
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sanghoon Lee | + 1371 word(s) | 1371 | 2020-11-11 08:55:25 | | | |
| 2 | Catherine Yang | Meta information modification | 1371 | 2020-12-18 03:05:18 | | |
Video Upload Options
This review focused on current challenges and future directions on the treatment and prevention of ovarian failure or infertility by chemotherapy in young reproductive women with cancer. We also address current knowledge on chemotherapy-induced ovarian toxicity and its mechanisms. We hope this review will help clinicians to prevent and treat girls and young women with cancer who desire to preserve their ovarian endocrine function and fertility.
1. Introduction
Cancer incidence is rapidly growing worldwide. In 2018, 8.6 million women were diagnosed with cancer globally [1]. Most women diagnosed with cancer are older, but 10% are <45 years of age [2]. Due to advances in cancer diagnosis and treatment, the survival rate for prepubertal and young women with cancer has significantly improved. In Europe, the five-year-survival rate is 79.1% in children diagnosed with cancer [3]. However, aggressive chemotherapy can cause impairment of reproductive functions and even fertility loss [4][5][6][7]. Although depletion of ovarian function is associated with improved survival outcomes in breast cancer patients of reproductive age, it has several side effects, such as hot flashes, osteoporosis, and sexual dysfunction [8]. Cardiovascular disease is the main cause of shortened life expectancy in women with premature ovarian insufficiency (POI) [9]. Moreover, chemotherapy-related POI and infertility may be associated with increased risk of neuro-degenerating disease and psychosocial distress [9].
In recent years, interest in fertility preservation has increased significantly among female cancer patients [10]. Despite the huge interest cancer patients have with respect to preserving fertility, there is an unmet need in children and young cancer survivors [11]. Oncofertility is a relatively innovative concept that describes a multidisciplinary network of experts focused on developing and providing the option of fertility preservation to young cancer patients. Currently, embryo and oocyte cryopreservation are the only established methods for fertility preservation [12]. However, there is accumulating evidence for other experimental techniques including ovarian tissue cryopreservation, artificial ovaries, and in vitro maturation [13].
2. The Effect of Chemotherapy on Ovarian Function
2.1. Risk of Ovarian Toxicity Due to Chemotherapy Agents
Although the survival rate of cancer patients has dramatically improved due to development of chemotherapy, ovarian toxicity induced by chemotherapy is a major complication. Gonadotoxic chemotherapy during reproductive age can lead to iatrogenic primary ovarian insufficiency (POI), and loss of follicular reserve that depends on the type, dose, duration, and combination of chemotherapeutic agents, and disease stage, as well as patient age [14][15]. It has been reported that 53–89% of chemotherapy-induced POI occurs in patients with breast cancer [16]. The combination of abdominal radiation and alkylating agents which are likely to cause gonadotoxicity induces POI in almost 100% of cancer patients [17][18]. In a large study of cancer survivors, the risk of POI was increased 9.2-fold for patients who received chemotherapy including alkylating agents and 27-fold in women who received combination alkylating agent-based chemotherapy and radiotherapy [19].
Figure 1 and Table 1 show the most common cancers and the risk of chemotherapy-induced ovarian toxicity in women according to chemotherapy protocol and age [3][20][21]. Notice that the course of chemotherapy and its related risks of gonadotoxicity can be unpredictable and variable due to treatment response and disease prognosis, i.e., refractory or recurrent cases [13][22]. In order to prevent POI due to chemotherapy and subsequent complications, effective and comprehensive oncofertility strategies should be undertaken to preserve fertility in young reproductive age women before initiation of cancer treatment [17][23][24][25][26].
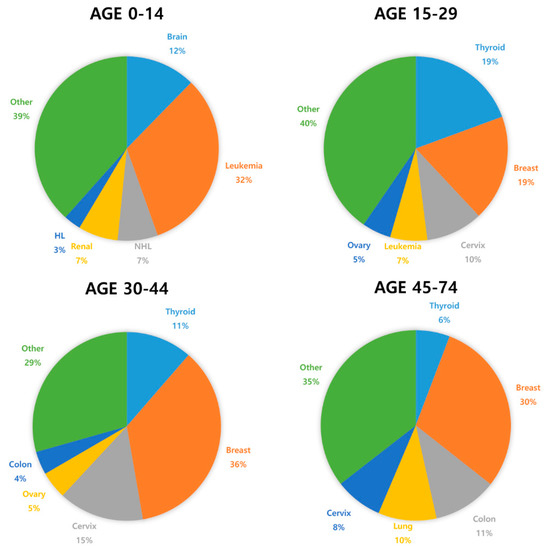
Figure 1. The most commonly diagnosed cancer types in females by age worldwide (2018) [27].
Table 1. Common malignancies occurring in prepubertal girls and women at reproductive age and the risk of chemotherapy-induced gonadotoxicity.
| Diagnosis | Chemotherapy Protocol | Risk of Iatrogenic POI |
|---|---|---|
| Non-Hodgkin lymphoma |
Cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisone (CHOP) (four to six cycles) Rituximab, cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisone (R-CHOP) (four to six cycles) |
<20% [3][28] |
| Hodgkin lymphoma |
Adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD) |
<20% [3] |
| Mustargen, oncovin, prednisone, and procarbazine (MOPP) |
10–50% [29][30][31][32] | |
| Bleomycin, etoposide, adriamycin, cyclophosphamide, oncovin, procarbazine, and prednisone (BEACOPP) (eight cycles) |
50–95% (age dependent) [33] | |
| Acute lympho- cytic leukemia |
Most standard chemotherapy protocols do not include a gonadotoxic multi-agent | <20% [3][13][14] |
| Acute myeloid leukemia |
Most standard chemotherapy protocols do not include gonadotoxic anthracycline/cytarabine | <20% [3][13][14] |
| Breast cancer | Cyclophosphamide, methotrexate, fluorouracil (CMF) (six cycles) Cyclophosphamide, epirubicin, fluorouracil (CEF) (six cycles) Cyclophosphamide, eoxorubicin (adriamycin), fluorouracil (CAF) (six cycles) |
>80% [3] (≥age 40) |
| 30–70% [3] (age 30–39) |
||
| Doxorubicin (adriamycin), cyclophosphamide (AC) (four cycles) |
30–70% [3] (≥age 40) |
|
| >20 [3] (age 30–39) |
||
| Others | Cyclophosphamide ≥ 7 g/m2 in females < 20 years Cyclophosphamide ≥ 5 g/m2 in females > 40 years Any alkylating agent (e.g., cyclophosphamide, ifosfamide, busulfan, carmustine, lomustine) |
>80% [34][35] |
| Cyclophosphamide ≥ 5 g/m2 in females 30–40 years | 30–70% [34][35] | |
| Taxanes Oxaliplatin Irinotecan Monoclonal antibodies (trastuzumab, bevacizumab, cetuximab) Tyrosine kinase inhibitors (erlotinib, imatinib) |
Unknown |
2.2. Mechanisms of Ovarian Toxicity
Gonadotoxic chemotherapy leads to primordial follicle loss, resulting in POI and infertility. Both direct acute and indirect delayed mechanisms have been reported for the effects of anticancer agents that cause a decrease in ovarian reserve. The main mechanism is that anticancer drugs directly induce DNA double-strand breaks (DSBs), which activate apoptosis and/or autophagy-related pathways [36][37][38][39][40][41][42]. The second mechanism is that anticancer drugs can indirectly cause primordial follicle depletion by microvascular and stromal injury through ischemia, necrosis, or inflammation [38][42][43][44][45]. There is third hypothesis called the “burnout” effect. A few studies have shown that anticancer drugs induce activation of the phosphoinositide 3-kinase/protein kinase B/forkhead box protein O3a (PI3K/AKT/FOXO3a) pathway, which leads to follicle reduction by massive activation of primordial follicles in mice and cultured human ovarian tissue [36][46][47][48][49]. However, there is some question of methodology and biological mechanism of follicle loss based on studies supporting “burnout theory”. It has not been proven that primordial follicle growth is the main cause of chemotherapy-induced primordial follicle loss. Thus, the “burnout” theory of chemotherapy-induced follicle depletion is still lacking evidence and is under debate [36][46]. The main cause of the follicle depletion induced by chemotherapy seems to be DNA double-strand breaks and apoptosis.
3. Improving Oncofertility Care
Preserving fertility is important to most young cancer survivors. One study reported that more than half (51.7%) of young women undergoing cancer treatment felt that having children was the “most important” issue in their life [50]. The fear of treatment-related infertility may affect patients’ decision making in choosing cancer treatment among those who want to conceive their own genetic offspring [51][52]. Therefore, according to the ASCO, clinicians should refer cancer patients who are undecided or uncertain about their fertility intentions to a reproductive specialist for a fertility preservation consultation before initiating cancer treatment [53][54]. Established in 2007, the Oncofertility Consortium (OC) is a nationwide network of oncologists, reproductive specialists, and research scientists for fertility preservation in young cancer patients [55]. The National Physicians Cooperative, formed by the OC to share knowledge and resources, comprises 60 centers across the United States that provide oncofertility services to women [56]. In Japan, after establishment of the Japan Society for Fertility Preservation (JSFP) in 2012, there are 46 current medical institutions for preserving fertility. In Europe, the FertiPROTEKT network was founded in May 2006, and has included approximately 100 centers from Germany, Austria, and Switzerland since January 2014 [55].
Despite the increasing interest in and the advance of technologies available in the oncofertility field, accessibility to fertility preservation remains relatively low for young cancer patients, particularly those in low- and middle-income countries [57][58]. In a retrospective cohort study of women aged 18–42 years diagnosed with cancer, 20.6% received fertility preservation care [57]. In another study, only 9% of patients received any information on fertility preservation options [59].
Major barriers are lack of awareness among oncologists, lack of referrals from oncologists, lack of interinstitutional networks, and lack of oncofertility specialists [20]. In addition, many oncologists fail to have fertility discussions with their cancer patients and, thus, fail to make timely referrals due to patients’ lack of awareness of treatment-related infertility, together with time pressures, financial costs, and conflicting priorities of physicians [60][61].
To provide fertility preservation strategies to prepubertal and young women with cancer, each medical institution should be properly equipped, and should have a highly skilled and experienced oncofertility team which consists of medical oncologists, gynecologists, reproductive biologists, oncologic surgeons, patient navigators, and research scientists [13]. When oncofertility care is not available in institutions that treat women with cancer, immediate referral of patients to specialized oncofertility centers is encouraged to assure a high standard of care. In addition, individualized fertility preservation options should be considered based on patient age, marital status, economic status of patients, cancer type, staging upon diagnosis, chemotherapy regimen, and urgency of chemotherapy treatment (Figure 2).
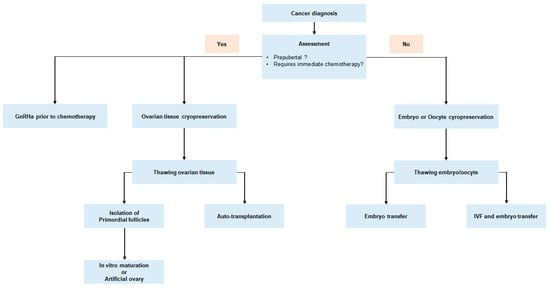
Figure 2. Fertility preservation approach for women with newly diagnosed malignancy.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer statistics, 2009. CA Cancer J. Clin. 2009, 59, 225–249.
- Lee, S.J.; Schover, L.R.; Partridge, A.H.; Patrizio, P.; Wallace, W.H.; Hagerty, K.; Beck, L.N.; Brennan, L.V.; Oktay, K.; American Society of Clinical Oncology. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J. Clin. Oncol. 2006, 24, 2917–2931.
- Hoekman, E.J.; Knoester, D.; Peters, A.A.W.; Jansen, F.W.; de Kroon, C.D.; Hilders, C. Ovarian survival after pelvic radiation: Transposition until the age of 35 years. Arch. Gynecol. Obstet. 2018, 298, 1001–1007.
- Anderson, R.A.; Mitchell, R.T.; Kelsey, T.W.; Spears, N.; Telfer, E.E.; Wallace, W.H. Cancer treatment and gonadal function: Experimental and established strategies for fertility preservation in children and young adults. Lancet Diabetes Endocrinol. 2015, 3, 556–567.
- Kort, J.D.; Eisenberg, M.L.; Millheiser, L.S.; Westphal, L.M. Fertility issues in cancer survivorship. CA Cancer J. Clin. 2014, 64, 118–134.
- Lee, S.; Song, J.Y.; Ku, S.Y.; Kim, S.H.; Kim, T. Fertility preservation in women with cancer. Clin. Exp. Reprod. Med. 2012, 39, 46–51.
- Partridge, A.H.; Pagani, O.; Abulkhair, O.; Aebi, S.; Amant, F.; Azim, H.A., Jr.; Costa, A.; Delaloge, S.; Freilich, G.; Gentilini, O.D.; et al. First international consensus guidelines for breast cancer in young women (BCY1). Breast 2014, 23, 209–220.
- Podfigurna-Stopa, A.; Czyzyk, A.; Grymowicz, M.; Smolarczyk, R.; Katulski, K.; Czajkowski, K.; Meczekalski, B. Premature ovarian insufficiency: The context of long-term effects. J. Endocrinol. Invest. 2016, 39, 983–990.
- Donnez, J.; Dolmans, M.-M. Fertility preservation in women. N. Engl. J. Med. 2017, 377, 1657–1665.
- Quinn, G.P.; Vadaparampil, S.T.; Gwede, C.K.; Miree, C.; King, L.M.; Clayton, H.B.; Wilson, C.; Munster, P. Discussion of fertility preservation with newly diagnosed patients: Oncologists’ views. J. Cancer Surviv. 2007, 1, 146–155.
- The Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in patients facing gonadotoxic therapies: A committee opinion. Fertil. Steril. 2013, 100, 1224–1231.
- Salama, M.; Anazodo, A.; Woodruff, T.K. Preserving fertility in female patients with hematological malignancies: A multidisciplinary oncofertility approach. Ann. Oncol. 2019, 30, 1760–1775.
- Salama, M.; Isachenko, V.; Isachenko, E.; Rahimi, G.; Mallmann, P. Advances in fertility preservation of female patients with hematological malignancies. Expert Rev. Hematol. 2017, 10, 951–960.
- Kim, S.; Lee, Y.; Lee, S.; Kim, T. Ovarian tissue cryopreservation and transplantation in patients with cancer. Obs. Gynecol. Sci. 2018, 61, 431–442.
- Han, H.S.; Ro, J.; Lee, K.S.; Nam, B.H.; Seo, J.A.; Lee, D.H.; Lee, H.; Lee, E.S.; Kang, H.S.; Kim, S.W. Analysis of chemotherapy-induced amenorrhea rates by three different anthracycline and taxane containing regimens for early breast cancer. Breast Cancer Res. Treat 2009, 115, 335–342.
- Donnez, J.; Dolmans, M.M. Preservation of fertility in females with haematological malignancy. Br. J. Haematol. 2011, 154, 175–184.
- Wallace, W.H.; Thomson, A.B.; Kelsey, T.W. The radiosensitivity of the human oocyte. Hum. Reprod. 2003, 18, 117–121.
- Byrne, J.; Fears, T.R.; Gail, M.H.; Pee, D.; Connelly, R.R.; Austin, D.F.; Holmes, G.F.; Holmes, F.F.; Latourette, H.B.; Meigs, J.W.; et al. Early menopause in long-term survivors of cancer during adolescence. Am. J. Obstet. Gynecol. 1992, 166, 788–793.
- Salama, M.; Woodruff, T.K. Anticancer treatments and female fertility: Clinical concerns and role of oncologists in oncofertility practice. Expert Rev. Anticancer Ther. 2017, 17, 687–692.
- Loren, A.W.; Mangu, P.B.; Beck, L.N.; Brennan, L.; Magdalinski, A.J.; Partridge, A.H.; Quinn, G.; Wallace, W.H.; Oktay, K. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 2013, 31, 2500–2510.
- Meirow, D.; Nugent, D. The effects of radiotherapy and chemotherapy on female reproduction. Hum. Reprod. Update 2001, 7, 535–543.
- Shapira, M.; Raanani, H.; Cohen, Y.; Meirow, D. Fertility preservation in young females with hematological malignancies. Acta Haematol. 2014, 132, 400–413.
- Loren, A.W. Fertility issues in patients with hematologic malignancies. Hematol. Am Soc. Hematol. Educ. Program. 2015, 2015, 138–145.
- Leader, A.; Lishner, M.; Michaeli, J.; Revel, A. Fertility considerations and preservation in haemato-oncology patients undergoing treatment. Br. J. Haematol. 2011, 153, 291–308.
- Anderson, R.A.; Remedios, R.; Kirkwood, A.A.; Patrick, P.; Stevens, L.; Clifton-Hadley, L.; Roberts, T.; Hatton, C.; Kalakonda, N.; Milligan, D.W.; et al. Determinants of ovarian function after response-adapted therapy in patients with advanced Hodgkin’s lymphoma (RATHL): A secondary analysis of a randomised phase 3 trial. Lancet Oncol. 2018, 19, 1328–1337.
- International Agency for Research on Cancer. Available online: https://gco.iarc.fr/today/online-analysis-multibars?v=2018&mode=cancer&mode_population=countries&population=900&populations=900&key=asr&sex=2&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=2&nb_items=10&group_cancer=1&include_nmsc=1&include_nmsc_other=1&type_multiple=%257B%2522inc%2522%253Atrue%252C%2522mort%2522%253Afalse%252C%2522prev%2522%253Afalse%257D&orientation=horizontal&type_sort=0&type_nb_items=%257B%2522top%2522%253Atrue%252C%2522bottom%2522%253Afalse%257D&population_group_globocan_id= (accessed on 1 October 2020).
- Elis, A.; Tevet, A.; Yerushalmi, R.; Blickstein, D.; Bairy, O.; Dann, E.J.; Blumenfeld, Z.; Abraham, A.; Manor, Y.; Shpilberg, O.; et al. Fertility status among women treated for aggressive non-Hodgkin’s lymphoma. Leuk Lymphoma 2006, 47, 623–627.
- Warne, G.L.; Fairley, K.F.; Hobbs, J.B.; Martin, F.I. Cyclophosphamide-induced ovarian failure. N. Engl. J. Med. 1973, 289, 1159–1162.
- Schilsky, R.L.; Sherins, R.J.; Hubbard, S.M.; Wesley, M.N.; Young, R.C.; DeVita, V.T. Long-term follow up of ovarian function in women treated with MOPP chemotherapy for Hodgkin‘s disease. Am. J. Med. 1981, 71, 552–556.
- Stillman, R.J.; Schinfeld, J.S.; Schiff, I.; Gelber, R.D.; Greenberger, J.; Larson, M.; Jaffe, N.; Li, F.P. Ovarian failure in long-term survivors of childhood malignancy. Am. J. Obs. Gynecol. 1981, 139, 62–66.
- Harel, S.; Fermé, C.; Poirot, C. Management of fertility in patients treated for Hodgkin‘s lymphoma. Haematologica 2011, 96, 1692–1699.
- Behringer, K.; Breuer, K.; Reineke, T.; May, M.; Nogova, L.; Klimm, B.; Schmitz, T.; Wildt, L.; Diehl, V.; Engert, A.; et al. Secondary amenorrhea after Hodgkin’s lymphoma is influenced by age at treatment, stage of disease, chemotherapy regimen, and the use of oral contraceptives during therapy: A report from the German Hodgkin’s Lymphoma Study Group. J. Clin. Oncol. 2005, 23, 7555–7564.
- Levine, J.; Canada, A.; Stern, C.J. Fertility preservation in adolescents and young adults with cancer. J. Clin. Oncol. 2010, 28, 4831–4841.
- Levine, J.M.; Kelvin, J.F.; Quinn, G.P.; Gracia, C.R. Infertility in reproductive-age female cancer survivors. Cancer 2015, 121, 1532–1539.
- Szymanska, K.J.; Tan, X.; Oktay, K. Unraveling the mechanisms of chemotherapy-induced damage to human primordial follicle reserve: Road to developing therapeutics for fertility preservation and reversing ovarian aging. Mol. Hum. Reprod. 2020, 26, 553–566.
- Hao, X.; Anastácio, A.; Liu, K.; Rodriguez-Wallberg, K.A. Ovarian Follicle Depletion Induced by Chemotherapy and the Investigational Stages of Potential Fertility-Protective Treatments-A Review. Int. J. Mol. Sci. 2019, 20, 4720.
- Soleimani, R.; Heytens, E.; Darzynkiewicz, Z.; Oktay, K. Mechanisms of chemotherapy-induced human ovarian aging: Double strand DNA breaks and microvascular compromise. Aging 2011, 3, 782–793.
- Rossi, V.; Lispi, M.; Longobardi, S.; Mattei, M.; Di Rella, F.; Salustri, A.; De Felici, M.; Klinger, F.G. LH prevents cisplatin-induced apoptosis in oocytes and preserves female fertility in mouse. Cell Death Differ. 2017, 24, 72–82.
- Petrillo, S.K.; Desmeules, P.; Truong, T.Q.; Devine, P.J. Detection of DNA damage in oocytes of small ovarian follicles following phosphoramide mustard exposures of cultured rodent ovaries in vitro. Toxicol Appl. Pharm. 2011, 253, 94–102.
- Nguyen, Q.N.; Zerafa, N.; Liew, S.H.; Findlay, J.K.; Hickey, M.; Hutt, K.J. Cisplatin- and cyclophosphamide-induced primordial follicle depletion is caused by direct damage to oocytes. Mol. Hum. Reprod. 2019, 25, 433–444.
- Luan, Y.; Edmonds, M.E.; Woodruff, T.K.; Kim, S.Y. Inhibitors of apoptosis protect the ovarian reserve from cyclophosphamide. J. Endocrinol. 2019, 240, 243–256.
- Meirow, D.; Dor, J.; Kaufman, B.; Shrim, A.; Rabinovici, J.; Schiff, E.; Raanani, H.; Levron, J.; Fridman, E. Cortical fibrosis and blood-vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum. Reprod. 2007, 22, 1626–1633.
- Luo, Q.; Yin, N.; Zhang, L.; Yuan, W.; Zhao, W.; Luan, X.; Zhang, H. Role of SDF-1/CXCR4 and cytokines in the development of ovary injury in chemotherapy drug induced premature ovarian failure mice. Life Sci. 2017, 179, 103–109.
- Bar-Joseph, H.; Ben-Aharon, I.; Tzabari, M.; Tsarfaty, G.; Stemmer, S.M.; Shalgi, R. In vivo bioimaging as a novel strategy to detect doxorubicin-induced damage to gonadal blood vessels. PLoS ONE 2011, 6, e23492.
- Sonigo, C.; Beau, I.; Binart, N.; Grynberg, M. The Impact of Chemotherapy on the Ovaries: Molecular Aspects and the Prevention of Ovarian Damage. Int. J. Mol. Sci. 2019, 20, 5342.
- Kalich-Philosoph, L.; Roness, H.; Carmely, A.; Fishel-Bartal, M.; Ligumsky, H.; Paglin, S.; Wolf, I.; Kanety, H.; Sredni, B.; Meirow, D. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci. Transl. Med. 2013, 5, 185ra62.
- Goldman, K.N.; Chenette, D.; Arju, R.; Duncan, F.E.; Keefe, D.L.; Grifo, J.A.; Schneider, R.J. mTORC1/2 inhibition preserves ovarian function and fertility during genotoxic chemotherapy. Proc. Natl. Acad. Sci. USA 2017, 114, 3186–3191.
- Chang, E.M.; Lim, E.; Yoon, S.; Jeong, K.; Bae, S.; Lee, D.R.; Yoon, T.K.; Choi, Y.; Lee, W.S. Cisplatin Induces Overactivation of the Dormant Primordial Follicle through PTEN/AKT/FOXO3a Pathway which Leads to Loss of Ovarian Reserve in Mice. PLoS ONE 2015, 10, e0144245.
- Reh, A.E.; Lu, L.; Weinerman, R.; Grifo, J.; Krey, L.; Noyes, N. Treatment outcomes and quality-of-life assessment in a university-based fertility preservation program: Results of a registry of female cancer patients at 2 years. J. Assist. Reprod. Genet. 2011, 28, 635–641.
- Deshpande, N.A.; Braun, I.M.; Meyer, F.L. Impact of fertility preservation counseling and treatment on psychological outcomes among women with cancer: A systematic review. Cancer 2015, 121, 3938–3947.
- Ruddy, K.J.; Gelber, S.I.; Tamimi, R.M.; Ginsburg, E.S.; Schapira, L.; Come, S.E.; Borges, V.F.; Meyer, M.E.; Partridge, A.H. Prospective study of fertility concerns and preservation strategies in young women with breast cancer. J. Clin. Oncol. 2014, 32, 1151–1156.
- Lee, S.; Ozkavukcu, S.; Heytens, E.; Moy, F.; Oktay, K. Value of early referral to fertility preservation in young women with breast cancer. J. Clin. Oncol. 2010, 28, 4683–4686.
- Oktay, K.; Harvey, B.E.; Partridge, A.H.; Quinn, G.P.; Reinecke, J.; Taylor, H.S.; Wallace, W.H.; Wang, E.T.; Loren, A.W. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1994–2001.
- Ataman, L.M.; Rodrigues, J.K.; Marinho, R.M.; Caetano, J.P.; Chehin, M.B.; Alves da Motta, E.L.; Serafini, P.; Suzuki, N.; Furui, T.; Takae, S.; et al. Creating a Global Community of Practice for Oncofertility. J. Glob. Oncol. 2016, 2, 83–96.
- Woodruff, T.K. The Oncofertility Consortium--addressing fertility in young people with cancer. Nat. Rev. Clin. Oncol. 2010, 7, 466–475.
- Goodman, L.R.; Balthazar, U.; Kim, J.; Mersereau, J.E. Trends of socioeconomic disparities in referral patterns for fertility preservation consultation. Hum. Reprod. 2012, 27, 2076–2081.
- Lee, S.; Heytens, E.; Moy, F.; Ozkavukcu, S.; Oktay, K. Determinants of access to fertility preservation in women with breast cancer. Fertil. Steril. 2011, 95, 1932–1936.
- Goldfarb, S.B.; Kamer, S.A.; Oppong, B.A.; Eaton, A.; Patil, S.; Junqueira, M.J.; Olcese, C.; Kelvin, J.F.; Gemignani, M.L. Fertility Preservation for the Young Breast Cancer Patient. Ann. Surg. Oncol. 2016, 23, 1530–1536.
- Linkeviciute, A.; Boniolo, G.; Chiavari, L.; Peccatori, F.A. Fertility preservation in cancer patients: The global framework. Cancer Treat Rev. 2014, 40, 1019–1027.
- Dolmans, M.M. Recent advances in fertility preservation and counseling for female cancer patients. Expert. Rev. Anticancer 2018, 18, 115–120.




