You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giorgio Ciprandi | -- | 1350 | 2022-11-24 11:05:27 | | | |
| 2 | Peter Tang | Meta information modification | 1350 | 2022-11-24 11:10:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ciprandi, G.; Damiani, V.; Cordara, V.; Tosca, M.A. Local Bacteriotherapy for Preventing Respiratory Infections. Encyclopedia. Available online: https://encyclopedia.pub/entry/36370 (accessed on 25 December 2025).
Ciprandi G, Damiani V, Cordara V, Tosca MA. Local Bacteriotherapy for Preventing Respiratory Infections. Encyclopedia. Available at: https://encyclopedia.pub/entry/36370. Accessed December 25, 2025.
Ciprandi, Giorgio, Valerio Damiani, Vittorio Cordara, Maria Angela Tosca. "Local Bacteriotherapy for Preventing Respiratory Infections" Encyclopedia, https://encyclopedia.pub/entry/36370 (accessed December 25, 2025).
Ciprandi, G., Damiani, V., Cordara, V., & Tosca, M.A. (2022, November 24). Local Bacteriotherapy for Preventing Respiratory Infections. In Encyclopedia. https://encyclopedia.pub/entry/36370
Ciprandi, Giorgio, et al. "Local Bacteriotherapy for Preventing Respiratory Infections." Encyclopedia. Web. 24 November, 2022.
Copy Citation
Recurrent respiratory infections (RRIs) account for relevant economic and social implications and significantly affect family life. Local Bacteriotherapy (LB) represents an innovative option in preventing RRIs. Local bacteriotherapy consists of administering “good” and safe bacteria (probiotics) by nasal or oral route.
recurrent respiratory infections
local bacteriotherapy
Streptococcus salivarius 24SMB
Streptococcus oralis 89a
nasal spray
oral spray
1. Introduction
Infections of the respiratory tract have a profound impact on society. Namely, respiratory infections (RIs) entail a relevant socioeconomic burden for society and for families [1][2][3]. In addition, RIs present a compelling challenge for pediatricians, otolaryngologists, general practitioners, and allergists [4]. Frequent RI configures a rather common clinical picture in childhood and is referred to as recurrent respiratory infection (RRI). Previously, inclusion criteria for RRI encompassed a history of more than six RIs yearly, excluding primary and secondary immunodeficiency, cystic fibrosis, primary ciliary dyskinesia, and airway malformations [5]. Successively, further RRI classifications were added, also considering localized infections [6][7][8][9][10][11].
Inadequate management of RRIs results in frequent doctor’s office visits, hospital admissions, overuse/abuse of antibiotics, and school and parental work absences.
Consequently, it is important to attempt to identify risk factors associated with RRI. In this regard, a relevant role is to be attributed to prematurity, preschool age (for relative immaturity of the immune system), early attendance at nursery school, indoor and outdoor pollution, home dampness, passive exposure to tobacco or vape fumes, low socio-economic level, overcrowding, and allergic diseases [12]. Indeed, allergic diseases may significantly predispose an individual to RI recurrence through different pathophysiologic mechanisms [13][14]. Type 2 immunity presumes a consequential defect of type 1 immune response that contributes to fighting infections [15]. As a result, allergic subjects are prone to contract infections more frequently and severely than healthy subjects [16]. As proof of this mechanism, allergen-specific immunotherapy, restoring a normal balance between type 1 and 2 immunity, consequently reduces predisposition to RI [17]. Namely, this research demonstrated that subjects undergoing allergen-specific immunotherapy had significantly less respiratory infections than control allergic subjects who did not undergo this treatment.
Another important concept should be noted: viral infections reduce the mechanisms of immune defense, thereby promoting infection recurrence [18]. Additionally, viral infections are frequently associated with bacterial super-infections, which in turn prompts doctors to prescribe antibiotics, further reducing immune defenses and promoting antibiotic resistance [19][20]. Furthermore, bacterial overgrowth induces the generation of biofilm, i.e., surface-attached, structured microbial communities containing sessile cells (bacteria and/or fungi) embedded in a self-produced extracellular matrix composed of polysaccharides, DNA, and other components, making them more resistant to bacteria and promoting their in situ permanence [21][22]. Notably, biofilm may be envisaged as an “influencer” of repeated infections that favors bacterial survival and contrasts host aggression [23].
Last but not least, infections per se cause dysbiosis both at intestinal and respiratory levels, thus contributing to the maintenance of the vicious circle that allows RRI [23]. Taking into consideration all these concepts, being able to prevent RRIs is crucial in reducing costs and complications, as well as improving socioeconomic aspects. In this regard, many attempts have been made to prevent RRI. Therefore, using “good” bacteria (probiotics) through local administration could be an attractive strategy [24].
2. The Meaning of Local Bacteriotherapy
Microbiota includes the microorganisms living inside the human body, mainly harboring on organs communicating with the outside environment [25][26]. A close relationship between the microbiota and human body (symbiosis) is well established [27][28]. Indeed, it is well known that probiotics interfere with pathogen growth [29]. Probiotics are live bacteria which, when given in adequate amounts, confer a benefit to the consumer, as stated by World Health Organization.
Consistent colonization by bacteria able to “interfere” with pathogen growth restores eubiosis and may prevent RI [30]. This phenomenon can occur as it has been evidenced that some strains of a-Streptococci produce substances characterized by microbicidal activity against pathogens, which have been called bacteriocin-like inhibitory substances (BLIS) [31]. As a result, the manipulation and use of probiotics in relation to the microbiota of upper airways could be an attractive strategy for preventing RI [32]. This practice was first proposed over 70 years ago, coining the term “Bacteriotherapy” [33].
Preliminary attempts were performed in the early 1950s [34][35][36]. As bacteria were usually administered directly into the airways, the term “Local Bacteriotherapy” became popular.
Recently, there has been a revival of interest in this medical practice, especially following the great success of the use of probiotics in medicine. Probiotics have been proposed to maintain or restore eubiosis in conditions characterized by dysbiosis.
To explain the efficacy of this practice, different mechanisms have been explored. The main mechanism consists of interference and/or inhibition of pathogens as a result of the production of proteins with microbicidal activity and mediators with immunomodulating properties, as outlined in Figure 1 [31].
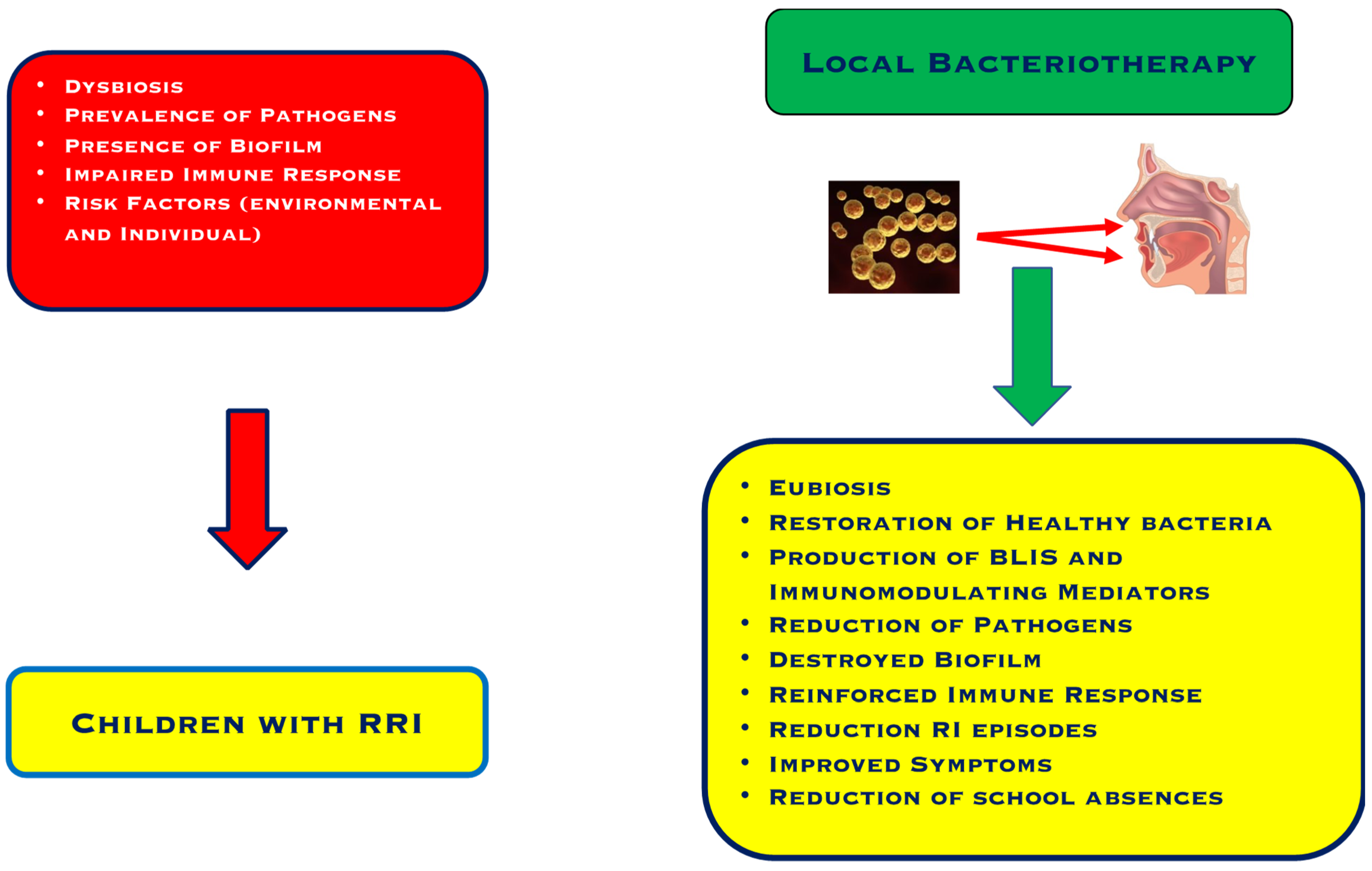
Figure 1. Characteristics of children with recurrent respiratory infections and the effects of Local Bacteriotherapy.
In particular, preliminary studies investigated the potential of one streptococcal strain, Streptococcus salivarius 24SMB. S salivarius is a non-pathogenic species able to colonize the oral cavity and can be considered a primary BLIS producer [37]. This strain exerted significant activity against S pneumoniae, was harmless to other S salivarius species, was non-pathogenic, and adhered to human larynx cells [38]. Successively, the topical administration of S salivarius by nasal spray allowed its colonization into the nasopharynx [39]. These preliminary experiences opened the way to new applications based on the local administration of probiotics, such as those in Local Bacteriotherapy. This approach allowed upper airways to be re-colonized with healthy microbes and led to pathogens being displaced by bacterial interference, in contrast with the present dysbiosis [39].
3. Local Bacteriotherapy: The Evidence
The first trial was designed as a randomized, double-blind, placebo-controlled study and included 100 children suffering from recurrent otitis media between the ages of 1 and 5 [40]. The study showed that Streptococcus salivarius 24SMB nasal spray reduced the number of otitis episodes and the use of antibiotics.
A real-life study was performed to confirm the previous trial and 267 children with predisposition to recurrent otitis were enrolled [41]. Children were stratified into two groups: 159 were treated with a nasal spray containing a bacterial mixture, containing Streptococcus salivarius 24SMB and Streptococcus oralis 89a, and 108 served as control. A previous study isolated Streptococcus oralis 89a from a child suffering from recalcitrant tonsillitis and showed that this strain inhibited the growth of Group A Streptococci [42]. The real-life study demonstrated that LB significantly reduced the frequency and severity of otitis episodes.
A further in vitro study demonstrated that both strains inhibited biofilm formation and dissolved preformed biofilms [43].
A prospective trial, conducted on 42 children presenting sleep-disordered breathing and RRI, showed that LB with both strains improved sleep characteristics and nasal airflow and reduced oral breathing after 3 months [44].
A further clinical study that enrolled 80 children with RRI reported that LB halved respiratory infections and reduced school and parental work absences [45].
Another study explored the possibility of preventing adenoidectomy using LB [46]. Forty-four children were waitlisted for adenoidectomy and tympanocentesis due to being diagnosed with adenoid hypertrophy or otitis media with effusion. Children were subdivided into two groups: 22 treated with LB nasal spray and 22 with hypertonic saline. The study evidenced that LB significantly reduced the need for surgery and improved tympanometry.
A successive study included 202 children with RRI that were treated with LB [47]. Local Bacteriotherapy significantly diminished RI episodes compared to the previous year.
An observational trial enrolled 79 children (1–6 years of age) with recurrent otitis and treated them with LB nasal spray, and a further 70 children were used as the control group [48]. Local bacteriotherapy significantly diminished otitis episodes.
The last study included 91 children suffering from RRI [49]. Local Bacteriotherapy nasal spray reduced respiratory symptoms and otalgia. Older children were more responsive than younger.
Recently, a new administration route was proposed for LB. It consists of an oral formulation containing Streptococcus salivarius 24SMB and Streptococcus oralis 89a, as well as the nasal spray formulation. Therefore, two trials explored the efficacy and safety of oral LB.
The first randomized controlled trial concerned 82 children suffering from recurrent streptococcal pharyngotonsillitis caused by Group A Streptococci [50]. The results confirmed that oral LB also diminished the prevalence and duration of GABHS infections, antibiotic consumption, and school absences.
The second trial was conducted as a real-life study and included 51 children (mean age of 4.8 years) with RRI [51]. This experience also confirmed that oral LB reduced RI and school absences.
References
- Mansback, A.I.; Brihave, P.; Casimir, G.; Dhooge, I.; Gordts, F.; Halewyck, S.; Hanssens, L. Clinical aspects of chronic ENT inflammation in children. B-ENT 2012, 8 (Suppl. S19), 83–101.
- Orlandi, R.R.; Kingdom, T.T.; Smith, T.L.; Bleier, B.; DeConde, A.; Luong, A.U.; Poetker, D.M.; Hwang, P.H. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis. Int. Forum Allergy Rhinol. 2016, 6 (Suppl. S1), S22–S209.
- Fokkens, W.J.; Lund, V.J.; Mullol, J.; Bachert, C.; Alobid, I.; Baroody, F.; Cohen, N.; Cervin, A.; Douglas, R.; Gevaert, P.; et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012, 50, 1–12.
- De Martino, M.; Vierucci, A.; Appendino, C. Children with recurrent respiratory infections. Immunol. Ped. 1981, 4, 76–81.
- De Martino, M.; Ballotti, S. The child with recurrent respiratory infections: Normal or not? Ped. Allergy. Immunol. 2007, 18 (Suppl. S18), 13–18.
- Paradise, J.L.; Bluestone, C.D.; Colborn, D.K.; Bernard, B.S.; Rockette, H.E.; Kurs-Lasky, M. Tonsillectomy and adenotonsillectomy for recurrent throat infection in moderately affected children. Pediatrics 2002, 110, 7–15.
- Greenberg, D.; Bilenko, N.; Liss, Z. The burden of acute otitis media on the patient and family. Eur. J. Pediatr. 2003, 162, 576–581.
- De Benedictis, F.M.; Bush, A. Recurrent lower respiratory tract infections in children. BMJ 2018, 362, k2698.
- Karevold, G.; Kvestad, E.; Nafstad, P.; Kvaerner, K.J. Respiratory infections in schoolchildren: Co-morbidity and risk factors. Arch. Dis. Child 2006, 91, 391–395.
- Thompson, M.; Vodicka, T.A.; Blair, P.S.; Buckley, D.I.; Heneghan, C.; Hay, B. ADTARGET Programme Team. Duration of symptoms of respiratory tract infections in children: Systematic review. BMJ 2013, 347, f7027.
- Toivonen, L.; Karppinen, S.; Schuez-Havupalo, L.; Teros-Jaakkola, T.; Vuononvirta, J. Burden of Recurrent Respiratory Tract Infections in Children: A Prospective Cohort Study. Pediatr. Infect. Dis. J. 2016, 35, e362–e369.
- Patria, M.F.; Esposito, S. Recurrent lower respiratory tract infections in children: A practical approach to diagnosis. Paediatr. Respir. Rev. 2013, 14, 53–60.
- Fahy, J.V. Type 2 inflammation in asthma—Present in most, absent in many. Nat. Rev. Immunol. 2015, 15, 57–65.
- Ciprandi, G.; Tosca, M.A.; Silvestri, M.; Ricciardolo, F.L.M. Inflammatory biomarkers for asthma endotyping and personalized therapy. Exp. Rev. Clin. Immunol. 2017, 13, 715–721.
- Ciprandi, G.; Tosca, M.A.; Fasce, L. Allergic children have more numerous and severe respiratory infections than non-allergic children. Pediatr. Allergy Immunol. 2006, 17, 389–391.
- Cirillo, I.; Marseglia, G.L.; Klersy, C.; Ciprandi, G. Allergic patients have more numerous and prolonged respiratory infections than non-allergic subjects. Allergy 2007, 62, 1087–1090.
- Ciprandi, G.; Sormani, M.P.; Cirillo, I. Upper respiratory infections and SLIT: Preliminary evidence. Ann. Allergy 2009, 102, 262–263.
- Griffin, M.R.; Walker, F.J.; Iwane, M.K. New vaccine surveillance network study group: Epidemiology of respiratory infections in young children: Insights from the new vaccine surveillance network. Pediatr. Infect. Dis. J. 2004, 23, 188–192.
- Li, J.; Song, X.; Yang, T.; Chen, Y.; Gong, Y.; Yin, X.; Lu, Z. A Systematic Review of Antibiotic Prescription Associated with Upper Respiratory Tract Infections in China. Medicine 2016, 95, e3587.
- Alexandrino, A.S.; Santos, R.; Melo, C.; Bastos, J.M.; Postiaux, G. Caregivers’ education vs. rhinopharyngeal clearance in children with upper respiratory infections: Impact on children’s health outcomes. Eur. J. Pediatr. 2017, 176, 1375–1383.
- Regli, A.; Becke, K.; von Ungern-Sternberg, B.S. An update on the perioperative management of children with upper respiratory tract infections. Curr. Opin. Anaesthesiol. 2017, 30, 362–367.
- Nazzari, E.; Torretta, S.; Pignataro, L. Role of biofilm in children with recurrent upper respiratory tract infections. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 421–429.
- Drago, L.; Pignataro, L.; Torretta, S. Microbiological aspects of acute and chronic pediatric rhinosinusitis. J. Clin. Med. 2019, 8, 149.
- Marchisio, P.; Nazzari, E.; Torretta, S.; Esposito, S.; Principi, N. Medical prevention of recurrent acute otitis media: An updated overview. Expert Rev. Anti-Infect. Ther. 2014, 12, 611–620.
- Yamanishi, S.; Pawankar, R. Current advances on the microbiome and role of probiotics in upper airways. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 30–35.
- Patel, R.; DuPont, H.L. New approaches for Bacteriotherapy: Prebiotics, new-generation probiotics, and synbiotics. Clin. Infect. Dis. 2015, 60 (Suppl. S2), S108–S121.
- Faden, H.; Waz, M.J.; Bernstein, J.M.; Brodsky, L.; Stanievich, J.; Ogra, P.L. Nasopharyngeal flora in the first three years of life in normal and otitis-prone children. Ann. Otol. Rhinol. Laryngol. 1991, 100, 612–615.
- Bernstein, J.M.; Faden, H.F.; Dryia, D.M.; Wactawski-Wende, J. Micro-ecology of the nasopharyngeal bacterial flora in otitis-prone and non-otitis prone children. Acta Otolaryngol. 1993, 113, 88–92.
- Gao, Z.; Kang, Y.; Yu, J.; Ren, L. Human pharyngeal microbiome may play a protective role in respiratory tract infections. Genom. Proteom. Bioinform. 2014, 12, 144–150.
- Roos, K.; Grahn Hakansson, E.; Holm, S. Effect of recolonization with “interfering” α streptococci on recurrences of acute and secretory otitis media in children: Randomised placebo-controlled trial. BMJ 2001, 322, 210–212.
- Walls, T.; Power, D.; Tagg, J. Bacteriocin-like inhibitory substances (BLIS) production by the normal flora of the nasopharynx: Potential to protect against otitis media? J. Med. Microbiol. 2003, 52, 829–833.
- Marsh, R.L.; Aho, C.; Beissbarth, J.; Bialasiewicz, S.; Binks, M.; Cervin, A. Recent advances in understanding the natural history of the otitis media microbiome and its response to environmental pressures. Int. J. Ped. Otorhinolaryngol. 2020, 130 (Suppl. S1), 109836.
- Torrent, H. La bacteriothérapie lactique; ses applications actuelles. Vie Med. 1949, 30, 39.
- Lanza Castelli, R.A.; Elkeles, G. Bacteriology and Bacteriotherapy in otorhinolaryngology. Ann. Otolaryngol. 1950, 67, 152–160.
- Larget, M.; Lamare, J.P. Bacterio-therapy Is Still Useful. Sem. Hosp. 1953, 29, 1331–1333.
- Lopez LaCarrere, E.; Viale del Carril, A. Remote results of Bacteriotherapy in otorhinolaryngology. Rev. Fac. Cienc. Med. 1953, 11, 453–461.
- Wescombe, P.A.; Heng, N.C.; Burton, J.P.; Chilcott, C.N.; Tagg, J.R. Streptococcal bacteriocins and the case for Streptococcus salivarius as model oral probiotics. Future Microbiol. 2009, 4, 819–835.
- Santagati, M.; Scillato, M.; Patanè, F.; Aiello, C.; Stefani, S. Bacteriocin-producing oral streptococci and inhibition of respiratory pathogens. FEMS Immunol. Med. Microbiol. 2012, 65, 23–31.
- Santagati, M.; Scillato, M.; Muscaridola, N.; Metoldo, V.; La Mantia, I.; Stefani, S. Colonization, safety, and tolerability study of the Streptococcus salivarius 24SMBc nasal spray for its application in upper respiratory tract infections. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 2075–2080.
- Marchisio, P.; Santagati, M.; Scillato, M.; Baggi, E.; Fattizzo, M.; Rosazza, C. Streptococcus salivarius 24SMB administered by nasal spray for the prevention of acute otitis media in otitis-prone children. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 2377–2383.
- Grahn, E.; Holm, S.E. Bacterial interference in the throat flora during a streptococcal tonsillitis outbreak in an apartment house area. Zentralbl. Bakteriol. Mikrobiologie Hyg. 1983, 256, 72–79.
- La Mantia, I.; Varricchio, A.; Ciprandi, G. Bacteriotherapy with Streptococcus salivarius 24SMB and Streptococcus oralis 89a nasal spray for preventing recurrent acute otitis media in children: A real-life clinical experience. Int. J. Gen. Med. 2017, 10, 171–175.
- Bidossi, A.; De Grandi, R.; Toscano, M.; Bottagisio, M.; De Vecchi, E.; Gelardi, M. Probiotics Streptococcus salivarius 24SMB and Streptococcus oralis 89a interfere with biofilm formation of pathogens of the upper respiratory tract. BMC Infect. Dis. 2018, 18, 653.
- Bellussi, L.M.; Villa, M.P.; Degiorgi, G.; Passali, F.M.; Evangelisti, M.; Innocenti Paganelli, I. Preventive nasal bacteriotherapy for the treatment of upper respiratory tract infections and sleep-disordered breathing in children. Int. J. Ped. Otorhinolaryngol. 2018, 110, 43–47.
- Tarantino, V.; Savaia, V.; D’Agostino, R.; Silvestri, M.; Ciprandi, G. Bacteriotherapy for preventing recurrent upper respiratory infections in children: A real-world experience. Otolaryngol. Pol. 2018, 72, 33–38.
- La Mantia, I.; Varricchio, A.; Di Girolamo, S.; Minni, A.; Passali, G.C.; Ciprandi, G. The role of bacteriotherapy in the prevention of adenoidectomy. Eur. Rev. Med. Pharmacol. Sci. 2019, 23 (Suppl. S1), 44–47.
- Passali, D.; Passali, G.C.; Vesperini, E.; Cocca, S.; Visconti, I.C.; Ralli, M. The efficacy and tolerability of Streptococcus salivarius 24SMB and Streptococcus oralis 89° administered as nasal spray in the treatment of recurrent upper respiratory tract infections in children. Eur. Rev. Med. Pharmacol. Sci. 2019, 23 (Suppl. S1), 67–72.
- Cantarutti, A.; Rea, F.; Donà, D.; Cantarutti, L.; Passarella, A.; Scamarcia, A. Preventing recurrent acute otitis media with Streptococcus salivarius 24SMB and Streptococcus oralis 89a five months intermittent treatment: An observational prospective cohort study. Int. J. Ped. Otorhinolaryngol. 2020, 132, 109921.
- Manti, S.; Parisi, G.F.; Papale, M.; Licari, A.; Salpietro, C.; Miraglia del Giudice, M.; Maresglia, G.L. Bacteriotherapy with Streptococcus salivarius 24SMB and Streptococcus oralis 89a nasal spray for treatment of upper respiratory tract infections in children: A pilot study on short-term efficacy. Ital. J. Pediatr. 2020, 46, 42.
- Andaloro, C.; Santagati, M.; Stefani, S.; La Mantia, I. Bacteriotherapy with Streptococcus salivarius 24SMB and Streptococcus oralis 89a oral spray for children with recurrent streptococcal pharyngotonsillitis: A randomized placebo-controlled clinical study. Eur. Arch. Otorhinolaryngol. 2019, 276, 879–887.
- Tarantino, V.; Savaia, V.; D’Agostino, R.; Damiani, V.; Ciprandi, G. Oral bacteriotherapy in children with recurrent respiratory infections: A real-life study. Acta Biomed. 2020, 91 (Suppl. S1), 73–76.
More
Information
Subjects:
Infectious Diseases
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
890
Revisions:
2 times
(View History)
Update Date:
24 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




