Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Infectious Diseases
The coronavirus disease 2019 (COVID-19) has spread worldwide and imposed a substantial burden on human health, the environment, and socioeconomic development, which has also accelerated the process of nucleic acid vaccine development and licensure. Nucleic acid vaccines are viral genetic sequence-based vaccines and third-generation vaccines after whole virus vaccines and recombinant subunit vaccines, including DNA vaccines and RNA vaccines.
- nucleic acid vaccines
- COVID-19
- development process
- advantages and shortcomings
- optimization
1. Introduction
Coronaviruses (CoVs) are widespread in nature and can cause multisystem disorders in humans [1,2,3,4,5,6], including the respiratory and alimentary tract, nervous system, etc., and lead to immense financial loss at the same time [7]. To date, CoV infections, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), have resulted in three global pandemics [8,9,10,11]. Due to its greater ability to recognize receptors [12], SARS-CoV-2 spreads more efficiently from person to person [13,14]. Currently, drug development is side-by-side with vaccine upgrading. Vaccination is the most effective strategy for preventing and controlling infectious diseases [15,16,17,18]. Multiple types of SARS-CoV-2 vaccines have been developed at the same time [19], and some of them have successively been granted emergency use authorization by the World Health Organization (WHO), mainly including inactivated vaccines [20,21], adenovirus-vectored vaccines [22,23], and nucleic acid vaccines [24,25]. Due to well-established technology, vaccination with inactivated vaccines is the primary method used during our epidemic prevention and control mechanisms. However, vaccination with inactivated vaccines, in most cases, result in the generation of humoral, but not cell-mediated, immune responses. With the deepening research of virology and the gradual maturation of vaccine technology, substantial progress has been made in the development and application of nucleic acid vaccines, which, as an emerging platform, have become a hotspot in the vaccine research and development field.
Nucleic acid vaccines, as an emerging concept, were established in the early 1990s [26] and include DNA vaccines and RNA vaccines, which were also third-generation vaccines after whole virus vaccines and recombinant subunit vaccines. After introducing foreign target genes, they use the protein synthesis systems of host cells to express target proteins and then induce immune responses. Before the outbreak of coronavirus disease 2019 (COVID-19), nucleic acid vaccines were not yet available for human use on the market. The unprecedented pandemic scenario has accelerated the vaccine development and licensure process.
2. The Research and Development Process of Nucleic Acid Vaccines
The research and development procedure of nucleic acid vaccines involves two main phases: early design stage and clinical experiment stage. More details are shown in Figure 1. The early design stage generally consists of searching for immunogens, designing vaccine structures, and determining toxicological effects and immune effects in animal models. The clinical experiments targeting primarily practical application mainly aim to provide definitive evidence for the safety and efficacy of vaccines. According to regulations of special approval processes, on the premise of guaranteeing the security and stability of COVID-19 vaccines, it is admissible to reduce certain approval processes accordingly [27]. Additionally, the vaccine life cycle includes production, supply, available on the market, and post-marketing research in the real world. Currently, the ZyCoV-D vaccine developed by Cadila in Ahmedabad, Gujarat, India is the first DNA vaccine for people to be approved anywhere in the world [28]. INO-4800, developed by Inovio (the leading global development corporation of DNA vaccines), is the first DNA vaccine to advance to clinical trials and is currently undergoing phase three clinical trials, having the prospect of being commercially available within one year. BioNTech/Pfizer and Moderna are two leading research teams for COVID-19 RNA vaccines. BNT162b2 from BioNTech/Pfizer and mRNA 1273 from Moderna were granted emergency use authorization by the WHO on 14 January 2021 and on 3 February 2021, respectively. An illustration of the current COVID-19 nucleic acid vaccines is presented in Table 1.
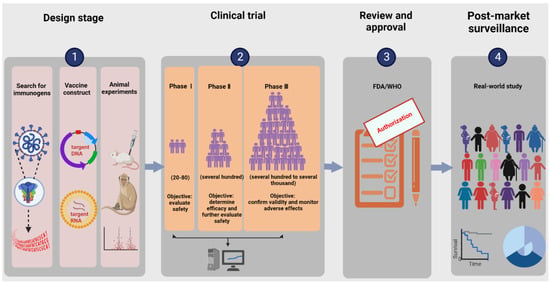
Figure 1. Illustration of the research and development process for nucleic acid vaccines. The procedure involves an early design stage, clinical trial stage, review and approval stage, and post-market surveillance. The early design stage generally consists of searching for immunogens, designing vaccine structures, and determining toxicological effects and immune effects in animal models. The clinical trials mainly include phases Ⅰ–Ⅲ, targeting primarily practical applications to provide definitive evidence for the safety and efficacy of vaccines.
Table 1. The current COVID-19 nucleic acid vaccines and details.
| Vaccine Name | Technology | Developer/Company | Immunization Protocol | Immunity | Effectiveness | Current Status |
|---|---|---|---|---|---|---|
| ZyCoV-D | DNA vaccine |
Zydus Cadila (Ahmedabad, India) | 3 doses (2.0 mg/dose), 4 weeks apart | humoral and cellular immunity | 66.6% | approved by India |
| INO-4800 | DNA vaccine |
Inovio (Plymouth Meeting, PA, USA) |
2 doses (2.0 mg/dose), 4 weeks apart | humoral and cellular immunity | unpublished results | phase Ⅱ/Ⅲ clinical trials |
| AG0302-COVID19 | DNA vaccine |
AnGes (Osaka, Japan) | 2 doses (2.0 mg/dose), 2/4 weeks apart | unpublished results | unpublished results | phase Ⅱ/Ⅲ clinical trials |
| GX-19N | DNA vaccine |
Genexine (Seoul, Korea) | unpublished results | unpublished results | unpublished results | phase Ⅱ/Ⅲ clinical trials |
| BNT162b2 | mRNA vaccine |
Pfizer (New York, NY, USA)/BioNTech (Mainz, Germany) | 2 doses (30 μg/0.3 mL/dose), 3 weeks apart | humoral and cellular immunity | 95.0% | approved by WHO |
| mRNA-1273 | mRNA vaccine |
Moderna (Cambridge, MA, USA) | 2 doses (100 μg/0.5 mL/dose), 28 days apart | humoral and cellular immunity | 94.1% | approved by WHO |
| ARCoV | mRNA vaccine |
WALVAX (Yunnan, China)/ABOGEN (Suzhou, China) | unpublished results | humoral and cellular immunity | unpublished results | phase Ⅱ/Ⅲ clinical trials |
3. The Pros and Cons of Nucleic Acid Vaccines
3.1. Comparison of Nucleic Acid Vaccines and Traditional Vaccines
Nucleic acid-based vaccines with theoretical advantages over conventional vaccines are attractive platforms with great opportunities and challenges. Compared to traditional vaccines, nucleic acid vaccines present several advantages. The major one is that the target genes can be anthropogenic modifications based on the dominant antigenic epitopes. Once new virus variants occur, targeted vaccines can be prepared rapidly and inexpensively on a large scale, which is essential for controlling an unexpected epidemic outbreak. Due to the singleness of antigen components, the phenomenon of antibody-dependent enhancement (ADE) is rare in nucleic acid-based vaccines [84]. As a consequence of containing partial, but not all, pathogen genome sequences, atavistic risk is absent. Moreover, nucleic acid vaccines can induce strong and long-lasting humoral and cellular immune responses simultaneously. It has been demonstrated that foreign plasmid DNA can still be detected by PCR in mice at 15 months after intramuscular injection [85].
Nucleic acid-based vaccines are highly promising. However, their development is nascent, and much remains to be further validated, such as safety. Synthetic raw materials and encrusting materials are likely to be toxic, presenting the risk of peripheral host cell damage. For example, it has been reported that the polyethylene glycol (PEG) used to conjugate lipids in mRNA vaccines is associated with anaphylaxis events [86]. The nucleic acid persisting in vivo contributes to the production of self-reactive antibodies and then induces autoimmune disease. Some patients with common autoimmune diseases are detected to have more than one anti-nucleic acid autoantibody, such as systemic lupus erythematosus [87], multiple sclerosis [88], rheumatoid arthritis [89], and polymyositis [90]. With rapid development, growing safety concerns are particularly apparent for DNA vaccines. This foreign DNA is likely to integrate randomly into the host chromosome, thereby leading to the activation of oncogenes, inactivation of tumor suppressor genes, or other chromosomal instability.
3.2. Comparison of DNA Vaccines and RNA Vaccines
Although similar in many ways, there are some important distinctions between DNA vaccines and RNA vaccines. First, the inoculation means are different in that DNA vaccines do not exert their function until they reach the nucleus, whereas RNA vaccines only need to enter the cytoplasm. Therefore, DNA vaccines struggle to induce potent immune responses in clinical trials after intramuscular vaccination, which is why research on their clinical application is progressing slowly. Many vaccination methods with gene guns [91] or electroporation apparatus [92] are now available; however, they are costly and experimentally more challenging. They all limit the application of DNA vaccines. Second, the risk of vaccination varies between DNA and RNA vaccines. RNA vaccines both preserve the advantages of intracellular expression of target antigens and overcome potential risks of integrating into the host DNA. In addition, their stability in vitro is different. DNA with a unique double-helix structure is strongly associated with good stability and an extended storage period, whereas mRNA is easily catabolized by host machinery under the physiologic conditions of ubiquitous ribonucleases. For example, the RNA vaccine of BNT162b2 requires storage at −70 °C, which introduces additional burdens for vaccine distribution and transportation.
This entry is adapted from the peer-reviewed paper 10.3390/vaccines10111849
This entry is offline, you can click here to edit this entry!
