
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vikash Kumar | -- | 3486 | 2022-11-23 10:00:34 | | | |
| 2 | Amina Yu | + 11 word(s) | 3497 | 2022-11-24 01:55:44 | | | | |
| 3 | Amina Yu | Meta information modification | 3497 | 2022-11-24 01:57:33 | | | | |
| 4 | Amina Yu | -2 word(s) | 3495 | 2022-11-25 07:24:46 | | |
Video Upload Options
Heat shock proteins (Hsps) are a family of ubiquitously expressed stress proteins and extrinsic chaperones that are required for viability and cell growth in all living organisms. These proteins are highly conserved and produced in all cellular organisms when exposed to stress. Hsps play a significant role in protein synthesis and homeostasis, as well as in the maintenance of overall health in crustaceans against various internal and external environmental stresses. Recent reports have suggested that enhancing in vivo Hsp levels via non-lethal heat shock, exogenous Hsps, or plant-based compounds, could be a promising strategy used to develop protective immunity in crustaceans against both abiotic and biotic stresses.
1. Regulation of the Heat Shock Protein Response
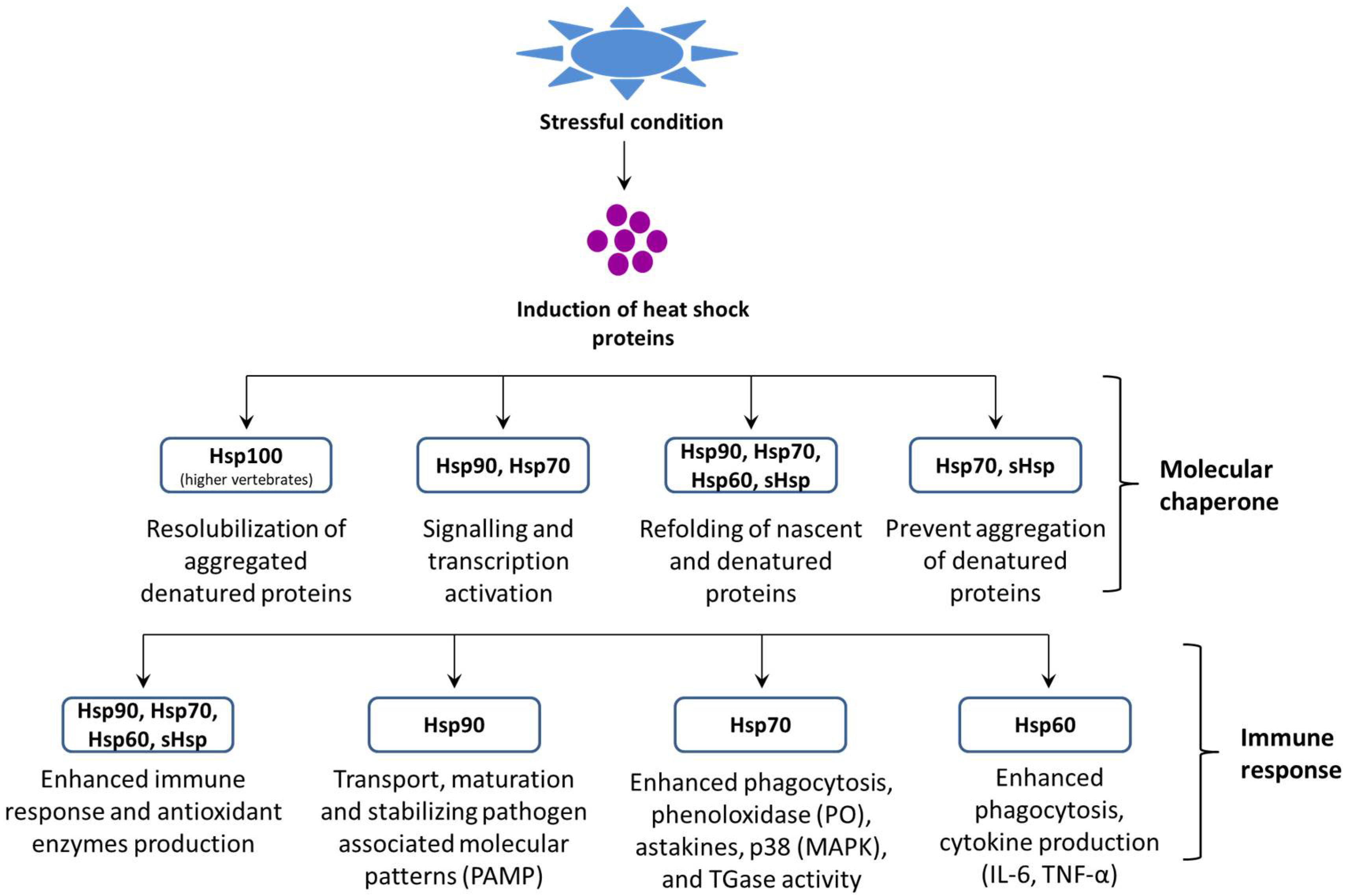
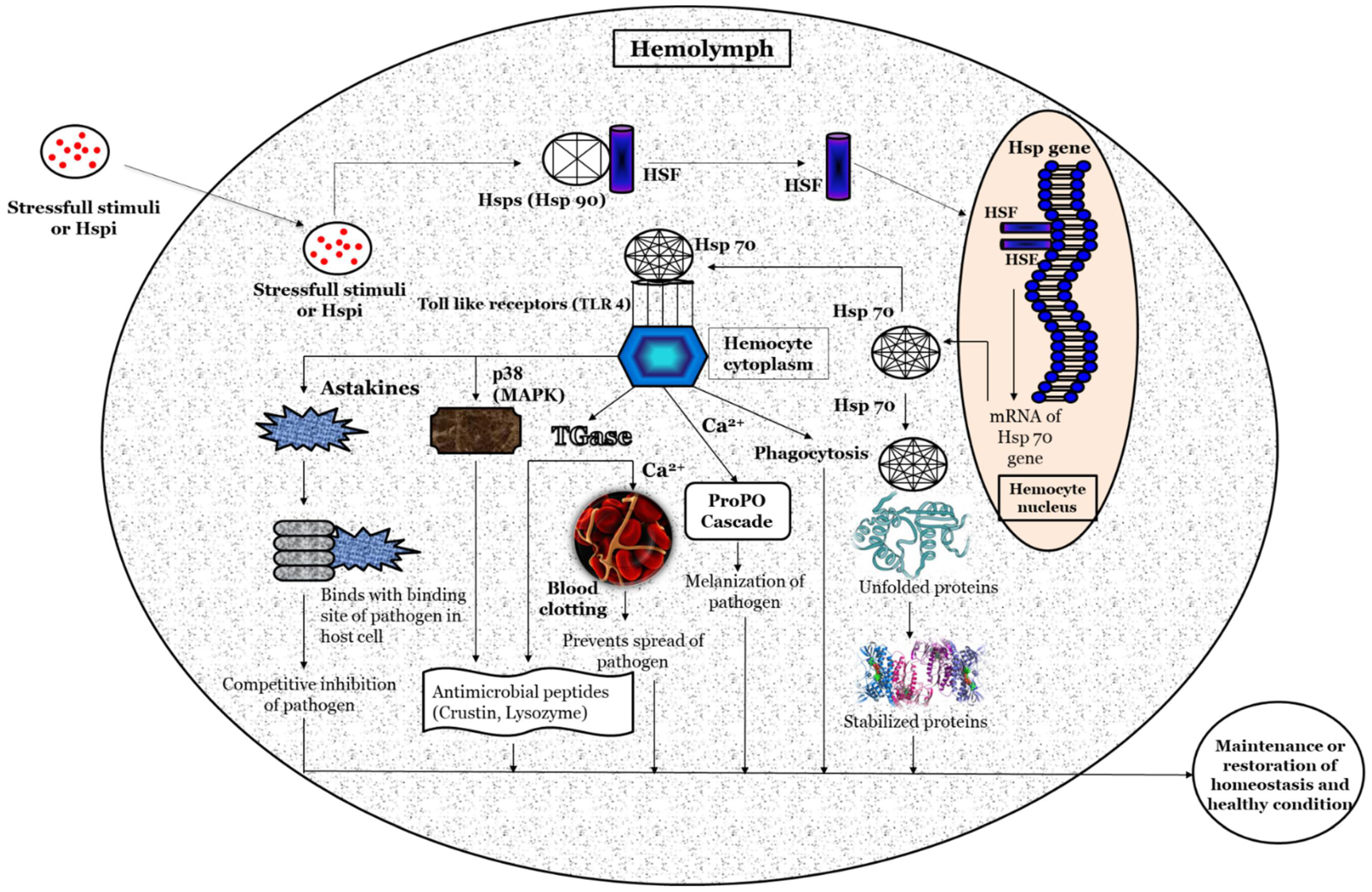
2. Factors Modulate Heat Shock Protein Response
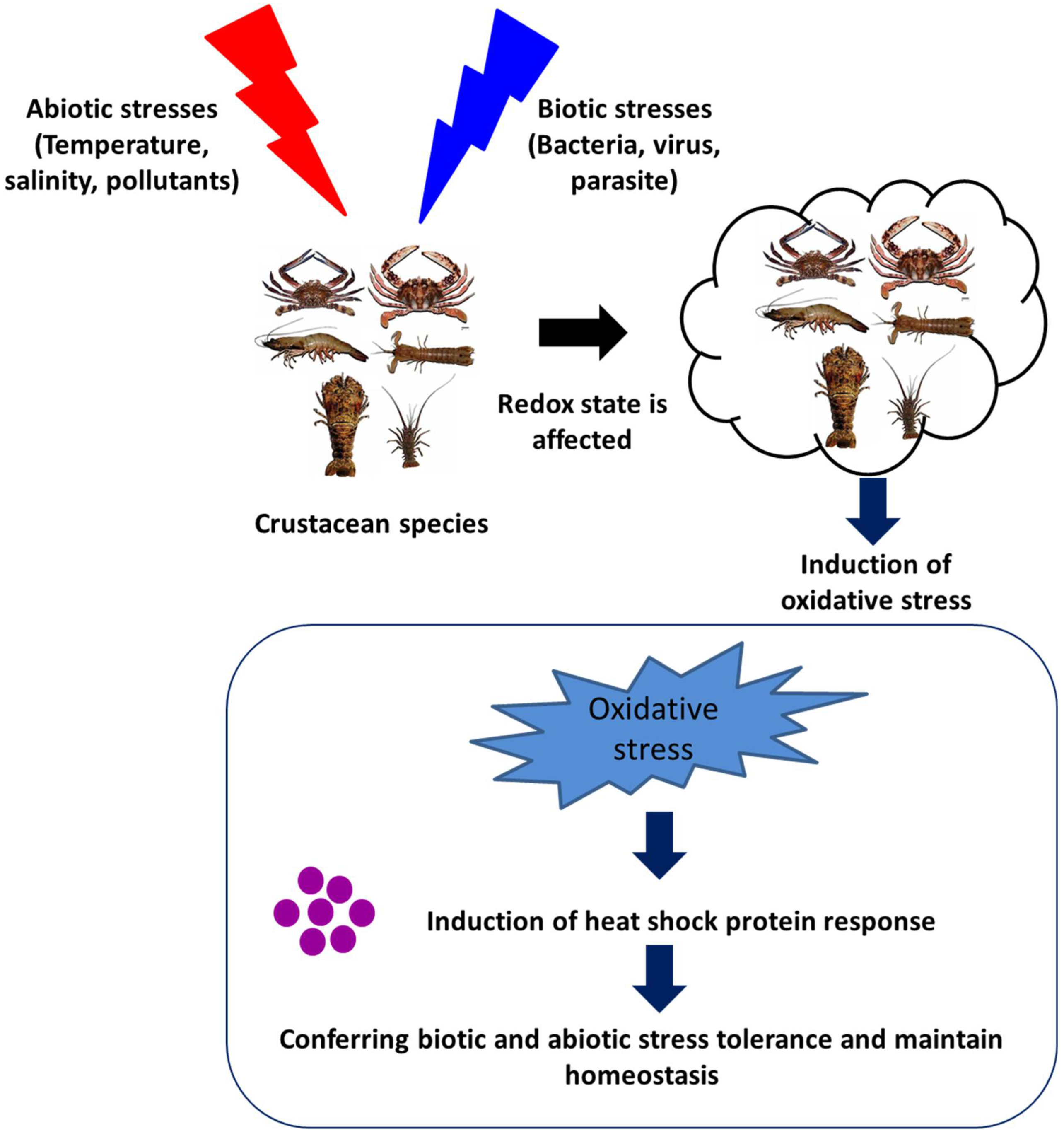
2.1. Environmental or Abiotic Stresses
2.1.1. Temperature
2.1.2. Salinity
2.1.3. Environmental Pollutants
2.2. Biotic Stresses
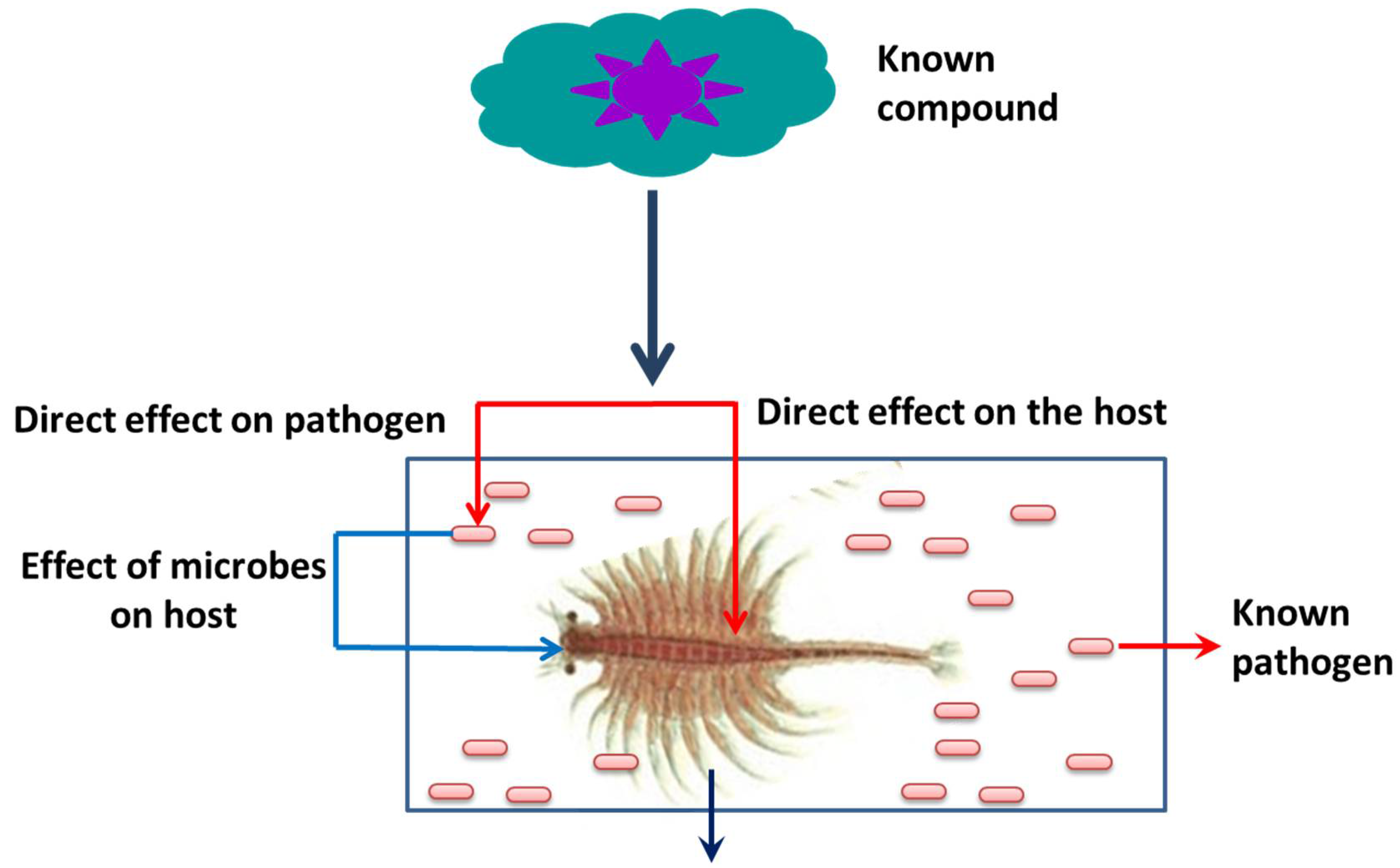
3. An A. franciscana Model System to Investigate the Role of Hsps in Crustaceans
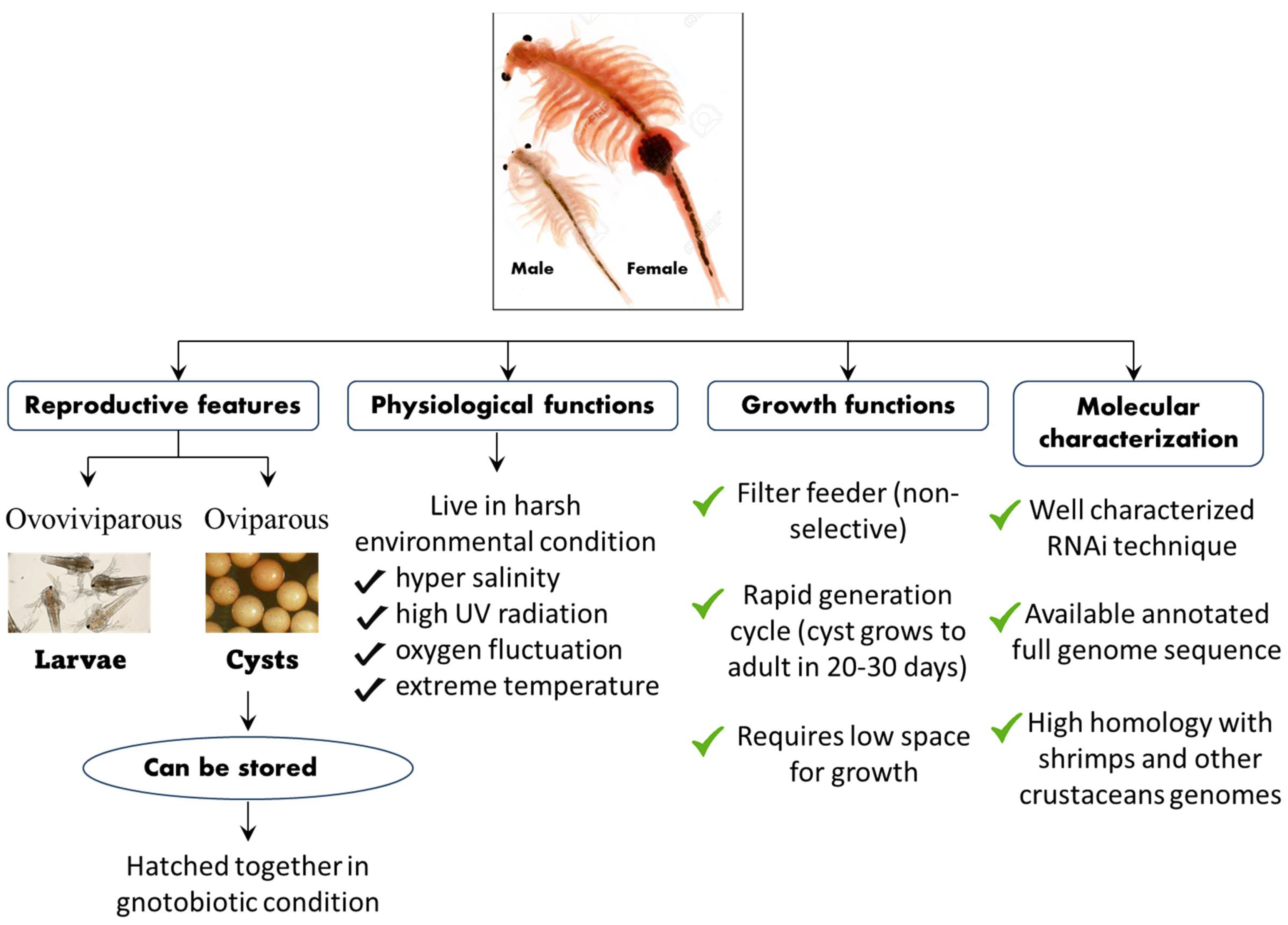
References
- Nover, L.; Scharf, K.D.; Neumann, D. Cytoplasmic heat shock granules are formed from precursor particles and are associated with a specific set of mRNAs. Mol. Cell. Biol. 1989, 9, 1298–1308.
- Parsell, D.A.; Lindquist, S. The Function of Heat-Shock Proteins in Stress Tolerance: Degradation and Reactivation of Damaged Proteins. Annu. Rev. Genet. 1993, 27, 437–496.
- Craig, E.A.; Weissman, J.S.; Horwich, A.L. Heat shock proteins and molecular chaperones: Mediators of protein conformation and turnover in the cell. Cell 1994, 78, 365–372.
- Dong, H.; Roy, S.; Zheng, X.; Kumar, V.; Das, B.K.; Duan, Y.; Sun, Y.; Zhang, J. Dietary teprenone enhances non-specific immunity, antioxidative response and resistance to hypoxia induced oxidative stress in Lateolabrax maculatus. Aquaculture 2021, 533, 736126.
- Dong, H.; Zheng, X.; Kumar, V.; Roy, S.; Duan, Y.; Gao, H.; Zhang, J. Dietary supplementation of teprenone potentiates thermal and hypoxia tolerance as well as cellular stress protection of Epinephelus coioides juveniles reared under multiple stressors. Aquaculture 2020, 514, 734413.
- Yang, Y.; Ye, H.; Huang, H.; Li, S.; Liu, X.; Zeng, X.; Gong, J. Expression of Hsp70 in the mud crab, Scylla paramamosain in response to bacterial, osmotic, and thermal stress. Cell Stress Chaperones 2013, 18, 475–482.
- Lindquist, S.; Craig, E.A. The Heat-Shock Proteins. Annu. Rev. Genet. 1988, 22, 631–677.
- Iryani, M.T.M.; MacRae, T.H.; Panchakshari, S.; Tan, J.; Bossier, P.; Wahid, M.E.A.; Sung, Y.Y. Knockdown of heat shock protein 70 (Hsp70) by RNAi reduces the tolerance of Artemia franciscana nauplii to heat and bacterial infection. J. Exp. Mar. Biol. Ecol. 2017, 487, 106–112.
- Roberts, R.J.; Agius, C.; Saliba, C.; Bossier, P.; Sung, Y.Y. Heat shock proteins (chaperones) in fish and shellfish and their potential role in relation to fish health: A review. J. Fish Dis. 2010, 33, 789–801.
- Sung, Y.Y.; Macrae, T.H.; Sorgeloos, P.; Bossier, P. Stress response for disease control in aquaculture. Rev. Aquac. 2011, 3, 120–137.
- Robert, J. Evolution of heat shock protein and immunity. Dev. Comp. Immunol. 2003, 27, 449–464.
- Norouzitallab, P.; Baruah, K.; Muthappa, D.M.; Bossier, P. Non-lethal heat shock induces HSP70 and HMGB1 protein production sequentially to protect Artemia franciscana against Vibrio campbellii. Fish Shellfish Immunol. 2015, 42, 395–399.
- Norouzitallab, P.; Baruah, K.; Biswas, P.; Vanrompay, D.; Bossier, P. Probing the phenomenon of trained immunity in invertebrates during a transgenerational study, using brine shrimp Artemia as a model system. Sci. Rep. 2016, 6, 21166.
- Lindquist, S. The Heat-Shock Response. Annu. Rev. Biochem. 1986, 55, 1151–1191.
- Richter, K.; Haslbeck, M.; Buchner, J. The Heat Shock Response: Life on the Verge of Death. Mol. Cell 2010, 40, 253–266.
- Courgeon, A.-M.; Maisonhaute, C.; Best-Belpomme, M. Heat shock proteins are induced by cadmium in Drosophila cells. Exp. Cell Res. 1984, 153, 515–521.
- Tan, J.; Macrae, T.H. Stress tolerance in diapausing embryos of Artemia franciscana is dependent on heat shock factor 1 (Hsf1). PLoS ONE 2018, 13, e0200153.
- Sornchuer, P.; Junprung, W.; Yingsunthonwattana, W.; Tassanakajon, A. Heat shock factor 1 regulates heat shock proteins and immune-related genes in Penaeus monodon under thermal stress. Dev. Comp. Immunol. 2018, 88, 19–27.
- Yura, T.; Tobe, T.; Ito, K.; Osawa, T. Heat shock regulatory gene (htpR) of Escherichia coli is required for growth at high temperature but is dispensable at low temperature. Proc. Natl. Acad. Sci. USA 1984, 81, 6803–6807.
- Michel, G.P.; Starka, J. Effect of ethanol and heat stresses on the protein pattern of Zymomonas mobilis. J. Bacteriol. 1986, 165, 1040–1042.
- Ananthan, J.; Goldberg, A.L.; Voellmy, R. Abnormal Proteins Serve as Eukaryotic Stress Signals and Trigger the Activation of Heat Shock Genes. Science 1986, 232, 522–524.
- Csermely, P.; Schnaider, T.; So, C.; Prohászka, Z.; Nardai, G. The 90-kDa Molecular Chaperone Family: Structure, function, and clinical applications. a comprehensive review. Pharmacol. Ther. 1998, 79, 129–168.
- Bozaykut, P.; Ozer, N.K.; Karademir, B. Regulation of protein turnover by heat shock proteins. Free Radic. Biol. Med. 2014, 77, 195–209.
- Aridon, P.; Geraci, F.; Turturici, G.; D’Amelio, M.; Savettieri, G.; Sconzo, G. Protective Role of Heat Shock Proteins in Parkinson’s Disease. Neurodegener. Dis. 2011, 8, 155–168.
- Capy, P.; Gasperi, G.; Biémont, C.; Bazin, C. Stress and transposable elements: Co-evolution or useful parasites? Heredity 2000, 85, 101–106.
- Horowitz, A.; Horowitz, S. Disease control in shrimp aquaculture from a microbial ecology perspective. In The New Wave, Proceedings of the Special Session on Sustainable Shrimp Culture, Aquaculture; Browdy, C.L., Jory, D.E., Eds.; World Aquaculture Society, 2001; pp. 199–218. Available online: https://ag.arizona.edu/azaqua/tilapia/tilapia_shrimp/moriarty.PDF (accessed on 27 August 2022).
- De La Vega, E.; Degnan, B.; Hall, M.R.; Cowley, J.A.; Wilson, K.J. Quantitative real-time RT-PCR demonstrates that handling stress can lead to rapid increases of gill-associated virus (GAV) infection levels in Penaeus monodon. Dis. Aquat. Org. 2004, 59, 195–203.
- de la Vega, E.; Hall, M.R.; Degnan, B.M.; Wilson, K.J. Short-term hyperthermic treatment of Penaeus monodon increases expression of heat shock protein 70 (HSP70) and reduces replication of gill associated virus (GAV). Aquaculture 2006, 253, 82–90.
- Vidal, O.M.; Granja, C.B.; Aranguren, F.; Brock, J.A.; Salazar, M. A Profound Effect of Hyperthermia on Survival of Litopenaeus vannamei Juveniles Infected with White Spot Syndrome Virus. J. World Aquac. Soc. 2001, 32, 364–372.
- Ellis, R.J.; Van der Vies, S.M. Molecular chaperones. Annu. Rev. Biochem. 1991, 60, 321–347.
- Tomanek, L.; Somero, G. Evolutionary and acclimation-induced variation in the heat-shock responses of congeneric marine snails (genus Tegula) from different thermal habitats: Implications for limits of thermotolerance and biogeography. J. Exp. Biol. 1999, 202, 2925–2936.
- Tomanek, L. Variation in the heat shock response and its implication for predicting the effect of global climate change on species’ biogeographical distribution ranges and metabolic costs. J. Exp. Biol. 2010, 213, 971–979.
- Xu, Q.; Qin, Y. Molecular cloning of heat shock protein 60 (PtHSP60) from Portunus trituberculatus and its expression response to salinity stress. Cell Stress Chaperones 2012, 17, 589–601.
- Baruah, K.; Ranjan, J.; Sorgeloos, P.; MacRae, T.H.; Bossier, P. Priming the prophenoloxidase system of Artemia franciscana by heat shock proteins protects against Vibrio campbellii challenge. Fish Shellfish Immunol. 2011, 31, 134–141.
- Zhu, Y.; Zhu, G.; Guo, Q.; Zhu, Z.; Wang, C.; Liu, Z. A Comparative Proteomic Analysis of Pinellia ternata Leaves Exposed to Heat Stress. Int. J. Mol. Sci. 2013, 14, 20614–20634.
- Sung, Y.Y.; Dhaene, T.; Defoirdt, T.; Boon, N.; MacRae, T.H.; Sorgeloos, P.; Bossier, P. Ingestion of bacteria overproducing DnaK attenuates Vibrio infection of Artemia franciscana larvae. Cell Stress Chaperones 2009, 14, 603–609.
- Jakob, U.; Muse, W.; Eser, M.; Bardwell, J.C.A. Chaperone Activity with a Redox Switch. Cell 1999, 96, 341–352.
- Kumsta, C.; Jakob, U. Redox-Regulated Chaperones. Biochemistry 2009, 48, 4666–4676.
- Sharma, S.; Chakraborty, K.; Müller, B.K.; Astola, N.; Tang, Y.-C.; Lamb, D.C.; Hayer-Hartl, M.; Hartl, F.U. Monitoring Protein Conformation along the Pathway of Chaperonin-Assisted Folding. Cell 2008, 133, 142–153.
- Walter, S.; Buchner, J. Molecular chaperones—Cellular machines for protein folding. Angew. Chem. Int. Ed. 2002, 41, 1098–1113.
- Mayer, M.P.; Bukau, B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell. Mol. Life Sci. 2005, 62, 670.
- Flaherty, K.M.; McKay, D.B.; Kabsch, W.; Holmes, K.C. Similarity of the three-dimensional structures of actin and the ATPase fragment of a 70-kDa heat shock cognate protein. Proc. Natl. Acad. Sci. USA 1991, 88, 5041–5045.
- Lo, J.; Hayashi, M.; Woo-Kim, S.; Tian, B.; Huang, J.; Fearns, C.; Takayama, S.; Zapata, J.M.; Yang, Y.; Lee, J. Tid1, a co-chaperone of the heat shock 70 protein and the mammalian counterpart of the Drosophila tumor suppressor l(2)tid, is critical for early embryonic development and cell survival. Mol. Cell. Biol. 2004, 24, 2226–2236.
- Flaherty, K.M.; DeLuca-Flaherty, C.; McKay, D.B. Three-dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein. Nature 1990, 346, 623–628.
- Kiang, J.G. Heat Shock Protein 70 kDa Molecular Biology, Biochemistry, and Physiology. Pharmacol. Ther. 1998, 80, 183–201.
- Kregel, K.C. Heat shock proteins: Modifying factors in physiological stress responses and acquired thermotolerance. J. Appl. Physiol. 2002, 92, 2177–2186.
- Cottin, D.; Shillito, B.; Chertemps, T.; Thatje, S.; Léger, N.; Ravaux, J. Comparison of heat-shock responses between the hydrothermal vent shrimp Rimicaris exoculata and the related coastal shrimp Palaemonetes varians. J. Exp. Mar. Biol. Ecol. 2010, 393, 9–16.
- Cottin, D.; Roussel, D.; Foucreau, N.; Hervant, F.; Piscart, C. Disentangling the effects of local and regional factors on the thermal tolerance of freshwater crustaceans. Die Nat. 2012, 99, 259–264.
- Yost, H.; Lindquist, S. RNA splicing is interrupted by heat shock and is rescued by heat shock protein synthesis. Cell 1986, 45, 185–193.
- Huang, L.-H.; Wang, C.-Z.; Kang, L. Cloning and expression of five heat shock protein genes in relation to cold hardening and development in the leafminer, Liriomyza sativa. J. Insect Physiol. 2009, 55, 279–285.
- Yang, J.; Mu, Y.; Dong, S.; Jiang, Q.; Yang, J. Changes in the expression of four heat shock proteins during the aging process in Brachionus calyciflorus (rotifera). Cell Stress Chaperones 2013, 19, 33–52.
- Hartl, F.U.; Martin, J. Molecular chaperones in cellular protein folding. Bioessays 1995, 16, 689–692.
- Bukau, B.; Horwich, A.L. The Hsp70 and Hsp60 Chaperone Machines. Cell 1998, 92, 351–366.
- Hartl, F.U.; Hayer-Hartl, M. Molecular Chaperones in the Cytosol: From Nascent Chain to Folded Protein. Science 2002, 295, 1852–1858.
- Beckmann, R.P.; Mizzen, L.E.; Welch, W.J. Interaction of Hsp 70 with Newly Synthesized Proteins: Implications for Protein Folding and Assembly. Science 1990, 248, 850–854.
- Palleros, D.R.; Welch, W.J.; Fink, A.L. Interaction of hsp70 with unfolded proteins: Effects of temperature and nucleotides on the kinetics of binding. Proc. Natl. Acad. Sci. USA 1991, 88, 5719–5723.
- Sadis, S.; Hightower, L.E. Unfolded proteins stimulate molecular chaperone Hsc70 ATPase by accelerating ADP/ATP exchange. Biochemistry 1992, 31, 9406–9412.
- Frydman, J.; Nimmesgern, E.; Ohtsuka, K.; Hartl, F.U. Folding of nascent polypeptide chains in a high molecular mass assembly with molecular chaperones. Nature 1994, 370, 111–117.
- Gething, M.-J.; Sambrook, J. Protein folding in the cell. Nature 1992, 335, 33–45.
- Chen, Z.; Zhou, T.; Wu, X.; Hong, Y.; Fan, Z.; Li, H. Influence of cytoplasmic heat shock protein 70 on viral infection of Nicotiana benthamiana. Mol. Plant Pathol. 2008, 9, 809–817.
- Park, H.; Lee, J.; Huh, S.; Seo, J.; Choi, E. Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. EMBO J. 2001, 20, 446–456.
- Dong, Y.; Dong, S. Induced thermotolerance and expression of heat shock protein 70 in sea cucumber Apostichopus japonicus. Fish. Sci. 2008, 74, 573–578.
- Wu, G.; Harris, M.K.; Guo, J.-Y.; Wan, F.-H. Response of multiple generations of beet armyworm, Spodoptera exigua (Hübner), feeding on transgenic Bt cotton. J. Appl. Èntomol. 2009, 133, 90–100.
- Tsan, M.-F.; Gao, B. Heat shock protein and innate immunity. Cell. Mol. Immunol. 2004, 1, 274–279.
- Jolesch, A.; Elmer, K.; Bendz, H.; Issels, R.D.; Noessner, E. Hsp70, a messenger from hyperthermia for the immune system. Eur. J. Cell Biol. 2012, 91, 48–52.
- Welch, W.J.; Feramisco, J.R. Purification of the major mammalian heat shock proteins. J. Biol. Chem. 1982, 257, 14949–14959.
- Aligue, R.; Akhavan-Niak, H.; Russell, P. A role for Hsp90 in cell cycle control: Wee1 tyrosine kinase activity requires inter-action with Hsp90. EMBO J. 1994, 13, 6099–6106.
- Jakob, U.; Lilie, H.; Meyer, I.; Buchner, J. Transient interaction of Hsp90 with early unfolding intermediates of citrate synthase: Implications for heat shock in vivo. J. Biol. Chem. 1995, 270, 7288–7294.
- Pearl, L.H.; Prodromou, C. Structure and Mechanism of the Hsp90 Molecular Chaperone Machinery. Annu. Rev. Biochem. 2006, 75, 271–294.
- Reddy, P.S.; Thirulogachandar, V.; Vaishnavi, C.; Aakrati, A.; Sopory, S.K.; Reddy, M.K. Molecular characterization and expression of a gene encoding cytosolic Hsp90 from Pennisetum glaucum and its role in abiotic stress adaptation. Gene 2011, 474, 29–38.
- Fu, D.; Chen, J.; Zhang, Y.; Yu, Z. Cloning and expression of a heat shock protein (HSP) 90 gene in the haemocytes of Crassostrea hongkongensis under osmotic stress and bacterial challenge. Fish Shellfish Immunol. 2011, 31, 118–125.
- Xu, D.; Sun, L.; Liu, S.; Zhang, L.; Yang, H. Polymorphisms of heat shock protein 90 (Hsp90) in the sea cucumber Apostichopus japonicus and their association with heat-resistance. Fish Shellfish Immunol. 2014, 41, 428–436.
- Zhao, H.; Yang, H.; Zhao, H.; Chen, M.; Wang, T. The molecular characterization and expression of heat shock protein 90 (Hsp90) and 26 (Hsp26) cDNAs in sea cucumber (Apostichopus japonicus). Cell Stress Chaperones 2011, 16, 481–493.
- Zhu, J.-Y.; Wu, G.-X.; Ye, G.-Y.; Hu, C. Heat shock protein genes (hsp20, hsp75 and hsp90) from Pieris rapae: Molecular cloning and transcription in response to parasitization by Pteromalus puparum. Insect Sci. 2012, 20, 183–193.
- Quintana, F.J.; Cohen, I.R. Heat Shock Proteins as Endogenous Adjuvants in Sterile and Septic Inflammation. J. Immunol. 2005, 175, 2777–2782.
- Vabulas, R.M.; Ahmad-Nejad, P.; da Costa, C.; Miethke, T.; Kirschning, C.J.; Häcker, H.; Wagner, H. Endocytosed HSP60s Use Toll-like Receptor 2 (TLR2) and TLR4 to Activate the Toll/Interleukin-1 Receptor Signaling Pathway in Innate Immune Cells. J. Biol. Chem. 2001, 276, 31332–31339.
- Choresh, O.; Ron, E.; Loya, Y. The 60-kDa Heat Shock Protein (HSP60) of the Sea Anemone Anemonia viridis: A Potential Early Warning System for Environmental Changes. Mar. Biotechnol. 2001, 3, 501–508.
- Choresh, O.; Loya, Y.; Müller, W.E.; Wiedenmann, J.; Azem, A. The mitochondrial 60-kDa heat shock protein in marine invertebrates: Biochemical purification and molecular characterization. Cell Stress Chaperones 2004, 9, 38–48.
- Clayton, M.E.; Steinmann, R.; Fent, K. Different expression patterns of heat shock proteins hsp 60 and hsp 70 in zebra mussels (Dreissena polymorpha) exposed to copper and tributyltin. Aquat. Toxicol. 2000, 47, 213–226.
- Zhou, J.; Wang, W.-N.; He, W.-Y.; Zheng, Y.; Wang, L.; Xin, Y.; Liu, Y.; Wang, A.-L. Expression of HSP60 and HSP70 in white shrimp, Litopenaeus vannamei in response to bacterial challenge. J. Invertebr. Pathol. 2010, 103, 170–178.
- Huang, W.-J.; Leu, J.-H.; Tsau, M.-T.; Chen, J.-C.; Chen, L.-L. Differential expression of LvHSP60 in shrimp in response to environmental stress. Fish Shellfish Immunol. 2011, 30, 576–582.
- Sun, Y.; Macrae, T.H. Small heat shock proteins: Molecular structure and chaperone function. Cell. Mol. Life Sci. 2005, 62, 2460–2476.
- Mchaourab, H.S.; Godar, J.A.; Stewart, P.L. Structure and Mechanism of Protein Stability Sensors: Chaperone Activity of Small Heat Shock Proteins. Biochemistry 2009, 48, 3828–3837.
- Laganowsky, A.; Benesch, J.; Landau, M.; Ding, L.; Sawaya, M.; Cascio, D.; Huang, Q.; Robinson, C.; Horwitz, J.; Eisenberg, D. Crystal structures of truncated alphaA and alphaB crystallins reveal structural mechanisms of polydispersity important for eye lens function. Protein Sci. 2010, 19, 1031–1043.
- Hilario, E.; Martin, F.J.M.; Bertolini, M.C.; Fan, L. Crystal Structures of Xanthomonas Small Heat Shock Protein Provide a Structural Basis for an Active Molecular Chaperone Oligomer. J. Mol. Biol. 2011, 408, 74–86.
- Waters, E.R. The evolution, function, structure, and expression of the plant sHSPs. J. Exp. Bot. 2013, 64, 391–403.
- Kriehuber, T.; Rattei, T.; Weinmaier, T.; Bepperling, A.; Haslbeck, M.; Buchner, J. Independent evolution of the core domain and its flanking sequences in small heat shock proteins. FASEB J. 2010, 24, 3633–3642.
- Van Montfort, R.L.; Basha, E.; Friedrich, K.L.; Slingsby, C.; Vierling, E. Crystal structure and assembly of a eukaryotic small heat shock protein. Nat. Genet. 2001, 8, 1025–1030.
- Horwitz, J. Alpha-crystallin. Exp. Eye Res. 2003, 76, 145–153.
- Haslbeck, M.; Franzmann, T.; Weinfurtner, D.; Buchner, J. Some like it hot: The structure and function of small heat-shock proteins. Nat. Struct. Mol. Biol. 2005, 12, 842–846.
- Mogk, A.; Deuerling, E.; Vorderwülbecke, S.; Vierling, E.; Bukau, B. Small heat shock proteins, ClpB and the DnaK system form a functional triade in reversing protein aggregation. Mol. Microbiol. 2003, 50, 585–595.
- Lee, G.J.; Roseman, A.M.; Saibil, H.R.; Vierling, E. A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J. 1997, 16, 659–671.
- Cashikar, A.G.; Duennwald, M.; Lindquist, S.L. A Chaperone Pathway in Protein Disaggregation: Hsp26 alters the nature of protein aggregates to facilitate reactivation by Hsp104s. J. Biol. Chem. 2005, 280, 23869–23875.
- Liberek, K.; Lewandowska, A.; Ziętkiewicz, S. Chaperones in control of protein disaggregation. EMBO J. 2008, 27, 328–335.
- Jackson, S.A.; Clegg, J.S. Ontogeny of low molecular weight stress protein p26 during early embryogenesis of the brine shrimp, Artemia franciscana. Dev. Growth Differ. 1996, 38, 153–160.
- Clegg, J.S. Stress-related proteins compared in diapause and in activated, anoxic encysted embryos of the animal extremophile, Artemia franciscana. J. Insect Physiol. 2011, 57, 660–664.
- King, A.M.; Macrae, T.H. The Small Heat Shock Protein p26 Aids Development of Encysting Artemia Embryos, Prevents Spontaneous Diapause Termination and Protects against Stress. PLoS ONE 2012, 7, e43723.
- King, A.M.; Toxopeus, J.; MacRae, T.H. Functional differentiation of small heat shock proteins in diapause-destined Artemia embryos. FEBS J. 2013, 280, 4761–4772.
- Chen, T.; Villeneuve, T.S.; Garant, K.A.; Amons, R.; MacRae, T.H. Functional characterization of artemin, a ferritin homolog synthesized in Artemia embryos during encystment and diapause. FEBS J. 2007, 274, 1093–1101.
- Hu, Y.; Bojikova-Fournier, S.; King, A.M.; MacRae, T.H. The structural stability and chaperone activity of artemin, a ferritin homologue from diapause-destined Artemia embryos, depend on different cysteine residues. Cell Stress Chaperones 2010, 16, 133–141.
- King, A.M.; Toxopeus, J.; MacRae, T.H. Artemin, a Diapause-Specific Chaperone, Contributes to the Stress Tolerance of Artemia franciscana Cysts and Influences Their Release from Females. J. Exp. Biol. 2014, 217, 1719–1724.
- Macrae, T.H. Stress tolerance during diapause and quiescence of the brine shrimp, Artemia. Cell Stress Chaperones 2015, 21, 9–18.
- Dai, L.; Chen, D.-F.; Liu, Y.-L.; Zhao, Y.; Yang, F.; Yang, J.-S.; Yang, W.-J. Extracellular Matrix Peptides of Artemia Cyst Shell Participate in Protecting Encysted Embryos from Extreme Environments. PLoS ONE 2011, 6, e20187.
- Wu, B.J.; Kingston, R.E.; Morimoto, R.I. Human HSP70 promoter contains at least two distinct regulatory domains. Proc. Natl. Acad. Sci. USA 1986, 83, 629–633.
- Köhler, H.R.; Triebskorn, R.; Stöcker, W.; Kloetzel, P.-M.; Alberti, G. The 70 kD heat shock protein (hsp 70) in soil invertebrates: A possible tool for monitoring environmental toxicants. Arch. Environ. Contam. Toxicol. 1992, 22, 334–338.
- Viswanathan, C.; Khanna-Chopra, R. Heat shock proteins-Role in thermotolerance of crop plants. Curr. Sci. 1996, 71, 275–284.
- Morimoto, R.I. Cells in Stress: Transcriptional Activation of Heat Shock Genes. Science 1993, 259, 1409–1410.
- Amin, J.; Ananthan, J.; Voellmy, R. Key features of heat shock regulatory elements. Mol. Cell. Biol. 1988, 8, 3761–3769.
- Grossman, A.D.; Erickson, J.W.; Gross, C.A. The htpR gene product of E. coli is a sigma factor for heat-shock promoters. Cell 1984, 38, 383–390.
- Sorger, P.K.; Pelham, H.R. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell 1988, 54, 855–864.
- Kong, X.-C.; Zhang, D.; Qian, C.; Liu, G.-T.; Bao, X.-Q. FLZ, a novel HSP27 and HSP70 inducer, protects SH-SY5Y cells from apoptosis caused by MPP+. Brain Res. 2011, 1383, 99–107.
- Fang, H.; Wu, Y.; Huang, X.; Wang, W.; Ang, B.; Cao, X.; Wan, T. Toll-like Receptor 4 (TLR4) Is Essential for Hsp70-like Protein 1 (HSP70L1) to Activate Dendritic Cells and Induce Th1 Response. J. Biol. Chem. 2011, 286, 30393–30400.
- Triantafilou, M.; Triantafilou, K. Heat-shock protein 70 and heat-shock protein 90 associate with Toll-like receptor 4 in response to bacterial lipopolysaccharide. Biochem. Soc. Trans. 2004, 32, 636–639.
- Sato, S.; Fujita, N.; Tsuruo, T. Modulation of Akt kinase activity by binding to Hsp90. Proc. Natl. Acad. Sci. USA 2000, 97, 10832–10837.
- Lin, X.; Söderhäll, I. Crustacean hematopoiesis and the astakine cytokines. Blood 2011, 117, 6417–6424.
- Maningas, M.B.B.; Kondo, H.; Hirono, I.; Saito-Taki, T.; Aoki, T. Essential function of transglutaminase and clotting protein in shrimp immunity. Mol. Immunol. 2008, 45, 1269–1275.
- Fagutao, F.F.; Maningas, M.B.B.; Kondo, H.; Aoki, T.; Hirono, I. Transglutaminase regulates immune-related genes in shrimp. Fish Shellfish Immunol. 2012, 32, 711–715.
- Zhu, Y.-T.; Li, D.; Zhang, X.; Li, X.-J.; Li, W.-W.; Wang, Q. Role of transglutaminase in immune defense against bacterial pathogens via regulation of antimicrobial peptides. Dev. Comp. Immunol. 2016, 55, 39–50.
- Yu, Z.; Geng, Y.; Huang, A.; Wang, K.; Huang, X.; Chen, D.; Ou, Y.; Wang, J. Molecular characterization of a p38 mitogen-activated protein kinase gene from Scylla paramamosain and its expression profiles during pathogenic challenge. J. Invertebr. Pathol. 2017, 144, 32–36.
- He, S.; Qian, Z.; Yang, J.; Wang, X.; Mi, X.; Liu, Y.; Hou, F.; Liu, Q.; Liu, X. Molecular characterization of a p38 MAPK from Litopenaeus vannamei and its expression during the molt cycle and following pathogen infection. Dev. Comp. Immunol. 2013, 41, 217–221.
- Yan, H.; Zhang, S.; Li, C.-Z.; Chen, Y.-H.; Chen, Y.-G.; Weng, S.-P.; He, J.-G. Molecular characterization and function of a p38 MAPK gene from Litopenaeus vannamei. Fish Shellfish Immunol. 2013, 34, 1421–1431.
- Sanders, B.M. Stress Proteins in Aquatic Organisms: An Environmental Perspective. Crit. Rev. Toxicol. 1993, 23, 49–75.
- Feder, M.E.; Hofmann, G.E. Heat-Shock Proteins, Molecular Chaperones, and The Stress Response: Evolutionary and Ecological Physiology. Annu. Rev. Physiol. 1999, 61, 243–282.
- Huang, A.-M.; Geng, Y.; Wang, K.-Y.; Zeng, F.; Liu, Q.; Wang, Y.; Sun, Y.; Liu, X.-X.; Zhou, Y. Molecular Cloning and Expression Analysis of Heat Shock Protein 90 (Hsp90) of the Mud Crab, Scylla Paramamosain. J. Agric. Sci. 2013, 5, 1.
- Junprung, W.; Norouzitallab, P.; De Vos, S.; Tassanakajon, A.; Viet, D.N.; Van Stappen, G.; Bossier, P. Sequence and expression analysis of HSP70 family genes in Artemia franciscana. Sci. Rep. 2019, 9, 8391.
- Ravi, V.; Kubofcik, J.; Bandopathyaya, S.; Geetha, M.; Narayanan, R.; Nutman, T.; Kaliraj, P. Wuchereria bancrofti: Cloning and characterization of heat shock protein 70 from the human lymphatic filarial parasite. Exp. Parasitol. 2004, 106, 1–10.
- Luan, W.; Li, F.; Zhang, J.; Wen, R.; Li, Y.; Xiang, J. Identification of a novel inducible cytosolic Hsp70 gene in Chinese shrimp Fenneropenaeus chinensis and comparison of its expression with the cognate Hsc70 under different stresses. Cell Stress Chaperones 2009, 15, 83–93.
- Gbotsyo, Y.A. The Effect of Cold Stress on Heat Shock Proteins in Nauplii (larvae) of the Brine Shrimp, Artemia franciscana. Honours Thesis, Saint Mary’s University, Halifax, NS, Canada, 2017; pp. 1–39.
- Chapple, J.; Smerdon, G.R.; Berry, R.; Hawkins, A.J. Seasonal changes in stress-70 protein levels reflect thermal tolerance in the marine bivalve Mytilus edulis L. J. Exp. Mar. Biol. Ecol. 1998, 229, 53–68.
- Browne, R.; Bowen, S. Taxonomy and population genetics of Artemia. In Artemia Biology; Browne, R.A., Sorgeloos, P., Trotman, C.N.A., Eds.; CRC Press: Boca Raton, FL, USA, 1991; pp. 221–235.
- Triantaphyllidis, G.; Abatzopoulos, T.; Sorgeloos, P. Review of the biogeography of the genus Artemia (Crustacea, Anostraca). J. Biogeogr. 1998, 25, 213–226.
- Sorgeloos, P.; Bossuyt, E.; Laviña, E.; Baeza-Mesa, M.; Persoone, G. Decapsulation of Artemia cysts: A simple technique for the improvement of the use of brine shrimp in aquaculture. Aquaculture 1977, 12, 311–315.
- Kumar, V.; Das, B.K.; Swain, H.S.; Chowdhury, H.; Roy, S.; Bera, A.K.; Das, R.; Parida, S.N.; Dhar, S.; Jana, A.K.; et al. Outbreak of Ichthyophthirius multifiliis associated with Aeromonas hydrophila in Pangasianodon hypophthalmus: The role of turmeric oil in enhancing immunity and inducing resistance against co-infection. Front. Immunol. 2022, 13, 956478.
- Clegg, J.S.; Drinkwater, L.E.; Sorgeloos, P. The Metabolic Status of Diapause Embryos of Artemia franciscana (SFB). Physiol. Zool. 1996, 69, 49–66.
- Tran, P.T.N.; Kumar, V.; Bossier, P. Do acute hepatopancreatic necrosis disease-causing PirABVP toxins aggravate vibriosis? Emerg. Microbes Infect. 2020, 9, 1919–1932.
- Criel, G.R.J.; Macrae, T.H. Artemia morphology and structure. In Artemia: Basic and Applied Biology. Biology of Aquatic Organisms; Abatzopoulos, T.J., Beardmore, J.A., Clegg, J.S., Sorgeloos, P., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 1–37. ISBN 978-94-017-0791-6.
- Van Stappen, G. Zoogeography. In Artemia: Basic and Applied Biology. Biology of Aquatic Organisms; Abatzopoulos, T.J., Beardmore, J.A., Clegg, J.S., Sorgeloos, P., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 171–224.
- Robbins, H.M.; Van Stappen, G.; Sorgeloos, P.; Sung, Y.Y.; MacRae, T.H.; Bossier, P. Diapause termination and development of encysted Artemia embryos: Roles for nitric oxide and hydrogen peroxide. J. Exp. Biol. 2010, 213, 1464–1470.
- Kumar, V.; De Bels, L.; Couck, L.; Baruah, K.; Bossier, P.; Broeck, W.V.D. PirABVP Toxin Binds to Epithelial Cells of the Digestive Tract and Produce Pathognomonic AHPND Lesions in Germ-Free Brine Shrimp. Toxins 2019, 11, 717.
- Han, B.; Kaur, V.I.; Baruah, K.; Nguyen, V.D.; Bossier, P. High doses of sodium ascorbate act as a prooxidant and protect gnotobiotic brine shrimp larvae (Artemia franciscana) against Vibrio harveyi infection coinciding with heat shock protein 70 activation. Dev. Comp. Immunol. 2018, 92, 69–76.
- Kumar, V.; Nguyen, D.V.; Baruah, K.; Bossier, P. Probing the mechanism of VPAHPND extracellular proteins toxicity purified from Vibrio parahaemolyticus AHPND strain in germ-free Artemia test system. Aquaculture 2019, 504, 414–419.
- Kumar, V.; Baruah, K.; Bossier, P. Bamboo powder protects gnotobiotically-grown brine shrimp against AHPND-causing Vibrio parahaemolyticus strains by cessation of PirABVP toxin secretion. Aquaculture 2021, 539, 736624.
- De Vos, S.; Bossier, P.; Vuylsteke, M. Genomic Tools and Sex Determination in the Extremophile Brine Shrimp Artemia franciscana; Ghent University: Ghent, Belgium, 2014.
- Defoirdt, T.; Crab, R.; Wood, T.K.; Sorgeloos, P.; Verstraete, W.; Bossier, P. Quorum Sensing-Disrupting Brominated Furanones Protect the Gnotobiotic Brine Shrimp Artemia franciscana from Pathogenic Vibrio harveyi, Vibrio campbellii, and Vibrio parahaemolyticus Isolates. Appl. Environ. Microbiol. 2006, 72, 6419–6423.
- Crab, R.; Lambert, A.; Defoirdt, T.; Bossier, P.; Verstraete, W. The application of bioflocs technology to protect brine shrimp (Artemia franciscana) from pathogenic Vibrio harveyi. J. Appl. Microbiol. 2010, 109, 1643–1649.
- Miller, D.; McLennan, A.G. The heat shock response of the cryptobiotic brine Shrimp Artemia—I. A comparison of the thermotolerance of cysts and larvae. J. Therm. Biol. 1988, 13, 119–123.
- Seebaugh, D.R.; Wallace, W. Importance of metal-binding proteins in the partitioning of Cd and Zn as trophically available metal (TAM) in the brine shrimp Artemia franciscana. Mar. Ecol. Prog. Ser. 2004, 272, 215–230.
- Marques, A.; Ollevier, F.; Verstraete, W.; Sorgeloos, P.; Bossier, P. Gnotobiotically grown aquatic animals: Opportunities to investigate host-microbe interactions. J. Appl. Microbiol. 2006, 100, 903–918.
- El-Magsodi, M.O.; Bossier, P.; Sorgeloos, P.; Van Stappen, G. Effect of Light Colour, Timing, and Duration of Light Exposure on the Hatchability of Artemia Spp. (Branchiopoda: Anostraca). Eggs. J. Crustac. Biol. 2016, 36, 515–524.
- Clegg, J.S.; Jackson, S.A.; Van Hoa, N.; Sorgeloos, P. Thermal resistance, developmental rate and heat shock proteins in Artemia franciscana, from San Francisco Bay and southern Vietnam. J. Exp. Mar. Biol. Ecol. 2000, 252, 85–96.
- Kumar, V.; Bossier, P. Importance of plant—Derived compounds and/or natural products in aquaculture. Aquafeed 2018, 10, 28–31.
- Kumar, V.; Roy, S. Aquaculture Drugs: Sources, Active Ingredients, Pharmaceutic Preparations and Methods of Administration. J. Aquac. Res. Dev. 2017, 8, 510.
- Kumar, V.; Bossier, P. Novel plant-based compounds could be useful in protecting shrimp species against AHPND Vibrio parahaemolyticus. J. Inland Fish. Soc. India 2019, 51, 3–5.
- Kumar, V.; Wille, M.; Lourenço, T.M.; Bossier, P. Biofloc-Based Enhanced Survival of Litopenaeus vannamei Upon AHPND-Causing Vibrio parahaemolyticus Challenge Is Partially Mediated by Reduced Expression of Its Virulence Genes. Front. Microbiol. 2020, 11, 1270.
- Baruah, K.; Norouzitallab, P.; Phong, H.P.P.D.; Smagghe, G.; Bossier, P. Enhanced resistance against Vibrio harveyi infection by carvacrol and its association with the induction of heat shock protein 72 in gnotobiotic Artemia franciscana. Cell Stress Chaperones 2017, 22, 377–387.
- Frankenberg, M.; Jackson, S.; Clegg, J. The heat shock response of adult Artemia franciscana. J. Therm. Biol. 2000, 25, 481–490.
- Wandinger, S.K.; Richter, K.; Buchner, J. The Hsp90 Chaperone Machinery. J. Biol. Chem. 2008, 283, 18473–18477.
- Vos, M.; Hageman, J.; Carra, S.; Kampinga, H.H. Structural and Functional Diversities between Members of the Human HSPB, HSPH, HSPA, and DNAJ Chaperone Families. Biochemistry 2008, 47, 7001–7011.
- Kumar, V.; Roy, S.; Behera, B.K.; Swain, H.S.; Das, B.K. Biofloc Microbiome With Bioremediation and Health Benefits. Front. Microbiol. 2021, 12, 3499.
- Roy, S.; Baruah, K.; Bossier, P.; Vanrompay, D.; Norouzitallab, P. Induction of transgenerational innate immune memory against Vibrio infections in a brine shrimp (Artemia franciscana) model. Aquaculture 2022, 557, 738309.
- Roy, S.; Kumar, V.; Behera, B.K.; Parhi, J.; Mohapatra, S.; Chakraborty, T.; Das, B.K. CRISPR/Cas Genome Edit-ing-Can It Become a Game Changer in Future Fisheries Sector? Front. Mar. Sci. 2022, 9, 924475.
- Meimaridou, E.; Gooljar, S.B.; Chapple, J.P. From hatching to dispatching: The multiple cellular roles of the Hsp70 molecular chaperone machinery. J. Mol. Endocrinol. 2008, 42, 1–9.
- Vabulas, R.M.; Raychaudhuri, S.; Hayer-Hartl, M.; Hartl, F.U. Protein Folding in the Cytoplasm and the Heat Shock Response. Cold Spring Harb. Perspect. Biol. 2010, 2, a004390.
- Hartl, F.U.; Hayer-Hartl, M. Converging concepts of protein folding in vitro and in vivo. Nat. Struct. Mol. Biol. 2009, 16, 574–581.
- Yam, A.Y.; Xia, Y.; Lin, H.-T.J.; Burlingame, A.; Gerstein, M.; Frydman, J. Defining the TRiC/CCT interactome links chaperonin function to stabilization of newly made proteins with complex topologies. Nat. Struct. Mol. Biol. 2008, 15, 1255–1262.
- Shi, J.; Fu, M.; Zhao, C.; Zhou, F.; Yang, Q.; Qiu, L. Characterization and function analysis of Hsp60 and Hsp10 under different acute stresses in black tiger shrimp, Penaeus monodon. Cell Stress Chaperones 2015, 21, 295–312.
- Sung, Y.Y.; Ashame, M.F.; Chen, S.; MacRae, T.H.; Sorgeloos, P.; Bossier, P. Feeding Artemia franciscana (Kellogg) larvae with bacterial heat shock protein, protects from Vibrio campbellii infection. J. Fish Dis. 2009, 32, 675–685.
- Kumar, V.; Roy, S.; Behera, B.K.; Das, B.K. RNA Interference and Its Potential Applications in Aquatic Animal Health Management. In Biotechnological Advances in Aquaculture Health Management; Springer: Singapore, 2021; pp. 25–41.
- Kumar, V.; Roy, S.; Behera, B.K.; Das, B.K. Disease Diagnostic Tools for Health Management in Aquaculture. In Advances in Fisheries Biotechnology; Springer: Singapore, 2021; pp. 363–382.
- Roy, S.; Kumar, V.; Behera, B.K.; Das, B.K. Epigenetics: Perspectives and Potential in Aquaculture. In Advances in Fisheries Biotechnology; Springer: Singapore, 2021; pp. 133–150.





