
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gabriela Elena Badea | -- | 1173 | 2022-11-22 19:40:47 | | | |
| 2 | Camila Xu | Meta information modification | 1173 | 2022-11-23 04:02:29 | | |
Video Upload Options
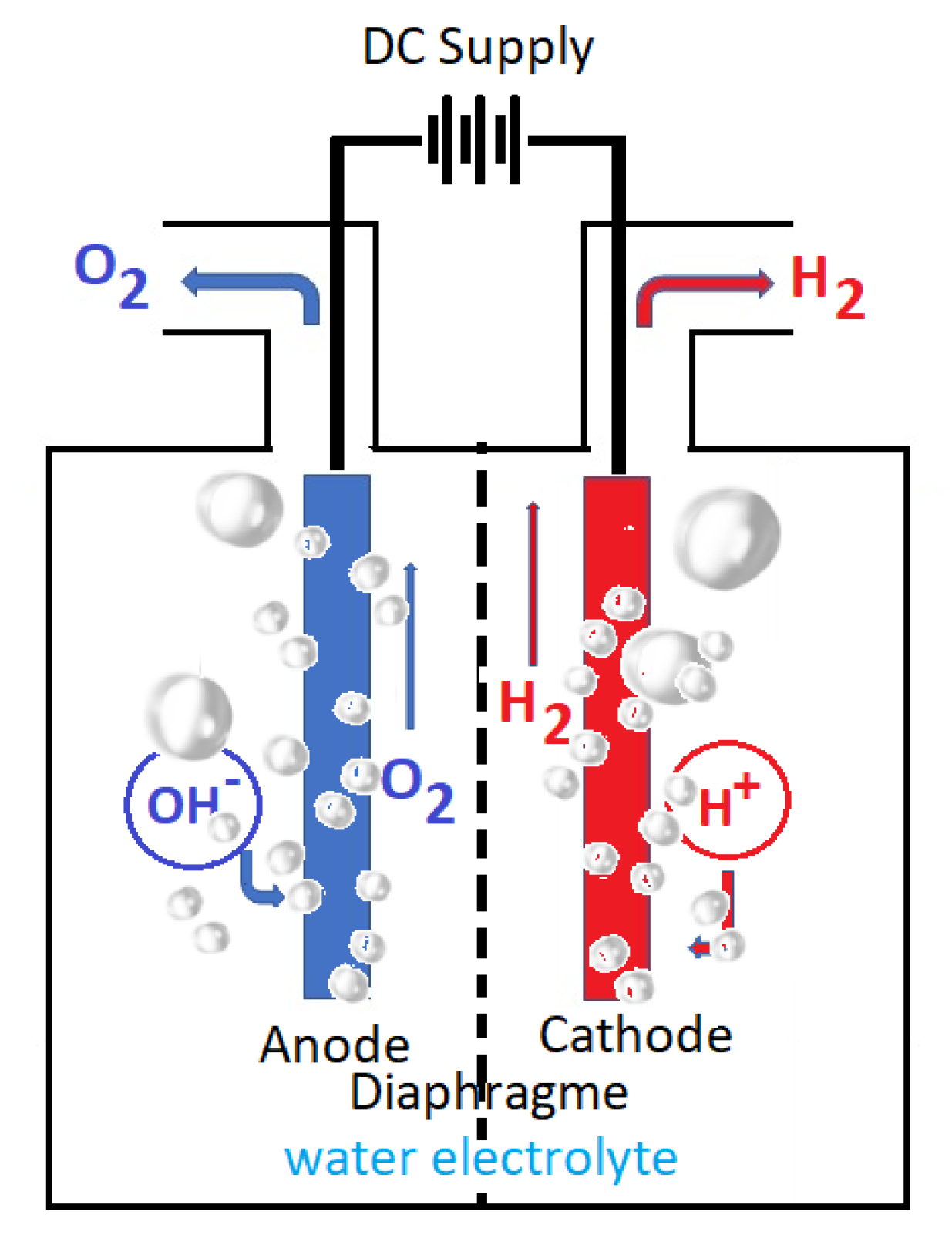
Seawater is the most abundant supply of water and the ideal and cheapest electrolyte. Because it is a green and renewable chemical process, water electrolysis has earned a lot of interest among the different hydrogen production techniques. Basis of water electrolysis include general theoretical concepts: chemical, physical, and electrochemical concepts. Research has focused on the specific seawater electrolysis parameters: cathodic evolution of hydrogen; concurrent anodic evolution of oxygen and chlorine; specific seawater catalyst electrodes, and seawater electrolyzer efficiency. A sustainable technology development must also capitalize on known and emerging technologies; protecting the environment; utilization of green, renewable energies as sources of electricity; and above all, economic efficiency as a whole.
1. Introduction
2. An Overview of Water Electrochemistry
References
- Wang, S.; Lu, A.; Zhong, C.-J. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 4.
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934.
- Naimi, Y.; Antar, A. Hydrogen Generation by Water Electrolysis. In Advances In Hydrogen Generation Technologies; IntechOpen: London, UK, 2018.
- Rashid, M.; Khaloofah Al Mesfer, M.; Naseem, H.; Danish, M. Hydrogen Production by Water Electrolysis: A Review of Alkaline Water Electrolysis, PEM Water Electrolysis and High Temperature Water Electrolysis. Int. J. Eng. Adv. Technol. (IJEAT) 2015, 4, 3.
- Chisholm, G.; Cronin, L. Hydrogen from Water Electrolysi s School of Chemistry, University of Glasgow, Glasgow, United Kingdom. 2016. Available online: http://www.chem.gla.ac.uk/cronin/images/pubs/Chisholm-Chapter_16_2016.pdf (accessed on 27 April 2022).
- Hora, C.; Dan, F.C.; Rancov, N.; Badea, G.E.; Secui, C. Main Trends and Research Directions in Hydrogen Generation Using Low Temperature Electrolysis: A Systematic Literature Review. Energies 2022, 15, 6076.
- Pérez Orosa, L.; Chinarro, E.; Guinea, D.; García-Alegre, M.C. Hydrogen Production by Wastewater Alkaline Electro-Oxidation. Energies 2022, 15, 5888.
- Jenkins, B.; Squires, D.; Barton, J.; Strickland, D.; Wijayantha, K.G.U.; Carroll, J.; Wilson, J.; Brenton, M.; Thomson, M. Techno-Economic Analysis of Low Carbon Hydrogen Production from Offshore Wind Using Battolyser Technology. Energies 2022, 15, 5796.
- Lee, C.H.; Lee, S.U. Theoretical Basis of Electrocatalysis. In Electrocatalysts for Fuel Cells and Hydrogen Evolution-Theory to Design; Ray, A., Mukhopadhyay, I., Pati, R.K., Eds.; IntechOpen: London, UK, 2018.
- Chen, Z.; Wei, W.; Song, L.; Ni, B.-J. Hybrid Water Electrolysis: A New Sustainable Avenue for Energy-Saving Hydrogen Production. Sustain. Horiz. 2021, 1, 100002.
- Saravanan, A.; Karishma, S.; Senthil Kumar, P.; Yaashikaa, P.R.; Jeevanantham, S.; Gayathri, B. Microbial electrolysis cells and microbial fuel cells for biohydrogen production: Current advances and emerging challenges. Biomass Conv. Bioref. 2020.
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Hefny, M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Hydrogen production, storage, utilisation and environmental impacts: A review. Environ. Chem. Lett. 2021, 20, 153–188.
- Elgarahy, A.M.; Eloffy, M.G.; Hammad, A.; Saber, A.N.; El-Sherif, D.M.; Mohsen, A.; Abouzid, M.; Elwakeel, K.Z. Hydrogen production from wastewater, storage, economy, governance and applications: A review. Environ. Chem. Lett. 2022.
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869.
- Liu, Y.; Wang, F.; Jiao, Z.; Bai, S.; Qiu, H.; Guo, L. Photochemical Systems for Solar-to-Fuel Production. Electrochem. Energy Rev. 2022, 5, 5.
- Wang, J.; Zhang, Z.; Ding, J.; Zhong, C.; Deng, Y.; Han, X.; Hu, W. Recent progresses of micro-nanostructured transition metal compound-based electrocatalysts for energy conversion technologies. Sci. China Mater. 2021, 64, 1–26.
- Wang, H.-Y.; Weng, C.-C.; Ren, J.-T.; Yuan, Z.-Y. An overview and recent advances in electrocatalysts for direct seawater splitting. Front. Chem. Sci. Eng. 2021, 15, 1408–1426.
- Khan, M.A.; Zhao, H.; Zou, W.; Chen, Z.; Cao, W.; Fang, J.; Xu, J.; Zhang, L.; Zhang, J. Recent Progresses in Electrocatalysts for Water Electrolysis. Electrochem. Energy Rev. 2018, 1, 483–530.
- Wang, T.; Cao, X.; Jiao, L. PEM water electrolysis for hydrogen production: Fundamentals, advances, and prospects. Carbon Neutrality 2022, 1, 21.
- Li, X.; Zhao, L.; Yu, J.; Liu, X.; Zhang, X.; Liu, H.; Zhou, W. Water Splitting: From Electrode to Green Energy System. Nano-Micro Lett. 2020, 12, 131.
- Gong, M.; Wang, D.-Y.; Chen, C.-C.; Hwang, B.-J.; Dai, H. A mini review on nickel-based electrocatalysts for alkaline hydrogen evolution reaction. Nano Res. 2015, 9, 28–46.
- Saba, S.M.; Müller, M.; Robinius, M.; Stolten, D. The investment costs of electrolysis–A comparison of cost studies from the past 30 years. Int. J. Hydrogen Energy 2018, 43, 1209–1223.
- Platzer, M.F.; Sarigul-Klijn, N. Hydrogen Production Methods. In The Green Energy Ship Concept; SpringerBriefs in Applied Sciences and Technology; Springer: Berlin/Heidelberg, Germany, 2021.
- Esposito, D.V. Membraneless Electrolyzers for Low-Cost Hydrogen Production in a Renewable Energy Future. Joule 2017, 1, 887.
- Esmaeilion, F. Hybrid renewable energy systems for desalination. Appl. Water Sci. 2020, 10, 84.





