
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nimer najib Assy | -- | 1279 | 2022-11-22 11:40:52 | | | |
| 2 | Beatrix Zheng | Meta information modification | 1279 | 2022-11-23 02:27:42 | | | | |
| 3 | Beatrix Zheng | + 4 word(s) | 1283 | 2022-11-23 02:29:11 | | |
Video Upload Options
Non-alcoholic fatty liver disease (NAFLD) is defined by the presence of >5% of hepatic steatosis demonstrated, either radiographically or histologically, in the absence of significant alcohol consumption. NAFLD is the most common liver disease worldwide, with a continuously growing prevalence. The pathophysiology of the disease is complex and includes several mechanisms, with metabolic syndrome and insulin resistance playing a major role. It is crucial to diagnose NAFLD before it advances to nonalcoholic steatohepatitis (NASH), which can progress to cirrhosis, presented by its complications which include ascites, portal hypertension, bleeding varices and encephalopathy. Another important complication of NAFLD and cirrhosis is hepatocellular carcinoma (HCC), a cancer with increasing incidence and poor prognosis. Even with the growing prevalence of NAFLD, diagnosis via liver biopsies is unrealistic, considering the costs and complications. Noninvasive tests, including serum biomarkers and elastography, are cost-effective and convenient, thereby replacing liver biopsies in diagnosing and excluding liver fibrosis
1. NAFLD Represents a Wide Spectrum of Diseases
2. Metabolic Profile of NAFLD
2.1. The Role of Adipose Tissue in NAFLD
2.2. Lipotoxicity in NAFLD
2.3. The Role of Glucose in NAFLD
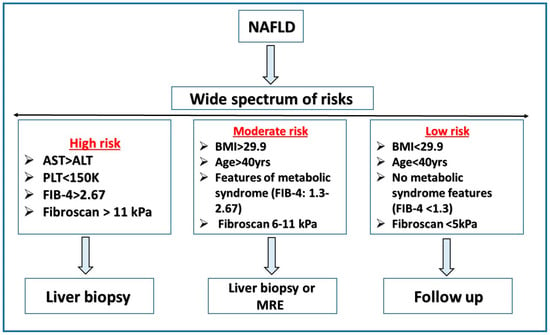
3. Risk Stratification
References
- Targher, G.; Corey, K.E.; Byrne, C.D.; Roden, M. The complex link between NAFLD and type 2 diabetes mellitus—Mechanisms and treatments. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 599–612.
- Juanola, O.; Martínez-López, S.; Francés, R.; Gómez-Hurtado, I. Non-alcoholic fatty liver disease: Metabolic, genetic, epigenetic and environmental risk factors. Int. J. Environ. Res. Public Health 2021, 18, 5227.
- Cardoso, A.C.; de Figueiredo-Mendes, C.; Villela-Nogueira, C.A. Current management of NAFLD/NASH. Liver Int. 2021, 41, 89–94.
- Armandi, A.; Bugianesi, E. Natural history of NASH. Liver Int. 2021, 41 (Suppl. 1), 78–82.
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224.
- Taylor, R.S.; Taylor, R.J.; Bayliss, S.; Hagström, H.; Nasr, P.; Schattenberg, J.M.; Ishigami, M.; Toyoda, H.; Wong, V.W.-S.; Peleg, N.; et al. Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology 2020, 158, 1611–1625.e12.
- Ioannou, G.N. Epidemiology and risk-stratification of NAFLD-associated HCC. J. Hepatol. 2021, 75, 1476–1484.
- Calzadilla Bertot, L.; Adams, L.A. The Natural Course of Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2016, 17, 774.
- Zheng, Q.; Martin, R.C.; Shi, X.; Pandit, H.; Yu, Y.; Liu, X.; Guo, W.; Tan, M.; Bai, O.; Meng, X.; et al. Lack of FGF21 promotes NASH-HCC transition via hepatocyte-TLR4-IL-17A signaling. Theranostics 2020, 10, 9923–9936.
- Allen, A.M.; Hicks, S.B.; Mara, K.C.; Larson, J.J.; Therneau, T.M. The risk of incident extrahepatic cancers is higher in non-alcoholic fatty liver disease than obesity—A longitudinal cohort study. J. Hepatol. 2019, 71, 1229–1236.
- Lombardi, R.; Iuculano, F.; Pallini, G.; Fargion, S.; Fracanzani, A.L. Nutrients, Genetic Factors, and Their Interaction in Non-Alcoholic Fatty Liver Disease and Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 8761.
- Sakurai, Y.; Kubota, N.; Yamauchi, T.; Kadowaki, T. Role of Insulin Resistance in MAFLD. Int. J. Mol. Sci. 2021, 22, 4156.
- Watt, M.J.; Miotto, P.M.; De Nardo, W.; Montgomery, M. The Liver as an Endocrine Organ—Linking NAFLD and Insulin Resistance. Endocr. Rev. 2019, 40, 1367–1393.
- Maurice, J.; Manousou, P. Non-alcoholic fatty liver disease. Clin. Med. 2018, 18, 245–250.
- Huang, R.; Zhu, L.; Wang, J.; Xue, L.; Liu, L.; Yan, X.; Huang, S.; Li, Y.; Yan, X.; Zhang, B.; et al. Clinical features of COVID-19 patients with non-alcoholic fatty liver disease. Hepatol. Commun. 2020, 4, 1758–1768.
- Ji, D.; Qin, E.; Xu, J.; Zhang, D.; Cheng, G.; Wang, Y.; Lau, G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J. Hepatol. 2020, 73, 451–453.
- Berlanga, A.; Guiu-Jurado, E.; Porras, J.A.; Auguet, T. Molecular Pathways in Non-Alcoholic Fatty Liver Disease. Clin. Exp. Gastroenterol. 2014, 7, 221–239.
- Abenavoli, L.; Milic, N.; Di Renzo, L.; Preveden, T.; Medić-Stojanoska, M.; De Lorenzo, A. Metabolic aspects of adult patients with nonalcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 7006–7016.
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048.
- Makri, E.; Goulas, A.; Polyzos, S.A. Epidemiology, Pathogenesis, Diagnosis and Emerging Treatment of Nonalcoholic Fatty Liver Disease. Arch. Med. Res. 2020, 52, 25–37.
- Milić, S.; Lulić, D.; Štimac, D. Non-alcoholic fatty liver disease and obesity: Biochemical, metabolic and clinical presentations. World J. Gastroenterol. 2014, 20, 9330–9337.
- Shabalala, S.C.; Dludla, P.V.; Mabasa, L.; Kappo, A.P.; Basson, A.K.; Pheiffer, C.; Johnson, R. The Effect of Adiponectin in the Pathogenesis of Non-Alcoholic Fatty Liver Disease (NAFLD] and the Potential Role of Polyphenols in the Modulation of Adiponectin Signaling. Biomed. Pharmacother. 2020, 131, 110785.
- Polyzos, S.A.; Aronis, K.; Kountouras, J.; Raptis, D.D.; Vasiloglou, M.; Mantzoros, C.S. Circulating leptin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Diabetologia 2015, 59, 30–43.
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 2019, 92, 82–97.
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Leptin in nonalcoholic fatty liver disease: A narrative review. Metabolism 2015, 64, 60–78.
- Stojsavljević, S.; Gomerčić Palčić, M.; Virović Jukić, L.; Smirčić Duvnjak, L.; Duvnjak, M. Adipokines and proinflammatory cytokines, the key mediators in the pathogenesis of nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 18070–18091.
- Svegliati-Baroni, G.; Pierantonelli, I.; Torquato, P.; Marinelli, R.; Ferreri, C.; Chatgilialoglu, C.; Bartolini, D.; Galli, F. Lipidomic biomarkers and mechanisms of lipotoxicity in non-alcoholic fatty liver disease. Free Radic. Biol. Med. 2019, 144, 293–309.
- Branković, M.; Jovanović, I.; Dukić, M.; Radonjić, T.; Oprić, S.; Klašnja, S.; Zdravković, M. Lipotoxicity as the Leading Cause of Non-Alcoholic Steatohepatitis. Int. J. Mol. Sci. 2022, 23, 5146.
- Basaranoglu, M.; Basaranoglu, G.; Bugianesi, E. Carbohydrate intake and nonalcoholic fatty liver disease: Fructose as a weapon of mass destruction. Hepatobiliary Surg. Nutr. 2015, 4, 109–116.
- Gjorgjieva, M.; Mithieux, G.; Rajas, F. Hepatic stress associated with pathologies characterized by disturbed glucose production. Cell Stress 2019, 3, 86–99.
- Xian, Y.X.; Weng, J.P.; Xu, F. MAFLD vs. NAFLD: Shared Features and Potential Changes in Epidemiology, Pathophysiology, Diagnosis, and Pharmacotherapy. Chin. Med. J. 2020, 134, 8–19.
- Dyson, J.K.; McPherson, S.; Anstee, Q.M. Republished: Non-alcoholic fatty liver disease: Non-invasive investigation and risk stratification. Postgrad. Med. J. 2014, 90, 254–266.
- Blank, V.; Petroff, D.; Beer, S.; Böhlig, A.; Heni, M.; Berg, T.; Bausback, Y.; Dietrich, A.; Tönjes, A.; Hollenbach, M.; et al. Current NAFLD guidelines for risk stratification in diabetic patients have poor diagnostic discrimination. Sci. Rep. 2020, 10, 18345.
- Rinella, M.E.; Sanyal, A.J. Management of NAFLD: A stage-based approach. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 196–205.




