Seawater is the most abundant supply of water and the ideal and cheapest electrolyte. Because it is a green and renewable chemical process, water electrolysis has earned a lot of interest among the different hydrogen production techniques. Basis of water electrolysis include general theoretical concepts: chemical, physical, and electrochemical concepts. Research has focused on the specific seawater electrolysis parameters: cathodic evolution of hydrogen; concurrent anodic evolution of oxygen and chlorine; specific seawater catalyst electrodes, and seawater electrolyzer efficiency. A sustainable technology development must also capitalize on known and emerging technologies; protecting the environment; utilization of green, renewable energies as sources of electricity; and above all, economic efficiency as a whole.
- seawater electrolysis for hydrogen production
- electrocatalyst
- sustainability
1. Introduction
2. An Overview of Water Electrochemistry
From a broad standpoint, seawater and water electrolysis have very similar electrochemical behavior: At the cathode, reduction reactions (electrons acceptance) take place, while at the anode, oxidation reactions take place (electrons releasing). Figure 1 presents a general scheme of water electrolysis that is also valid for an alkaline electrolyzer (AE). What creates a difference is the electrolyte, which can be water with additional bases, acids, or salts (as in seawater). Depending on the physico-chemical and technological operating parameters of the electrolysis cell, different secondary reactions may take place at both electrodes depending on the nature of the electrolyte. These reactions may affect the efficiency of the cell, the yield of hydrogen production, and the consumption of raw materials and electricity. The abovementioned issues regarding hydrogen production from water electrolysis lead to the conclusion that this solution is far from an optimum one even though it appears to be a straightforward electrochemical reaction. The subject will remain of high interest for researchers in order to discover the best answer in terms of energy efficiency and costs, even though research and technical advancement in recent years has brought technology extremely near commercial solutions. The electrochemical process called water electrolysis produces extremely pure hydrogen and oxygen. Due to its high purity, electrolytic hydrogen is frequently utilized in the chemical industry, particularly in the energy sector, or for smaller applications such as the semiconductor and food sectors. Hydrogen is also employed in catalytic hydrogenation reactions and ammonia production.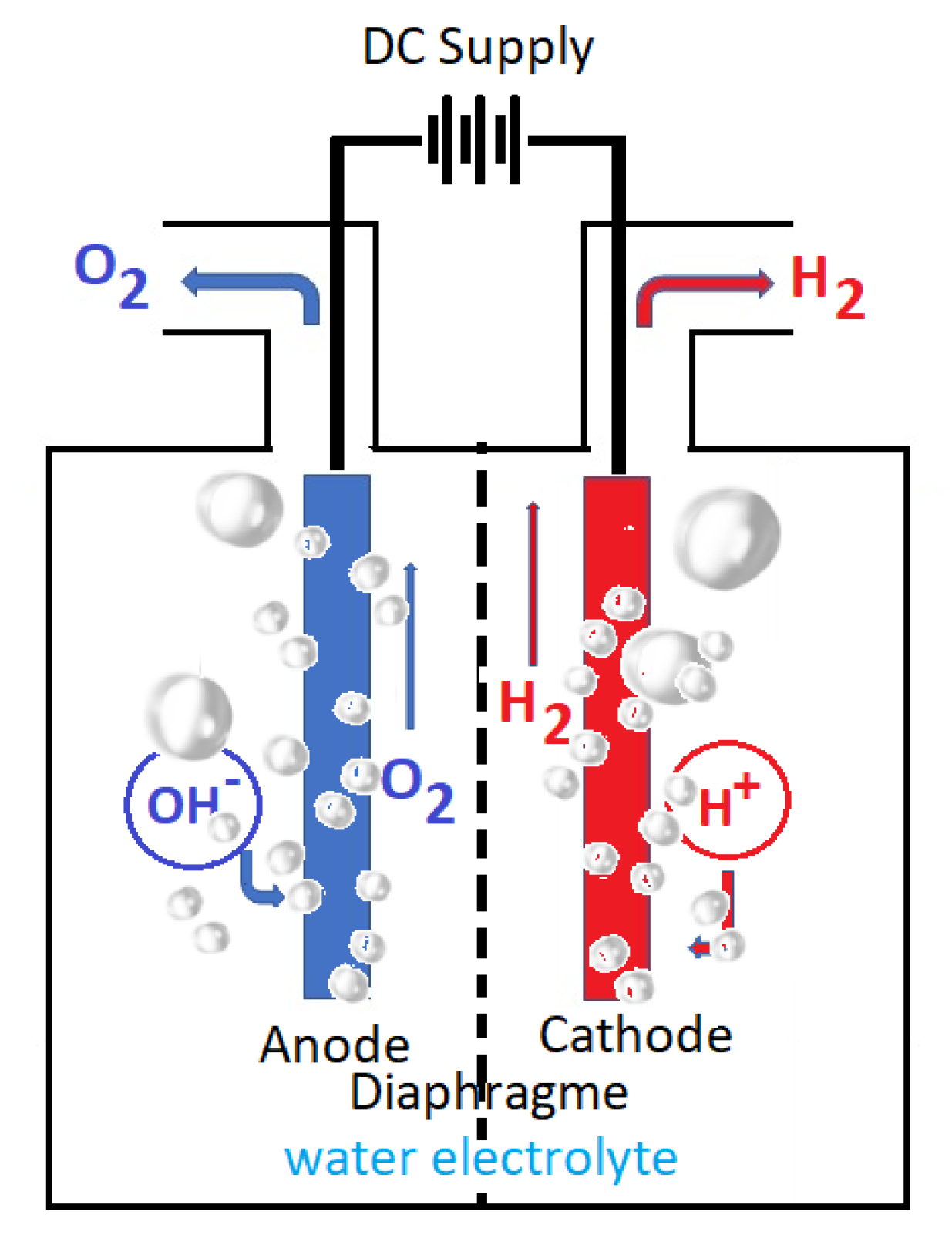
References
- Wang, S.; Lu, A.; Zhong, C.-J. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 4.
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934.
- Naimi, Y.; Antar, A. Hydrogen Generation by Water Electrolysis. In Advances In Hydrogen Generation Technologies; IntechOpen: London, UK, 2018.
- Rashid, M.; Khaloofah Al Mesfer, M.; Naseem, H.; Danish, M. Hydrogen Production by Water Electrolysis: A Review of Alkaline Water Electrolysis, PEM Water Electrolysis and High Temperature Water Electrolysis. Int. J. Eng. Adv. Technol. (IJEAT) 2015, 4, 3.
- Chisholm, G.; Cronin, L. Hydrogen from Water Electrolysi s School of Chemistry, University of Glasgow, Glasgow, United Kingdom. 2016. Available online: http://www.chem.gla.ac.uk/cronin/images/pubs/Chisholm-Chapter_16_2016.pdf (accessed on 27 April 2022).
- Hora, C.; Dan, F.C.; Rancov, N.; Badea, G.E.; Secui, C. Main Trends and Research Directions in Hydrogen Generation Using Low Temperature Electrolysis: A Systematic Literature Review. Energies 2022, 15, 6076.
- Pérez Orosa, L.; Chinarro, E.; Guinea, D.; García-Alegre, M.C. Hydrogen Production by Wastewater Alkaline Electro-Oxidation. Energies 2022, 15, 5888.
- Jenkins, B.; Squires, D.; Barton, J.; Strickland, D.; Wijayantha, K.G.U.; Carroll, J.; Wilson, J.; Brenton, M.; Thomson, M. Techno-Economic Analysis of Low Carbon Hydrogen Production from Offshore Wind Using Battolyser Technology. Energies 2022, 15, 5796.
- Lee, C.H.; Lee, S.U. Theoretical Basis of Electrocatalysis. In Electrocatalysts for Fuel Cells and Hydrogen Evolution-Theory to Design; Ray, A., Mukhopadhyay, I., Pati, R.K., Eds.; IntechOpen: London, UK, 2018.
- Chen, Z.; Wei, W.; Song, L.; Ni, B.-J. Hybrid Water Electrolysis: A New Sustainable Avenue for Energy-Saving Hydrogen Production. Sustain. Horiz. 2021, 1, 100002.
- Saravanan, A.; Karishma, S.; Senthil Kumar, P.; Yaashikaa, P.R.; Jeevanantham, S.; Gayathri, B. Microbial electrolysis cells and microbial fuel cells for biohydrogen production: Current advances and emerging challenges. Biomass Conv. Bioref. 2020.
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Hefny, M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Hydrogen production, storage, utilisation and environmental impacts: A review. Environ. Chem. Lett. 2021, 20, 153–188.
- Elgarahy, A.M.; Eloffy, M.G.; Hammad, A.; Saber, A.N.; El-Sherif, D.M.; Mohsen, A.; Abouzid, M.; Elwakeel, K.Z. Hydrogen production from wastewater, storage, economy, governance and applications: A review. Environ. Chem. Lett. 2022.
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869.
- Liu, Y.; Wang, F.; Jiao, Z.; Bai, S.; Qiu, H.; Guo, L. Photochemical Systems for Solar-to-Fuel Production. Electrochem. Energy Rev. 2022, 5, 5.
- Wang, J.; Zhang, Z.; Ding, J.; Zhong, C.; Deng, Y.; Han, X.; Hu, W. Recent progresses of micro-nanostructured transition metal compound-based electrocatalysts for energy conversion technologies. Sci. China Mater. 2021, 64, 1–26.
- Wang, H.-Y.; Weng, C.-C.; Ren, J.-T.; Yuan, Z.-Y. An overview and recent advances in electrocatalysts for direct seawater splitting. Front. Chem. Sci. Eng. 2021, 15, 1408–1426.
- Khan, M.A.; Zhao, H.; Zou, W.; Chen, Z.; Cao, W.; Fang, J.; Xu, J.; Zhang, L.; Zhang, J. Recent Progresses in Electrocatalysts for Water Electrolysis. Electrochem. Energy Rev. 2018, 1, 483–530.
- Wang, T.; Cao, X.; Jiao, L. PEM water electrolysis for hydrogen production: Fundamentals, advances, and prospects. Carbon Neutrality 2022, 1, 21.
- Li, X.; Zhao, L.; Yu, J.; Liu, X.; Zhang, X.; Liu, H.; Zhou, W. Water Splitting: From Electrode to Green Energy System. Nano-Micro Lett. 2020, 12, 131.
- Gong, M.; Wang, D.-Y.; Chen, C.-C.; Hwang, B.-J.; Dai, H. A mini review on nickel-based electrocatalysts for alkaline hydrogen evolution reaction. Nano Res. 2015, 9, 28–46.
- Saba, S.M.; Müller, M.; Robinius, M.; Stolten, D. The investment costs of electrolysis–A comparison of cost studies from the past 30 years. Int. J. Hydrogen Energy 2018, 43, 1209–1223.
- Platzer, M.F.; Sarigul-Klijn, N. Hydrogen Production Methods. In The Green Energy Ship Concept; SpringerBriefs in Applied Sciences and Technology; Springer: Berlin/Heidelberg, Germany, 2021.
- Esposito, D.V. Membraneless Electrolyzers for Low-Cost Hydrogen Production in a Renewable Energy Future. Joule 2017, 1, 887.
- Esmaeilion, F. Hybrid renewable energy systems for desalination. Appl. Water Sci. 2020, 10, 84.
