Activity-dependent protein (ADNP) is a neuroprotective protein of 123.56 kDa molecular weight, widely expressed throughout the body, including the eye. Morphological and proteomic studies showed that ADNP is distributed in the retina and cornea of different species, including humans. ADNP was originally discovered as an astroglial secreted protein, able to modulate the neurotrophic/neuroprotective activity of vasoactive intestinal peptide (VIP), as well as of pituitary adenylate cyclase-activating peptide (PACAP). PACAP and VIP perform their effects through the activation of G protein-coupled receptors, pituitary adenylate cyclase-activating polypeptide receptor type 1 (PAC1R), vasoactive intestinal polypeptide receptor 1 (VPAC1R), and vasoactive intestinal polypeptide receptor 2 (VPAC2R). In particular, the PAC1 receptor shows eight different splice variants (Null, Hip, Hop1, Hop2, Hiphop1, Hiphop2, short, and very short isoforms), whose activation by the binding to PACAP/VIP activates phospholipase C (PLC) and adenylate cyclase (AC), or calcium-regulated mechanisms. It is worth noting that a subpicomolar concentration of PACAP stimulated ADNP expression mainly through the MAPK signaling pathway and cAMP-dependent protein kinase activation. Both VIP and PACAP showed important protective effects against different ocular diseases.
1. Activity-Dependent Neuroprotective Protein (ADNP): Expression and Functions
The human
ADNP gene, discovered in 1999 by Bassan et al. [
33], spans ~40 kilobases and includes five exons and four introns with alternative splicing of an untranslated second exon. There is a striking degree of homology (90%) between human and mouse mRNA, and the region is highly conserved between vertebrates. The
ADNP gene is located on the q13.13 band of chromosome 20 [
34]. The
ADNP-containing locus is frequently amplified in several cancers. Moreover, the down-regulation of
ADNP by antisense oligodeoxynucleotides increases the expression of tumor suppressor p53 and decreases intestinal cancer cells’ vitality up to 90%, suggesting the involvement of
ADNP in cell survival, probably through the modulation of p53 [
34]. According to the Genotype-Tissue Expression (GTEx) database, the
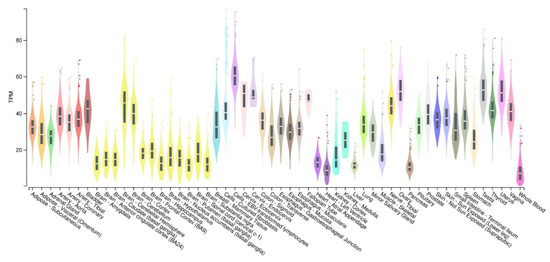
ADNP gene is found in central and peripheral nervous systems, as well as in different tissues and cells of various organs (
Figure 2).
Figure 2. Boxplot of transcripts per million (TPM) showing the bulk tissue gene expression for ADNP. GTEx Portal on 29 September 2022.
The
ADNP gene encodes a protein containing nine zinc fingers, a homeobox domain and a bipartite nuclear localization signal, indicating transcription factor activity. The ADNP protein is mainly expressed in the cytoplasm of neuronal cells, whereas it is predominantly localized in the nucleus of non-neural cells [
35,
36]. Moreover, ADNP-like immunoreactivity was found in the extracellular milieu of astrocytes following stimulation with vasoactive intestinal peptide (VIP) [
37].
ADNP, by interacting with nuclear chromatin, modulates the transcription of hundreds of genes involved in different biological events, such as embryogenesis [
38], dendritic spine plasticity, autophagy, autism-linked protein translation and axonal transport [
39]. Regarding the latter aspect, through the manganese (Mn
2+)-enhanced magnetic resonance imaging technique, it was found that in
Adnp+/+ mice, the signal intensity was significantly increased in the lateral part of the olfactory nerve and glomerular layer of the olfactory bulb. The signal intensity was significantly decreased in
Adnp+/− mice, suggesting an alteration in the axonal transport [
40,
41].
ADNP is essential for brain formation and maintenance [
42,
43,
44], and its expression is altered in different neurodegenerative diseases. In particular, the down-regulation of
ADNP may concur with dopaminergic neurodegeneration in Parkinson’s Disease [
45], and the
ADNP plasma/serum and lymphocyte mRNA levels were correlated to clinical stage and Alzheimer’s disease (AD) biomarkers [
46].
ADNP somatic mutations were found in the brains of AD-affected patients [
47], as well as it being one of three genes frequently associated with autism spectrum disorders (ASD). The ADNP syndrome, also known as Helsmoortel–Van der Aa syndrome (HVAS), is characterized by a plethora of clinical symptoms, including global developmental delays, motor dysfunctions, hypotonia, and repetitive infections, as well as ophthalmic abnormalities, which suggest the multisystem nature of this disorder [
48,
49,
50,
51,
52,
53].
In 1999, a small peptide of eight amino acids derived from ADNP was synthesized, known as NAP (davunetide, NAPVSIPQ/Asn-Ala-Pro-Val-Ser-Ile-Pro-Gln) [
33], and found to play a protective role at femtomolar concentrations [
33]. The SIP motif in NAP interacts with the microtubule end-binding proteins, such as end-binding proteins 1 and 3 (EB1 and EB3), promoting microtubule intervention on neuroplasticity and neuroprotection [
54,
55]. In fact, EB3 plays a pivotal role in dendritic spine formation, and the positive effect of NAP in this process is EB3-dependent. Furthermore, in rat pheochromocytoma (PC12) cells and in rat cortical astrocytes, NAP treatment significantly increased the microtubule network area in the cell, an event preceding neurite outgrowth [
56]. NAP is also involved in Tau–microtubule interaction, avoiding aberrant hyperphosphorylation and aggregation of Tau, which impairs cognitive functions [
57]. Accordingly,
Adnp+/− mice exhibited tauopathy features with a significant increase in phosphorylated Tau [
48]. Moreover, NAP was found to enhance the autophagic process and preserve the cells against the accumulation of misfolded proteins, by promoting ADNP interaction with MAP1-associated protein 1 light chain 3 (LC3), representing the fundamental constituent of the autophagosome [
58]. The chemical structure of this small fragment peptide allows it to enter the cells by dynamin-associated endocytosis [
59], exerting protective effects, both in vitro and in vivo [
60,
61,
62,
63]. NAP protected neuronal-like cells against oxidative stress [
64] and counteracted apoptotic cell death in neurons exposed to β-amyloid or tetrodotoxin treatment or glucose deprivation [
65,
66]. NAP ameliorated injury response in mice exposed to a closed head injury [
67,
68] whereas in a diabetes rat model NAP treatment partially rescued memory deficits by preventing the reduction of gray matter density [
69]. Moreover, NAP intranasal treatment seems to exert moderate improvements on some cognitive deficits of schizophrenia patients [
70,
71].
Very recently, Karmon et al. [
72] generated transgenic mice carrying the most common human p.Tyr719 (Tyr)
ADNP mutation. This mouse model, as compared to the
Adnp+/− model, showed greater severity of the phenotype due to heterozygous expression of 50%
WT-Adnp (loss of function) and 50%
Tyr-Adnp (potential gain of toxic function) alleles. For example, hyperphosphorylated tau deposits associated with visually evoked potential impairments were found in the hippocampus of ∼2-month-old
Tyr-Adnp with respect to 11-month-old
Adnp+/− male mouse brains [
48]. Moreover, the
Tyr-Adnp mice model reflects even more sexual dichotomy, as compared to
Adnp+/− mice, as confirmed by the early developmental and motor delay in females, rather than males, with ASD [
73].
2. The Role of ADNP in the Eye
ADNP shows widespread tissue and organ distribution. It has been detected in the brain, endocrine, respiratory, gastrointestinal and reproductive systems, as well as in skin, bone marrow and lymphoid tissues. In the eye, it was first reported in reference to its expression in the rat retina [
1]. Here, the octapeptide NAP was shown to increase RGC survival after neurotrophic factor deprivation and to promote neurite outgrowth, confirming previous studies which demonstrated its ability to enhance neuronal survival and support axonal elongation [
33,
74,
75]. Its pro-survival effects on RGCs were also displayed in vivo after retinal ischemia and optic nerve crush in rats after its intravitreal injection [
76]. This immediate method of NAP administration also showed significant ameliorative effects of rat retinal damage after laser photocoagulation [
77]. Moreover, stable transfection of NAP in rat retinal Müller cells exerted a protective role, not only in these cells against hypoxia-induced apoptosis, but also in other retinal neural cells, including neurons, astrocytes, and photoreceptors exerting nourishing effects against hypoxia-induced injuries [
78]. Retinal diseases associated with hypoxia mainly include glaucoma, retinal ischemia and diabetic retinopathy [
79,
80]. The latter is characterized by vessel impairment induced by hyperglycemia, which contributes to the development of a hypoxic microenvironment in the retina [
81,
82]. The hypoxic event induces vascular-endothelial growth factor (VEGF) over release, responsible for aberrant neo-angiogenesis leading to blood–retinal barrier (BRB) breakdown [
83]. Treatment with NAP was shown to keep the integrity of the outer BRB, counteracting human RPE apoptotic cell death induced by hyperglycemic/hypoxic insult [
84]. NAP exerted these effects by modulating the expression of hypoxic inducible factors (HIFs). In particular, the peptide affected HIF-1α and HIF-2α expression, which, under hypoxia, elude the proteasome degradation system, translocating into the nucleus and triggering many target genes, including VEGF [
85,
86]. The ability of NAP to modulate key elements associated with hyperglycemic/hypoxic damage was also demonstrated in the retina of diabetic rats. In particular, a single intravitreal injection of NAP significantly reduced the expression of HIF-1α, HIF-2α, and VEGF [
87]. It is well known that the hyperglycemic/hypoxic event also promotes the release of inflammatory cytokines, which concur with the impairment of BRB. NAP treatment was shown to modulate the inflammatory event during the early phase of DR. In fact, the intravitreal administration of NAP interfered with the expression of IL-1family members. Moreover, the peptide preserved outer BRB integrity after hyperglycemic–inflammatory insult in an in vitro model of DR [
88].
The expression of ADNP was also observed in human and rabbit corneal epithelium [
2]. In particular, ADNP was mainly expressed in the basal layer that is characterized by limbal epithelial stem cells involved in the corneal epithelium regeneration [
89]. This finding suggested a possible role of ADNP in corneal regeneration. Furthermore, NAP treatment of corneal epithelial cells exposed to UV-B radiations prevented ROS generation by reducing apoptotic cell death via JNK signaling pathway inhibition [
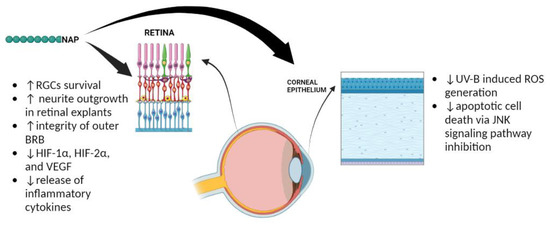
2]. Overall, these data are summarized in
Figure 3.
Figure 3. Protective effects played by NAP in the eye. The ↑ and ↓ refer to increase and decrease, respectively.
This entry is adapted from the peer-reviewed paper 10.3390/ijms232113654