
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Grazia Maugeri | -- | 1491 | 2022-11-24 09:50:59 | | | |
| 2 | Amina Yu | Meta information modification | 1491 | 2022-11-25 01:41:38 | | |
Video Upload Options
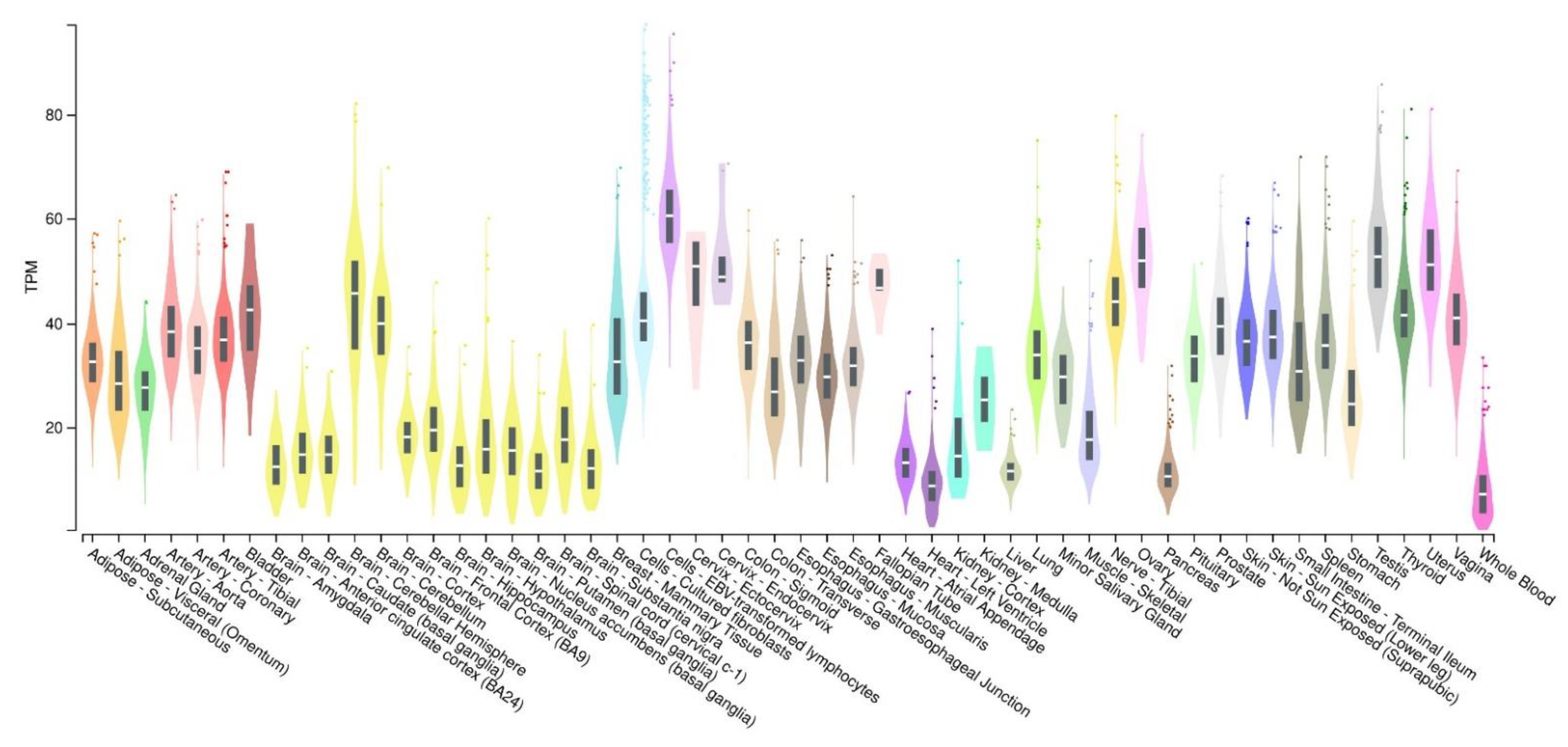
Activity-dependent protein (ADNP) is a neuroprotective protein of 123.56 kDa molecular weight, widely expressed throughout the body, including the eye. Morphological and proteomic studies showed that ADNP is distributed in the retina and cornea of different species, including humans. ADNP was originally discovered as an astroglial secreted protein, able to modulate the neurotrophic/neuroprotective activity of vasoactive intestinal peptide (VIP), as well as of pituitary adenylate cyclase-activating peptide (PACAP). PACAP and VIP perform their effects through the activation of G protein-coupled receptors, pituitary adenylate cyclase-activating polypeptide receptor type 1 (PAC1R), vasoactive intestinal polypeptide receptor 1 (VPAC1R), and vasoactive intestinal polypeptide receptor 2 (VPAC2R). In particular, the PAC1 receptor shows eight different splice variants (Null, Hip, Hop1, Hop2, Hiphop1, Hiphop2, short, and very short isoforms), whose activation by the binding to PACAP/VIP activates phospholipase C (PLC) and adenylate cyclase (AC), or calcium-regulated mechanisms. It is worth noting that a subpicomolar concentration of PACAP stimulated ADNP expression mainly through the MAPK signaling pathway and cAMP-dependent protein kinase activation. Both VIP and PACAP showed important protective effects against different ocular diseases.
1. Activity-Dependent Neuroprotective Protein (ADNP): Expression and Functions
2. The Role of ADNP in the Eye
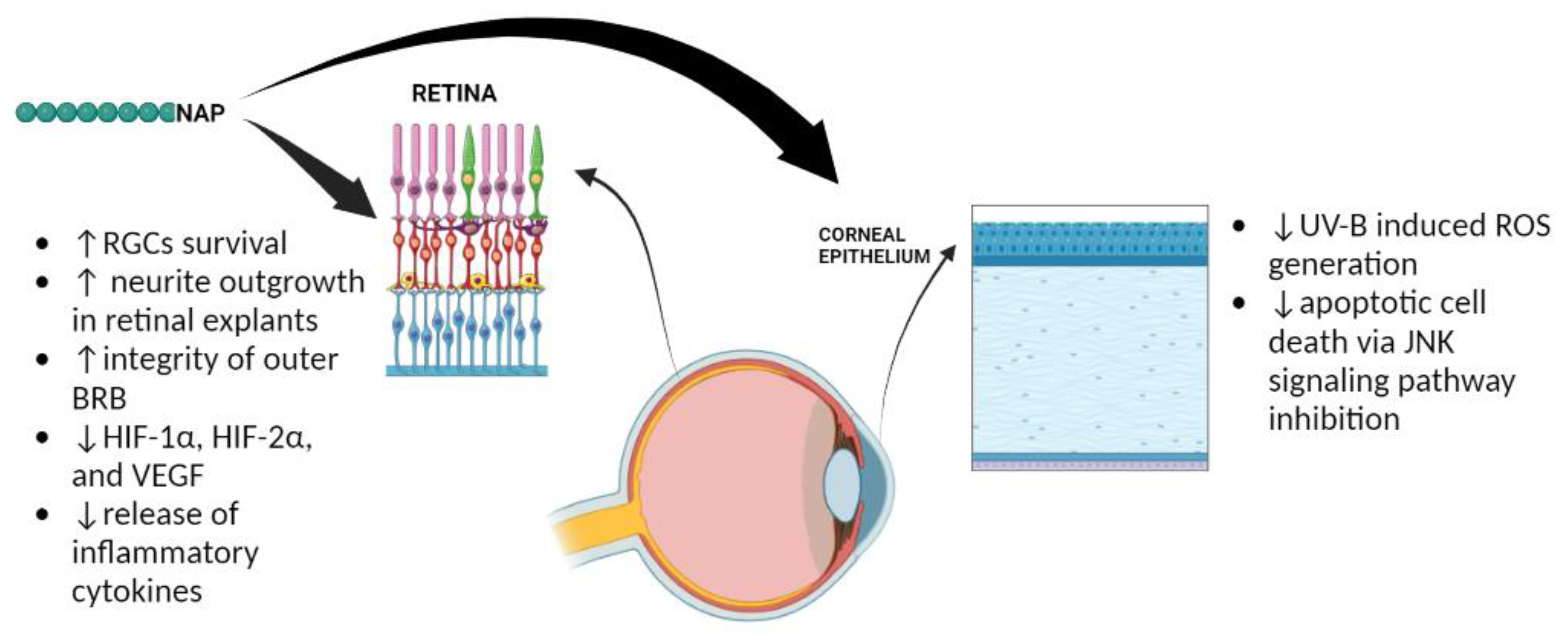
References
- Bassan, M.; Zamostiano, R.; Davidson, A.; Pinhasov, A.; Giladi, E.; Perl, O.; Bassan, H.; Blat, C.; Gibney, G.; Glazner, G.; et al. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J. Neurochem. 1999, 72, 1283–1293.
- Zamostiano, R.; Pinhasov, A.; Gelber, E.; Steingart, R.A.; Seroussi, E.; Giladi, E.; Bassan, M.; Wollman, Y.; Eyre, H.J.; Mulley, J.C.; et al. Cloning and characterization of the human activity-dependent neuroprotective protein. J. Biol. Chem. 2001, 276, 708–714.
- Gennet, N.; Herden, C.; Bubb, V.J.; Quinn, J.P.; Kipar, A. Expression of activity-dependent neuroprotective protein in the brain of adult rats. Histol. Histopathol. 2008, 23, 309–317.
- Mandel, S.; Spivak-Pohis, I.; Gozes, I. ADNP differential nucleus/cytoplasm localization in neurons suggests multiple roles in neuronal differentiation and maintenance. J. Mol. Neurosci. 2008, 35, 127–141.
- Furman, S.; Steingart, R.A.; Mandel, S.; Hauser, J.M.; Brenneman, D.E.; Gozes, I. Subcellular localization and secretion of activity-dependent neuroprotective protein in astrocytes. Neuron Glia Biol. 2004, 1, 193–199.
- Mandel, S.; Rechavi, G.; Gozes, I. Activity-dependent neuroprotective protein (ADNP) differentially interacts with chromatin to regulate genes essential for embryogenesis. Dev. Biol. 2007, 303, 814–824.
- Malishkevich, A.; Amram, N.; Hacohen-Kleiman, G.; Magen, I.; Giladi, E.; Gozes, I. Activity-dependent neuroprotective protein (ADNP) exhibits striking sexual dichotomy impacting on autistic and Alzheimer’s pathologies. Transl. Psychiatry 2015, 5, e501.
- Jouroukhin, Y.; Ostritsky, R.; Assaf, Y.; Pelled, G.; Giladi, E.; Gozes, I. NAP (davunetide) modifies disease progression in a mouse model of severe neurodegeneration: Protection against impairments in axonal transport. Neurobiol. Dis. 2013, 56, 79–94.
- Amram, N.; Hacohen-Kleiman, G.; Sragovich, S.; Malishkevich, A.; Katz, J.; Touloumi, O.; Lagoudaki, R.; Grigoriadis, N.C.; Giladi, E.; Yeheskel, A.; et al. Sexual divergence in microtubule function: The novel intranasal microtubule targeting SKIP normalizes axonal transport and enhances memory. Mol. Psychiatry 2016, 21, 1467–1476.
- Pinhasov, A.; Mandel, S.; Torchinsky, A.; Giladi, E.; Pittel, Z.; Goldsweig, A.M.; Servoss, S.J.; Brenneman, D.E.; Gozes, I. Activity-dependent neuroprotective protein: A novel gene essential for brain formation. Brain Res. Dev. Brain Res. 2003, 144, 83–90.
- Gozes, I.; Zaltzman, R.; Hauser, J.; Brenneman, D.E.; Shohami, E.; Hill, J.M. The expression of activity-dependent neuroprotective protein (ADNP) is regulated by brain damage and treatment of mice with the ADNP derived peptide, NAP, reduces the severity of traumatic head injury. Curr. Alzheimer Res. 2005, 2, 149–153.
- Zaltzman, R.; Alexandrovich, A.; Beni, S.M.; Trembovler, V.; Shohami, E.; Gozes, I. Brain injury-dependent expression of activity-dependent neuroprotective protein. J. Mol. Neurosci. 2004, 24, 181–187.
- Chu, Y.; Morfini, G.A.; Kordower, J.H. Alterations in Activity-Dependent Neuroprotective Protein in Sporadic and Experimental Parkinson’s Disease. J. Park. Dis. 2016, 6, 77–97.
- Malishkevich, A.; Marshall, G.A.; Schultz, A.P.; Sperling, R.A.; Aharon-Peretz, J.; Gozes, I. Blood-Borne Activity-Dependent Neuroprotective Protein (ADNP) is Correlated with Premorbid Intelligence, Clinical Stage, and Alzheimer’s Disease Biomarkers. J. Alzheimers Dis. 2016, 50, 249–260.
- Ivashko-Pachima, Y.; Hadar, A.; Grigg, I.; Korenková, V.; Kapitansky, O.; Karmon, G.; Gershovits, M.; Sayas, C.L.; Kooy, R.F.; Attems, J.; et al. Discovery of autism/intellectual disability somatic mutations in Alzheimer’s brains: Mutated ADNP cytoskeletal impairments and repair as a case study. Mol. Psychiatry 2021, 26, 1619–1633.
- Vulih-Shultzman, I.; Pinhasov, A.; Mandel, S.; Grigoriadis, N.; Touloumi, O.; Pittel, Z.; Gozes, I. Activity-dependent neuroprotective protein snippet NAP reduces tau hyperphosphorylation and enhances learning in a novel transgenic mouse model. J. Pharmacol. Exp. Ther. 2007, 323, 438–449.
- Gozes, I. Sexual divergence in activity-dependent neuroprotective protein impacting autism, schizophrenia, and Alzheimer’s disease. J. Neurosci. Res. 2017, 95, 652–660.
- Hacohen-Kleiman, G.; Sragovich, S.; Karmon, G.; Gao, A.Y.L.; Grigg, I.; Pasmanik-Chor, M.; Le, A.; Korenková, V.; McKinney, R.A.; Gozes, I. Activity-dependent neuroprotective protein deficiency models synaptic and developmental phenotypes of autism-like syndrome. J. Clin. Investig. 2018, 128, 4956–4969.
- Van Dijck, A.; Vulto-van Silfhout, A.T.; Cappuyns, E.; van der Werf, I.M.; Mancini, G.M.; Tzschach, A.; Bernier, R.; Gozes, I.; Eichler, E.E.; Romano, C.; et al. Clinical Presentation of a Complex Neurodevelopmental Disorder Caused by Mutations in ADNP. Biol. Psychiatry 2019, 85, 287–297.
- Gozes, I.; Patterson, M.C.; Van Dijck, A.; Kooy, R.F.; Peeden, J.N.; Eichenberger, J.A.; Zawacki-Downing, A.; Bedrosian-Sermone, S. The Eight and a Half Year Journey of Undiagnosed AD: Gene Sequencing and Funding of Advanced Genetic Testing Has Led to Hope and New Beginnings. Front. Endocrinol. (Lausanne) 2017, 8, 107.
- Mollinedo, P.; Kapitansky, O.; Gonzalez-Lamuño, D.; Zaslavsky, A.; Real, P.; Gozes, I.; Gandarillas, A.; Fernandez-Luna, J.L. Cellular and animal models of skin alterations in the autism-related ADNP syndrome. Sci. Rep. 2019, 9, 736.
- Oz, S.; Kapitansky, O.; Ivashco-Pachima, Y.; Malishkevich, A.; Giladi, E.; Skalka, N.; Rosin-Arbesfeld, R.; Mittelman, L.; Segev, O.; Hirsch, J.A.; et al. The NAP motif of activity-dependent neuroprotective protein (ADNP) regulates dendritic spines through microtubule end binding proteins. Mol. Psychiatry 2014, 19, 1115–1124.
- Sayas, C.L.; Tortosa, E.; Bollati, F.; Ramírez-Ríos, S.; Arnal, I.; Avila, J. Tau regulates the localization and function of End-binding proteins 1 and 3 in developing neuronal cells. J. Neurochem. 2015, 133, 653–667.
- Oz, S.; Ivashko-Pachima, Y.; Gozes, I. The ADNP derived peptide, NAP modulates the tubulin pool: Implication for neurotrophic and neuroprotective activities. PLoS ONE 2012, 7, e51458.
- Ivashko-Pachima, Y.; Sayas, C.L.; Malishkevich, A.; Gozes, I. ADNP/NAP dramatically increase microtubule end-binding protein-Tau interaction: A novel avenue for protection against tauopathy. Mol. Psychiatry 2017, 22, 1335–1344.
- Merenlender-Wagner, A.; Malishkevich, A.; Shemer, Z.; Udawela, M.; Gibbons, A.; Scarr, E.; Dean, B.; Levine, J.; Agam, G.; Gozes, I. Autophagy has a key role in the pathophysiology of schizophrenia. Mol. Psychiatry 2015, 20, 126–132.
- Ivashko-Pachima, Y.; Gozes, I. Deciphering the Enigma: NAP (CP201) the Active ADNP Drug Candidate Enters Cells by Dynamin-Associated Endocytosis. J. Mol. Neurosci. 2020, 70, 993–998.
- D’Amico, A.G.; Scuderi, S.; Maugeri, G.; Cavallaro, S.; Drago, F.; D’Agata, V. NAP reduces murine microvascular endothelial cells proliferation induced by hyperglycemia. J. Mol. Neurosci. 2014, 54, 405–413.
- Spong, C.Y.; Abebe, D.T.; Gozes, I.; Brenneman, D.E.; Hill, J.M. Prevention of fetal demise and growth restriction in a mouse model of fetal alcohol syndrome. J. Pharmacol. Exp. Ther. 2001, 297, 774–779.
- Steingart, R.A.; Gozes, I. Recombinant activity-dependent neuroprotective protein protects cells against oxidative stress. Mol. Cell. Endocrinol. 2006, 252, 148–153.
- D’Amico, A.G.; Maugeri, G.; Musumeci, G.; Reglodi, D.; D’Agata, V. PACAP and NAP: Effect of Two Functionally Related Peptides in Diabetic Retinopathy. J. Mol. Neurosci. 2021, 71, 1525–1535.
- Divinski, I.; Mittelman, L.; Gozes, I. A femtomolar acting octapeptide interacts with tubulin and protects astrocytes against zinc intoxication. J. Biol. Chem. 2004, 279, 28531–28538.
- Gozes, I.; Divinski, I.; Piltzer, I. NAP and D-SAL: Neuroprotection against the beta amyloid peptide (1-42). BMC Neurosci. 2008, 9 (Suppl. 3), S3.
- Zemlyak, I.; Manley, N.; Sapolsky, R.; Gozes, I. NAP protects hippocampal neurons against multiple toxins. Peptides 2007, 28, 2004–2008.
- Beni-Adani, L.; Gozes, I.; Cohen, Y.; Assaf, Y.; Steingart, R.A.; Brenneman, D.E.; Eizenberg, O.; Trembolver, V.; Shohami, E. A peptide derived from activity-dependent neuroprotective protein (ADNP) ameliorates injury response in closed head injury in mice. J. Pharmacol. Exp. Ther. 2001, 296, 57–63.
- Zaltzman, R.; Alexandrovich, A.; Trembovler, V.; Shohami, E.; Gozes, I. The influence of the peptide NAP on Mac-1-deficient mice following closed head injury. Peptides 2005, 26, 1520–1527.
- Idan-Feldman, A.; Schirer, Y.; Polyzoidou, E.; Touloumi, O.; Lagoudaki, R.; Grigoriadis, N.C.; Gozes, I. Davunetide (NAP) as a preventative treatment for central nervous system complications in a diabetes rat model. Neurobiol. Dis. 2011, 44, 327–339.
- Javitt, D.C.; Buchanan, R.W.; Keefe, R.S.; Kern, R.; McMahon, R.P.; Green, M.F.; Lieberman, J.; Goff, D.C.; Csernansky, J.G.; McEvoy, J.P.; et al. Effect of the neuroprotective peptide davunetide (AL-108) on cognition and functional capacity in schizophrenia. Schizophr. Res. 2012, 136, 25–31.
- Jarskog, L.F.; Dong, Z.; Kangarlu, A.; Colibazzi, T.; Girgis, R.R.; Kegeles, L.S.; Barch, D.M.; Buchanan, R.W.; Csernansky, J.G.; Goff, D.C.; et al. Effects of davunetide on N-acetylaspartate and choline in dorsolateral prefrontal cortex in patients with schizophrenia. Neuropsychopharmacology 2013, 38, 1245–1252.
- Karmon, G.; Sragovich, S.; Hacohen-Kleiman, G.; Ben-Horin-Hazak, I.; Kasparek, P.; Schuster, B.; Sedlacek, R.; Pasmanik-Chor, M.; Theotokis, P.; Touloumi, O.; et al. Novel ADNP Syndrome Mice Reveal Dramatic Sex-Specific Peripheral Gene Expression With Brain Synaptic and Tau Pathologies. Biol. Psychiatry 2022, 92, 81–95.
- Gabis, L.V.; Attia, O.L.; Roth-Hanania, R.; Foss-Feig, J. Motor delay—An early and more common “red flag” in girls rather than boys with autism spectrum disorder. Res. Dev. Disabil. 2020, 104, 103702.
- Teuchner, B.; Dimmer, A.; Humpel, C.; Amberger, A.; Fischer-Colbrie, R.; Nemeth, J.; Waschek, J.A.; Kieselbach, G.; Kralinger, M.; Schmid, E.; et al. VIP, PACAP-38, BDNF and ADNP in NMDA-induced excitotoxicity in the rat retina. Acta Ophthalmol. 2011, 89, 670–675.
- Zemlyak, I.; Furman, S.; Brenneman, D.E.; Gozes, I. A novel peptide prevents death in enriched neuronal cultures. Regul. Pept. 2000, 96, 39–43.
- Smith-Swintosky, V.L.; Gozes, I.; Brenneman, D.E.; D’Andrea, M.R.; Plata-Salaman, C.R. Activity-dependent neurotrophic factor-9 and NAP promote neurite outgrowth in rat hippocampal and cortical cultures. J. Mol. Neurosci. 2005, 25, 225–238.
- Jehle, T.; Dimitriu, C.; Auer, S.; Knoth, R.; Vidal-Sanz, M.; Gozes, I.; Lagrèze, W.A. The neuropeptide NAP provides neuroprotection against retinal ganglion cell damage after retinal ischemia and optic nerve crush. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 1255–1263.
- Belokopytov, M.; Shulman, S.; Dubinsky, G.; Gozes, I.; Belkin, M.; Rosner, M. Ameliorative effect of NAP on laser-induced retinal damage. Acta Ophthalmol. 2011, 89, e126–e131.
- Zheng, Y.; Zeng, H.; She, H.; Liu, H.; Sun, N. Expression of peptide NAP in rat retinal Müller cells prevents hypoxia-induced retinal injuries and promotes retinal neurons growth. Biomed. Pharmacother. 2010, 64, 417–423.
- Savagian, C.A.; Dubielzig, R.R.; Nork, T.M. Comparison of the distribution of glial fibrillary acidic protein, heat shock protein 60, and hypoxia-inducible factor-1alpha in retinas from glaucomatous and normal canine eyes. Am. J. Vet. Res. 2008, 69, 265–272.
- Chen, Y.N.; Yamada, H.; Mao, W.; Matsuyama, S.; Aihara, M.; Araie, M. Hypoxia-induced retinal ganglion cell death and the neuroprotective effects of beta-adrenergic antagonists. Brain Res. 2007, 1148, 28–37.
- Dong, X.; Wang, Y.S.; Dou, G.R.; Hou, H.Y.; Shi, Y.Y.; Zhang, R.; Ma, K.; Wu, L.; Yao, L.B.; Cai, Y.; et al. Influence of Dll4 via HIF-1α-VEGF signaling on the angiogenesis of choroidal neovascularization under hypoxic conditions. PLoS ONE 2011, 6, e18481.
- Frank, R.N. Diabetic retinopathy. N. Engl. J. Med. 2004, 350, 48–58.
- Yang, X.M.; Wang, Y.S.; Zhang, J.; Li, Y.; Xu, J.F.; Zhu, J.; Zhao, W.; Chu, D.K.; Wiedemann, P. Role of PI3K/Akt and MEK/ERK in mediating hypoxia-induced expression of HIF-1alpha and VEGF in laser-induced rat choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1873–1879.
- D’Amico, A.G.; Maugeri, G.; Rasà, D.M.; La Cognata, V.; Saccone, S.; Federico, C.; Cavallaro, S.; D’Agata, V. NAP counteracts hyperglycemia/hypoxia induced retinal pigment epithelial barrier breakdown through modulation of HIFs and VEGF expression. J. Cell. Physiol. 2018, 233, 1120–1128.
- Maugeri, G.; D’Amico, A.G.; Rasà, D.M.; La Cognata, V.; Saccone, S.; Federico, C.; Cavallaro, S.; D’Agata, V. Nicotine promotes blood retinal barrier damage in a model of human diabetic macular edema. Toxicol. Vitr. 2017, 44, 182–189.
- Zhang, P.; Zhang, X.; Hao, X.; Wang, Y.; Hui, Y.; Wang, H.; Hu, D.; Zhou, J. Rac1 activates HIF-1 in retinal pigment epithelium cells under hypoxia. Graefes Arch. Clin. Exp. Ophthalmol. 2009, 247, 633–639.
- D’Amico, A.G.; Maugeri, G.; Bucolo, C.; Saccone, S.; Federico, C.; Cavallaro, S.; D’Agata, V. Nap Interferes with Hypoxia-Inducible Factors and VEGF Expression in Retina of Diabetic Rats. J. Mol. Neurosci. 2017, 61, 256–266.
- D’Amico, A.G.; Maugeri, G.; Rasà, D.; Federico, C.; Saccone, S.; Lazzara, F.; Fidilio, A.; Drago, F.; Bucolo, C.; D’Agata, V. NAP modulates hyperglycemic-inflammatory event of diabetic retina by counteracting outer blood retinal barrier damage. J. Cell. Physiol. 2019, 234, 5230–5240.
- Maugeri, G.; D’Amico, A.G.; Giunta, S.; Giallongo, C.; Tibullo, D.; Bucolo, C.; Saccone, S.; Federico, C.; Scollo, D.; Longo, A.; et al. Activity-Dependent Neuroprotective Protein (ADNP)-Derived Peptide (NAP) Counteracts UV-B Radiation-Induced ROS Formation in Corneal Epithelium. Antioxidants 2022, 11, 128.
- Tseng, S.C. Concept and application of limbal stem cells. Eye 1989, 3, 141–157.




