
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Eryk Fernandes | -- | 5754 | 2022-11-25 17:01:30 | | | |
| 2 | Lindsay Dong | -43 word(s) | 5711 | 2022-11-28 03:25:51 | | |
Video Upload Options
Photocatalysis has been vastly applied for the removal of contaminants of emerging concern (CECs) and other micropollutants, with the aim of future water reclamation. As a process based upon photon irradiation, materials that may be activated through natural light sources are highly pursued, to facilitate their application and reduce costs. TiO2 is a reference material, and it has been greatly optimized. However, in its typical configuration, it is known to be mainly active under ultraviolet radiation. Thus, multiple alternative visible light driven (VLD) materials have been intensively studied recently. WO3 and g-C3N4 are currently attractive VLD catalysts, with WO3 possessing similarities with TiO2 as a metal oxide, allowing correlations between the knowledge regarding the reference catalyst, and g-C3N4 having an interesting and distinct non-metallic polymeric structure with the benefit of easy production.
1. Introduction
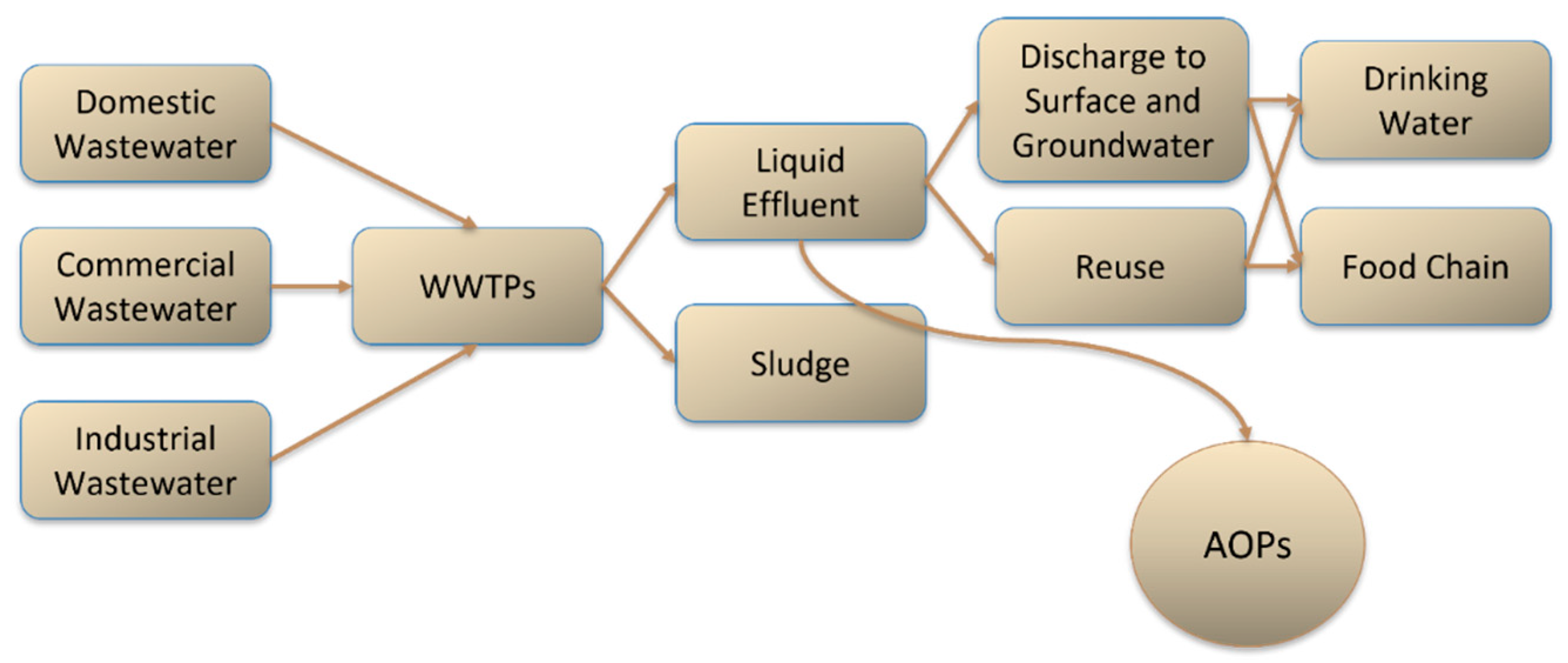
2. General Features of Catalysts for Photo-based Treatment Processes
Titanium dioxide is certainly the most applied catalyst for photocatalytic water treatment and possesses different crystalline phases, anatase, rutile, and brookite, with anatase having a higher general photocatalytic activity [21].
3. Catalyst Doping
3.1. TiO2
3.1.1. Transition Metals Doping
Both metal and non-metal elements may be incorporated in the catalyst structure and are capable of altering light absorption capacity and, more importantly, visible light, preventing the recombination of electron-hole [29]. Regarding metal doping, transition metals are more commonly applied, due to their partially filled d states, which promote the creation of intra-bandgap energy states and the absorption shift [30].
In reference to the catalyst synthesis, the sol-gel method is one of the main alternatives and allows better control of the product characteristics, such as porosity, structure, composition, and homogeneity, which is especially important in the incorporation of dopants. Moreover, a calcination step post-synthesis is usually applied as a simple technique to transform an amorphous structure into a crystalline (Anatase, Rutile, and Brookite). Karuppasamy et al. [31] attested that, during the investigation of the doping effect of different transition metals (Zn, Cu, and Zr) on TiO2, the presence of doping elements may also alter the crystalline phase formation, reducing the temperature at the calcination stage needed to achieve the different structures, which was confirmed by other studies [32][33]. Regarding the different elements used and their photocatalytic activity, Zn-TiO2 presented the best performance in methylene blue elimination under visible light. The interactions between Zn correspondent electronic states with the TiO2 conduction band may provoke a red shift in the bandgap, improving the material light absorption between 400 nm and 700 nm.
3.1.2. Noble and Rare-Earth Metals Doping
3.1.3. Non-Metals Doping
3.1.4. Co-Doping
3.2. WO3
3.2.1. Transition Metals Doping
3.2.2. Noble and Rare-Earth Metals Doping
3.2.3. Non-Metals Doping
3.2.4. Co-Doping
3.3. g-C3N4
3.3.1. Metals Doping
3.3.2. Noble and Rare-Earth Metals Doping
3.3.3. Non-Metals Doping
3.3.4. Co-Doping
3.4. Overall Considerations
4. Composite Catalysts
4.1. TiO2
4.2. WO3
4.3. g-C3N4
5. Conclusions
References
- McCance, W.; Jones, O.A.H.; Cendón, D.I.; Edwards, M.; Surapaneni, A.; Chadalavada, S.; Wang, S.; Currell, M. Combining Environmental Isotopes with Contaminants of Emerging Concern (CECs) to Characterise Wastewater Derived Impacts on Groundwater Quality. Water Res. 2020, 182, 1–15.
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Đolić, M.B.; Gernjak, W.; Heath, E.; Ivancev-Tumbas, I.; Karaolia, P.; Lado Ribeiro, A.R.; et al. Consolidated vs New Advanced Treatment Methods for the Removal of Contaminants of Emerging Concern from Urban Wastewater. Sci. Total Environ. 2019, 655, 986–1008.
- Valbonesi, P.; Pro, M.; Vasumini, I.; Fabbri, E. Contaminants of Emerging Concern in Drinking Water: Quality Assessment by Combining Chemical and Biological Analysis. Sci. Total Environ. 2021, 758, 143624.
- Olalla, A.; Moreno, L.; Valcárcel, Y. Prioritisation of Emerging Contaminants in the Northern Antarctic Peninsula Based on Their Environmental Risk. Sci. Total Environ. 2020, 742, 140417.
- Lencioni, V.; Bellamoli, F.; Paoli, F. Multi-Level Effects of Emerging Contaminants on Macroinvertebrates in Alpine Streams: From DNA to the Ecosystem. Ecol. Indic. 2020, 117, 106660.
- Llamas-dios, M.I.; Vadillo, I.; Jiménez-gavilán, P.; Candela, L.; Corada-fernández, C. Assessment of a Wide Array of Contaminants of Emerging Concern in a Mediterranean Water Basin (Guadalhorce River, Spain): Motivations for an Improvement of Water Management and Pollutants Surveillance. Sci. Total Environ. 2021, 788, 147822.
- Tian, Z.; Wark, D.A.; Bogue, K.; James, C.A. Suspect and Non-Target Screening of Contaminants of Emerging Concern in Streams in Agricultural Watersheds. Sci. Total Environ. 2021, 795, 148826.
- Shah, A.I.; Din Dar, M.U.; Bhat, R.A.; Singh, J.P.; Singh, K.; Bhat, S.A. Prospectives and Challenges of Wastewater Treatment Technologies to Combat Contaminants of Emerging Concerns. Ecol. Eng. 2020, 152, 105882.
- Liu, Z.; Demeestere, K.; Hulle, S. Van Comparison and Performance Assessment of Ozone-Based AOPs in View of Trace Organic Contaminants Abatement in Water and Wastewater: A Review. J. Environ. Chem. Eng. 2021, 9, 105599.
- Giwa, A.; Yusuf, A.; Balogun, H.A.; Sambudi, N.S.; Bilad, M.R.; Adeyemi, I.; Chakraborty, S.; Curcio, S. Recent Advances in Advanced Oxidation Processes for Removal of Contaminants from Water: A Comprehensive Review. Process Saf. Environ. Prot. 2021, 146, 220–256.
- Sgroi, M.; Snyder, S.; Roccaro, P. Comparison of AOPs at Pilot Scale: Energy Costs for Micro-Pollutants Oxidation, Disinfection by-Products Formation and Pathogens Inactivation. Chemosphere 2021, 273, 128527.
- Khan, J.A.; Sayed, M.; Khan, S.; Shah, N.S.; Dionysiou, D.D.; Boczkaj, G. Advanced Oxidation Processes for the Treatment of Contaminants of Emerging Concern. In Contaminants of Emerging Concern in Water and Wastewater: Advanced Treatment Processes; Elsevier Inc.: Cambridge, MA, USA, 2019; pp. 299–365. ISBN 9780128135617.
- Itzel, F.; Gehrmann, L.; Bielak, H.; Ebersbach, P.; Boergers, A.; Herbst, H.; Maus, C.; Simon, A.; Dopp, E.; Hammers-Wirtz, M.; et al. Investigation of Full-Scale Ozonation at a Municipal Wastewater Treatment Plant Using a Toxicity-Based Evaluation Concept. J. Toxicol. Environ. Health—Part A Curr. Issues 2017, 80, 1242–1258.
- Irani, R.; Khoshfetrat, A.B.; Forouzesh, M. Real Municipal Wastewater Treatment Using Simultaneous Pre and Post-Ozonation Combined Biological Attached Growth Reactor: Energy Consumption Assessment. J. Environ. Chem. Eng. 2021, 9, 104595.
- Gulde, R.; Rutsch, M.; Clerc, B.; Schollée, J.E.; von Gunten, U.; McArdell, C.S. Formation of Transformation Products during Ozonation of Secondary Wastewater Effluent and Their Fate in Post-Treatment: From Laboratory- to Full-Scale. Water Res. 2021, 200, 117200.
- Fernandes, E.; Contreras, S.; Medina, F.; Martins, R.C.; Gomes, J. N-Doped Titanium Dioxide for Mixture of Parabens Degradation Based on Ozone Action and Toxicity Evaluation: Precursor of Nitrogen and Titanium Effect. Process Saf. Environ. Prot. 2020, 138, 80–89.
- Sgroi, M.; Anumol, T.; Vagliasindi, F.G.A.; Snyder, S.A.; Roccaro, P. Comparison of the New Cl2/O3/UV Process with Different Ozone- and UV-Based AOPs for Wastewater Treatment at Pilot Scale: Removal of Pharmaceuticals and Changes in Fluorescing Organic Matter. Sci. Total Environ. 2021, 765, 142720.
- Cai, Q.Q.; Jothinathan, L.; Deng, S.H.; Ong, S.L.; Ng, H.Y.; Hu, J.Y. Fenton- and Ozone-Based AOP Processes for Industrial Effluent Treatment; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780128210116.
- Fernandes, E.; Martins, R.C.; Gomes, J. Photocatalytic Ozonation of Parabens Mixture Using 10% N-TiO2 and the Effect of Water Matrix. Sci. Total Environ. 2020, 718, 137321.
- Dong, P.; Xi, X.; Hou, G. Typical Non–TiO2-Based Visible-Light Photocatalysts. In Semiconductor Photocatalysis—Materials, Mechanisms and Applications; BoD–Books on Demand: Norderstedt, Germany, 2016; pp. 209–229.
- Barakat, M.A.; Kumar, R. Photocatalytic Activity Enhancement of Titanium Dioxide Nanoparticles; Springer: Cham, Switzerland, 2016; ISBN 9783319242699.
- Gomes, J.; Roccamante, M.; Contreras, S.; Medina, F.; Oller, I.; Martins, R.C. Scale-up Impact over Solar Photocatalytic Ozonation with Benchmark-P25 and N-TiO2 for Insecticides Abatement in Water. J. Environ. Chem. Eng. 2021, 9, 104915.
- García-López, E.I.; Palmisano, L. (Eds.) Material Science in Photocatalysis; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9781119130536.
- Nguyen, T.K.A.; Pham, T.T.; Nguyen-Phu, H.; Shin, E.W. The Effect of Graphitic Carbon Nitride Precursors on the Photocatalytic Dye Degradation of Water-Dispersible Graphitic Carbon Nitride Photocatalysts. Appl. Surf. Sci. 2021, 537, 148027.
- Fernandes, R.A.; Sampaio, M.J.; Dražić, G.; Faria, J.L.; Silva, C.G. Efficient Removal of Parabens from Real Water Matrices by a Metal-Free Carbon Nitride Photocatalyst. Sci. Total Environ. 2020, 716, 135346.
- Orge, C.A.; Sampaio, M.J.; Faria, J.L.; Pereira, M.F.R.; Silva, C.G. Efficiency and Stability of Metal-Free Carbon Nitride in the Photocatalytic Ozonation of Oxamic Acid under Visible Light. J. Environ. Chem. Eng. 2020, 8, 104172.
- Mena, E.; Rey, A.; Contreras, S.; Beltrán, F.J. Visible Light Photocatalytic Ozonation of DEET in the Presence of Different Forms of WO3. Catal. Today 2015, 252, 100–106.
- Huang, F.; Yan, A.; Zhao, H. Influences of Doping on Photocatalytic Properties of TiO2 Photocatalyst. In Semiconductor Photocatalysis: Materials, Mechanisms and Applications; InTech: Osaka, Japan, 2016; pp. 31–80.
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent Progress in Metal-Doped TiO2, Non-Metal Doped/Codoped TiO2 and TiO2 Nanostructured Hybrids for Enhanced Photocatalysis. Int. J. Hydrogen Energy 2020, 45, 7764–7778.
- Varma, K.S.; Tayade, R.J.; Shah, K.J.; Joshi, P.A.; Shukla, A.D.; Gandhi, V.G. Photocatalytic Degradation of Pharmaceutical and Pesticide Compounds (PPCs) Using Doped TiO2 Nanomaterials: A Review. Water-Energy Nexus 2020, 3, 46–61.
- Karuppasamy, P.; Ramzan Nilofar Nisha, N.; Pugazhendhi, A.; Kandasamy, S.; Pitchaimuthu, S. An Investigation of Transition Metal Doped TiO2 photocatalysts for the Enhanced Photocatalytic Decoloration of Methylene Blue Dye under Visible Light Irradiation. J. Environ. Chem. Eng. 2021, 9, 105254.
- Lee, H.; Jang, H.S.; Kim, N.Y.; Joo, J.B. Cu-Doped TiO2 Hollow Nanostructures for the Enhanced Photocatalysis under Visible Light Conditions. J. Ind. Eng. Chem. 2021, 99, 352–363.
- Moreira, A.J.; Malafatti, J.O.D.; Giraldi, T.R.; Paris, E.C.; Pereira, E.C.; de Mendonça, V.R.; Mastelaro, V.R.; Freschi, G.P.G. Prozac® Photodegradation Mediated by Mn-Doped TiO2 Nanoparticles: Evaluation of by-Products and Mechanisms Proposal. J. Environ. Chem. Eng. 2020, 8, 104543.
- Saber, N.B.; Mezni, A.; Alrooqi, A.; Altalhi, T. Fabrication of Efficient 2/RGO Heterojunction Nanocomposite: Boosted Photocatalytic Activity under Ultraviolet and Visible Light Irradiation. J. Mater. Res. Technol. 2021, 12, 2238–2246.
- Ellouzi, I.; Bouddouch, A.; Bakiz, B.; Benlhachemi, A.; Abou Oualid, H. Glucose-Assisted Ball Milling Preparation of Silver-Doped Biphasic TiO2 for Efficient Photodegradation of Rhodamine B: Effect of Silver-Dopant Loading. Chem. Phys. Lett. 2021, 770, 138456.
- Ribeiro, A.R.; Umbuzeiro, G. de A. Effects of a Textile Azo Dye on Mortality, Regeneration, and Reproductive Performance of the Planarian, Girardia Tigrina. Environ. Sci. Eur. 2014, 26, 1–8.
- Gusain, R.; Kumar, N.; Ray, S.S. Factors Influencing the Photocatalytic Activity of Photocatalysts in Wastewater Treatment. In Photocatalysts in Advanced Oxidation Processes for Wastewater Treatment; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 229–270.
- Mirzaei, A.; Eddah, M.; Roualdes, S.; Ma, D.; Chaker, M. Multiple-Homojunction Gradient Nitrogen Doped TiO2 for Photocatalytic Degradation of Sulfamethoxazole, Degradation Mechanism, and Toxicity Assessment. Chem. Eng. J. 2021, 422, 130507.
- Ramezanisani, S.; Rajabi, M.; Mohseni, F. Influence of Nitrogen Doping on Visible Light Photocatalytic Activity of TiO2 Nanowires with Anatase-Rutile Junction. Chem. Phys. Lett. 2020, 744, 137217.
- Suwannaruang, T.; Kamonsuangkasem, K.; Kidkhunthod, P.; Chirawatkul, P.; Saiyasombat, C.; Chanlek, N.; Wantala, K. Influence of Nitrogen Content Levels on Structural Properties and Photocatalytic Activities of Nanorice-like N-Doped TiO2 with Various Calcination Temperatures. Mater. Res. Bull. 2018, 105, 265–276.
- Erdogan, N.; Bouziani, A.; Park, J.; Micusik, M.; Kim, S.Y.; Majkova, E.; Omastova, M.; Ozturk, A. Synthesis and Enhanced Photocatalytic Activity of Nitrogen-Doped Triphasic TiO2 Nanoparticles. J. Photochem. Photobiol. A Chem. 2019, 377, 92–100.
- Toe, E.D.; Kurniawan, W.; Mariquit, E.G.; Hinode, H. Synthesis of N-Doped Mesoporous TiO2 by Facile One-Step Solvothermal Process for Visible Light Photocatalytic Degradation of Organic Pollutant. J. Environ. Chem. Eng. 2018, 6, 5125–5134.
- Yadav, V.; Saini, V.K.; Sharma, H. How Different Dopants Leads to Difference in Photocatalytic Activity in Doped TiO2? Ceram. Int. 2020, 46, 27308–27317.
- Bakre, P.V.; Tilve, S.G.; Shirsat, R.N. Influence of N Sources on the Photocatalytic Activity of N-Doped TiO2. Arab. J. Chem. 2020, 13, 7637–7651.
- Zhao, W.; Liu, S.; Zhang, S.; Wang, R.; Wang, K. Preparation and Visible-Light Photocatalytic Activity of N-Doped TiO2 by Plasma-Assisted Sol-Gel Method. Catal. Today 2019, 337, 37–43.
- Coronado, J.M.; Fresno, F.; Hernández-Alonso, M.D.; Portela, R. Design of Advanced Photocatalytic Materials for Energy and Environmental Applications; Springer: London, UK, 2013; Volume 71, ISBN 9781447150602.
- Assayehegn, E.; Solaiappan, A.; Chebude, Y.; Alemayehu, E. Fabrication of Tunable Anatase/Rutile Heterojunction N/TiO2 Nanophotocatalyst for Enhanced Visible Light Degradation Activity. Appl. Surf. Sci. 2020, 515, 145966.
- Mancuso, A.; Sacco, O.; Vaiano, V.; Sannino, D.; Pragliola, S.; Venditto, V.; Morante, N. Visible Light Active Fe-Pr Co-Doped TiO2 for Water Pollutants Degradation. Catal. Today 2021.
- Mancuso, A.; Sacco, O.; Sannino, D.; Pragliola, S.; Vaiano, V. Enhanced Visible-Light-Driven Photodegradation of Acid Orange 7 Azo Dye in Aqueous Solution Using Fe-N Co-Doped TiO2. Arab. J. Chem. 2020, 13, 8347–8360.
- Razali, N.A.M.; Salleh, W.N.W.; Aziz, F.; Jye, L.W.; Yusof, N.; Ismail, A.F. Review on Tungsten Trioxide as a Photocatalysts for Degradation of Recalcitrant Pollutants. J. Clean. Prod. 2021, 309, 127438.
- Quyen, V.T.; Kim, J.T.; Park, P.M.; Huong, P.T.; Viet, N.M.; Thang, P.Q. Enhanced the Visible Light Photocatalytic Decomposition of Antibiotic Pollutant in Wastewater by Using Cu Doped WO3. J. Environ. Chem. Eng. 2021, 9, 104737.
- Palharim, P.H.; Fusari, B.L.D.d.R.; Ramos, B.; Otubo, L.; Teixeira, A.C.S.C. Effect of HCl and HNO3 on the Synthesis of Pure and Silver-Based WO3 for Improved Photocatalytic Activity under Sunlight. J. Photochem. Photobiol. A Chem. 2022, 422, 113550.
- Han, F.; Li, H.; Fu, L.; Yang, J.; Liu, Z. Synthesis of S-Doped WO3 Nanowires with Enhanced Photocatalytic Performance towards Dye Degradation. Chem. Phys. Lett. 2016, 651, 183–187.
- Tijani, J.O.; Ugochukwu, O.; Fadipe, L.A.; Bankole, M.T.; Abdulkareem, A.S.; Roos, W.D. Photocatalytic Degradation of Local Dyeing Wastewater by Iodine-Phosphorus Co-Doped Tungsten Trioxide Nanocomposites under Natural Sunlight Irradiation. J. Environ. Manag. 2019, 236, 519–533.
- Ma, T.; Shen, Q.; Xue, B.Z.J.; Guan, R.; Liu, X.; Jia, H.; Xu, B. Facile Synthesis of Fe-Doped g-C3N4 for Enhanced Visible-Light Photocatalytic Activity. Inorg. Chem. Commun. 2019, 107, 107451.
- Pham, T.H.; Park, J.W.; Kim, T.Y. Enhanced Photodegradation of Paracetamol from Water by Cobalt Doped Graphitic Carbon Nitride. Sol. Energy 2021, 215, 151–156.
- Tri, N.L.M.; Kim, J.; Giang, B.L.; Tahtamouni, T.M.A.; Huong, P.T.; Lee, C.; Viet, N.M.; Trung, D.Q. Ag-Doped Graphitic Carbon Nitride Photocatalyst with Remarkably Enhanced Photocatalytic Activity towards Antibiotic in Hospital Wastewater under Solar Light. J. Ind. Eng. Chem. 2019, 80, 597–605.
- Zhang, B.; Li, X.; Zhao, Y.; Song, H.; Wang, H. Facile Synthesis of Oxygen Doped Mesoporous Graphitic Carbon Nitride with High Photocatalytic Degradation Efficiency under Simulated Solar Irradiation. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 580, 123736.
- Ganganboina, A.B.; Nguyen, M.D.; Nguyen, T.H.L.; Kuncoro, E.P.; Doong, R.-A. Boron and Phosphorus Co-Doped One-Dimensional Graphitic Carbon Nitride for Enhanced Visible-Light-Driven Photodegradation of Diclofenac. Chem. Eng. J. 2021, 425, 131520.
- Pandit, B.B.; Sonawane, S.; Aniruddha, V.P. (Eds.) Handbook of Nanomaterials for Wastewater Treatment: Fundamental and Scale Up Issues, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9789896540821.
- Khan, M.M.; Pradhan, D.; Sohn, Y. (Eds.) Nanocomposites for Visible Light-Induced Photocatalysis; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 9783319624457.
- Mugunthan, E.; Saidutta, M.B.; Jagadeeshbabu, P.E. Visible Light Assisted Photocatalytic Degradation of Diclofenac Using TiO2-WO3 Mixed Oxide Catalysts. Environ. Nanotechnol. Monit. Manag. 2018, 10, 322–330.
- El-Yazeed, W.S.A.; Ahmed, A.I. Photocatalytic Activity of Mesoporous WO3/TiO2 Nanocomposites for the Photodegradation of Methylene Blue. Inorg. Chem. Commun. 2019, 105, 102–111.
- Wang, Q.; Zhang, W.; Hu, X.; Xu, L.; Chen, G.; Li, X. Hollow Spherical WO3/TiO2 Heterojunction for Enhancing Photocatalytic Performance in Visible-Light. J. Water Process Eng. 2021, 40, 101943.
- Su, X.; Liu, C.j.; Liu, Y.; Yang, Y.; Liu, X.; Chen, S. Construction of BiVO4 3 Arrays Heterojunction Photoanodes by Versatile Phase Transformation Strategy. Trans. Nonferrous Met. Soc. China 2021, 31, 533–544.
- Meng, S.; Sun, W.; Zhang, S.; Zheng, X.; Fu, X.; Chen, S. Insight into the Transfer Mechanism of Photogenerated Carriers for WO3/TiO2 Heterojunction Photocatalysts: Is It the Transfer of Band-Band or Z-Scheme? Why? J. Phys. Chem. C 2018, 122, 26326–26336.
- Someswararao, M.V.; Dubey, R.S.; Subbarao, P.S.V. Electrospun Composite Nanofibers Prepared by Varying Concentrations of TiO2/ZnO Solutions for Photocatalytic Applications. J. Photochem. Photobiol. 2021, 6, 100016.
- Das, A.; Kumar, P.M.; Bhagavathiachari, M.; Nair, R.G. Hierarchical ZnO-TiO2 Nanoheterojunction: A Strategy Driven Approach to Boost the Photocatalytic Performance through the Synergy of Improved Surface Area and Interfacial Charge Transport. Appl. Surf. Sci. 2020, 534, 147321.
- Cipagauta-Díaz, S.; Estrella-González, A.; Navarrete-Magaña, M.; Gómez, R. N Doped-TiO2 Coupled to BiVO4 with High Performance in Photodegradation of Ofloxacin Antibiotic and Rhodamine B Dye under Visible Light. Catal. Today 2021, 394, 445–457.
- Sharma, S.; Kumar, N.; Mari, B.; Chauhan, N.S.; Mittal, A.; Maken, S.; Kumari, K. Solution Combustion Synthesized TiO2/Bi2O3/CuO Nano-Composites and Their Photocatalytic Activity Using Visible LEDs Assisted Photoreactor. Inorg. Chem. Commun. 2021, 125, 108418.
- Chawla, H.; Chandra, A.; Ingole, P.P.; Garg, S. Recent Advancements in Enhancement of Photocatalytic Activity Using Bismuth-Based Metal Oxides Bi2MO6 (M = W, Mo, Cr) for Environmental Remediation and Clean Energy Production. J. Ind. Eng. Chem. 2021, 95, 1–15.
- Li, H.; Zhang, Y.; Zhang, Q.; Wang, Y.; Fan, Y.; Gao, X.; Niu, J. Boosting Visible-Light Photocatalytic Degradation of Indomethacin by an Efficient and Photostable Ag3PO4/NG/WO3 Composites. Appl. Surf. Sci. 2019, 490, 481–491.
- Iqbal, S.; Bahadur, A.; Javed, M.; Hakami, O.; Irfan, R.M.; Ahmad, Z.; AlObaid, A.; Al-Anazy, M.M.; Baghdadi, H.B.; Abd-Rabboh, H.S.M.; et al. Design Ag-Doped ZnO Heterostructure Photocatalyst with Sulfurized Graphitic C3N4 Showing Enhanced Photocatalytic Activity. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2021, 272, 115320.
- Zhang, C.; Zhang, M.; Li, Y.; Shuai, D. Visible-Light-Driven Photocatalytic Disinfection of Human Adenovirus by a Novel Heterostructure of Oxygen-Doped Graphitic Carbon Nitride and Hydrothermal Carbonation Carbon. Appl. Catal. B Environ. 2019, 248, 11–21.




