; Rhodes, G.H.; Dwarki, V.J.; Gromkowski, S.H. Long-term anti-nucleoprotein cellular and humoral immunity is induced by intramuscular injection of plasmid DNA containing NP gene. DNA Cell Biol. 1993, 12, 771–776.
- Zhou, Z.H.; Stone, C.A., Jr.; Jakubovic, B.; Phillips, E.J.; Sussman, G.; Park, J.; Hoang, U.; Kirshner, S.L.; Levin, R.; Kozlowski, S. Anti-PEG IgE in anaphylaxis associated with polyethylene glycol. J. Allergy Clin. Immunol. Pract. 2021, 9, 1731–1733.e3.
- Howe, H.S.; Leung, B.P.L. Anti-Cytokine Autoantibodies in Systemic Lupus Erythematosus. Cells 2019, 9, 72.
- Comi, G.; Bar-Or, A.; Lassmann, H.; Uccelli, A.; Hartung, H.P.; Montalban, X.; Sørensen, P.S.; Hohlfeld, R.; Hauser, S.L. Role of B Cells in Multiple Sclerosis and Related Disorders. Ann. Neurol. 2021, 89, 13–23.
- Nielen, M.M.; van Schaardenburg, D.; Reesink, H.W.; Twisk, J.W.; van de Stadt, R.J.; van der Horst-Bruinsma, I.E.; de Koning, M.H.; Habibuw, M.R.; Dijkmans, B.A. Simultaneous development of acute phase response and autoantibodies in preclinical rheumatoid arthritis. Ann. Rheum. Dis. 2006, 65, 535–537.
- Bronner, I.M.; van der Meulen, M.F.; de Visser, M.; Kalmijn, S.; van Venrooij, W.J.; Voskuyl, A.E.; Dinant, H.J.; Linssen, W.H.; Wokke, J.H.; Hoogendijk, J.E. Long-term outcome in polymyositis and dermatomyositis. Ann. Rheum. Dis. 2006, 65, 1456–1461.
- Chang, M.L.; Chen, J.C.; Yeh, C.T.; Chang, M.Y.; Liang, C.K.; Chiu, C.T.; Lin, D.Y.; Liaw, Y.F. Gene gun bombardment with DNA-coated gold particles is a potential alternative to hydrodynamics-based transfection for delivering genes into superficial hepatocytes. Hum. Gene Ther. 2008, 19, 391–395.
- May, R.D.; Tekari, A.; Frauchiger, D.A.; Krismer, A.; Benneker, L.M.; Gantenbein, B. Efficient Nonviral Transfection of Primary Intervertebral Disc Cells by Electroporation for Tissue Engineering Application. Tissue Eng. Part C Methods 2017, 23, 30–37.
More
Information
Subjects:
Infectious Diseases
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
897
Revisions:
2 times
(View History)
Update Date:
29 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




