Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ilya D. Klabukov | -- | 1810 | 2022-11-21 11:17:09 | | | |
| 2 | Rita Xu | -3 word(s) | 1807 | 2022-11-22 03:18:18 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Krasilnikova, O.A.; Baranovskii, D.S.; Yakimova, A.O.; Arguchinskaya, N.; Kisel, A.; Sosin, D.; Sulina, Y.; Ivanov, S.A.; Shegay, P.V.; Kaprin, A.D.; et al. Tissue-Engineered Grafts with Minimally Manipulated Cells. Encyclopedia. Available online: https://encyclopedia.pub/entry/35546 (accessed on 07 March 2026).
Krasilnikova OA, Baranovskii DS, Yakimova AO, Arguchinskaya N, Kisel A, Sosin D, et al. Tissue-Engineered Grafts with Minimally Manipulated Cells. Encyclopedia. Available at: https://encyclopedia.pub/entry/35546. Accessed March 07, 2026.
Krasilnikova, Olga A., Denis S. Baranovskii, Anna O. Yakimova, Nadezhda Arguchinskaya, Anastas Kisel, Dmitry Sosin, Yana Sulina, Sergey A. Ivanov, Peter V. Shegay, Andrey D. Kaprin, et al. "Tissue-Engineered Grafts with Minimally Manipulated Cells" Encyclopedia, https://encyclopedia.pub/entry/35546 (accessed March 07, 2026).
Krasilnikova, O.A., Baranovskii, D.S., Yakimova, A.O., Arguchinskaya, N., Kisel, A., Sosin, D., Sulina, Y., Ivanov, S.A., Shegay, P.V., Kaprin, A.D., & Klabukov, I.D. (2022, November 21). Tissue-Engineered Grafts with Minimally Manipulated Cells. In Encyclopedia. https://encyclopedia.pub/entry/35546
Krasilnikova, Olga A., et al. "Tissue-Engineered Grafts with Minimally Manipulated Cells." Encyclopedia. Web. 21 November, 2022.
Copy Citation
Transfer of regenerative approaches into clinical practice is limited by strict legal regulation of in vitro expanded cells and risks associated with substantial manipulations. Isolation of cells for the enrichment of bone grafts directly in the Operating Room appears to be a promising solution for the translation of biomedical technologies into clinical practice. These intraoperative approaches could be generally characterized as a joint concept of tissue engineering in situ.
bone repair
cell therapy
in situ
minimally manipulated cells
1. Introduction
Translation of regenerative medical approaches into routine clinical practice is limited by strict legal regulations of cultured cells. Legal restrictions are intended to protect patients from the risks associated with substantial cell manipulation [1][2][3]. Therefore, tissue-engineered grafts created directly in the operating room (O.R.) with minimal manipulation of cells appear to be extremely promising.
Intraoperative tissue engineering in situ demonstrated its effectiveness in recent clinical studies [4]. Traditionally, the term ‘tissue engineering in situ’ means different approaches to creating grafts that will mature into functionally active tissue inside the recipient’s body using its own regenerative potential [5]. However, researchers believe that the term ‘tissue engineering in situ’ should be applied to the process of intraoperative creation of tissue-engineered grafts with their implantation during the same surgical procedure. The obvious advantage of in situ tissue-engineered grafts is off-the-shelf availability, while traditional full-cycle tissue engineering requires sufficient time and financial expenses for cell culture in vitro. Importantly, tissue-engineered grafts created during surgical procedures can be enriched with cells isolated intraoperatively with minimal manipulation.
Traditional tissue engineering in situ does not include scaffold enrichment with cells since it requires in vitro culturing [5]. However, existing techniques and devices allow intraoperative cell isolation and cell seeding on scaffolds. The osteoconductive and osteoinductive properties of scaffolds are required for successful bone regeneration. However, the addition of a cellular component to various types of scaffolds can bring additional therapeutic value. Enrichment of scaffolds with intraoperatively isolated cells is an effective tool to create a unique milieu around the graft, enhance its maturation, and stimulate the intrinsic regenerative potential of the recipient’s body. In particular, seeding with bone marrow-derived mononuclear cells can contribute to angiogenesis in the implantation area [6]. Mesenchymal stem cells (MSCs) are one of the most important cell types within mononuclear and stromal vascular fractions. Tissue engineering in situ may benefit from MSCs due to their ability to reduce inflammation and promote angiogenesis. Also, scaffold enrichment with MSCs in situ may be of value due to the MSC potential for osteogenic differentiation. Calcium phosphate-containing scaffolds are shown to promote MSC differentiation into osteoblasts [7]. Notably, MSCs seeded on calcium phosphate-containing scaffolds underwent osteogenic differentiation and formed bone tissue in vivo earlier than embryonic stem cells [8]. Enrichment with stromal vascular fraction cells has also been shown to enhance the effectiveness of osteoinductive scaffolds in a critical-size cranial defect in mice [9].
The understanding of tissue engineering in situ as a process of intraoperative creation of tissue-engineered graft comprises the possibility of scaffold enrichment with intraoperatively isolated minimally manipulated cells (Figure 1). These cells can be isolated intraoperatively from structural and non-structural tissues including bone marrow, adipose tissue, skin, mucous tissue, etc., seeded on the scaffold and implanted in a patient in a one-step procedure [10][11]. In contrast, tissue engineering with cultured cells requires two separate interventions (e.g., bone marrow aspiration/adipose tissue harvesting and surgical implantation of created tissue-engineered graft), authorized manufacturing facilities, and compliance with special regulations for advanced therapy medicinal products [2][12].
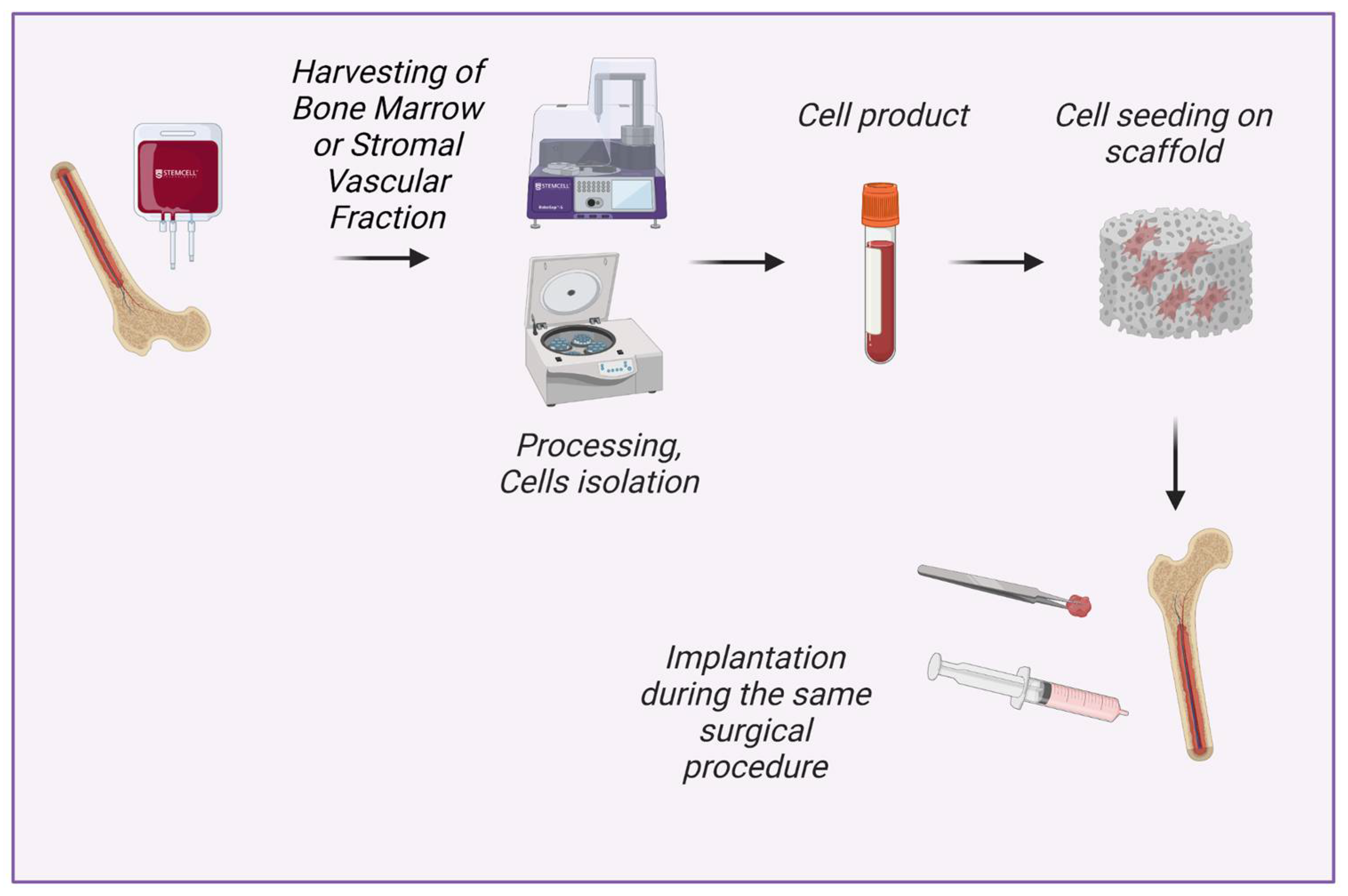
Figure 1. New concept of tissue engineering in situ: intraoperative isolation of cells with minimal manipulation, cell seeding on scaffold and implantation during the same surgical procedure. Created with BioRender.com.
The concept of intraoperative tissue engineering in situ does not contradict traditional tissue engineering in situ since the enrichment of scaffolds with intraoperatively isolated cells may contribute to the stimulation of the recipient’s own regenerative potential [13].
2. Cells Used for Bone Tissue Engineering In Situ
The use of in vitro cultured cells has been shown to enhance bone healing in multiple studies. In particular, it helped to treat non-unions [14][15]. Researchers will consider cells that have shown their effectiveness for bone regeneration and that can be isolated during a surgical procedure with minimal manipulations.
Cells contained in the bone marrow mononuclear fraction are often used to create tissue-engineered grafts in the operating room, although in some clinical studies scaffolds were enriched with bone marrow aspirate concentrate or with non-concentrated bone marrow aspirate [16][17].
Bone marrow mononuclear cells (BM-MNCs) are widely used in clinical practice alone or in combination with various scaffolds, including bone autografts, hydroxylapatite, β-tricalcium phosphate, etc. Multiple clinical and animal studies demonstrated results in improved bone healing [4][18][19]. Importantly, BM-MNCs are available as minimally-manipulated cells without preliminary cell culture [20]. BM-MNCs are a heterogeneous population of cells with a single nucleus, which includes mesenchymal stromal and stem cells, hematopoietic stem cells, endothelial progenitor cells, very small embryonic-like stem cells, embryonic stem cells, etc. The presence of different cell types in the mononuclear fraction causes combinative effects on regeneration. In particular, endothelial progenitor cells promote vascularization, and mesenchymal stem cells reduce inflammation.
Mesenchymal stem cells (MSCs) as well as mesenchymal stromal cells are components of BM-MNCs and one of the key players in tissue regeneration due to their ability to reduce inflammation, express growth factors, and differentiate into osteoblasts [21]. MSCs represent the population of osteoblasts progenitors in the bone marrow. Interestingly, ultrasound microwave irradiation was used for stimulation of MSCs osteo-differentiation in culture [22]. A combination of various scaffolds and cultured bone marrow-derived MSCs (BM-MSCs) has been used for the treatment of bone fractures or resorption in multiple clinical and animal studies [14][19][23][24]. Importantly, the combination of uncultured BM-MNCs and β-tricalcium phosphate resulted in enhanced bone regeneration and mineralization in vivo compared to MSCs cultured on the same scaffold [19].
For purposes of intraoperative tissue engineering in situ, MSCs can be isolated from other sources besides bone marrow. Intraoperative enrichment with adipose tissue-derived MSCs (AD-MSCs) could be achieved by the adhesion of stromal vascular fraction (SVF) cells to the scaffold. In that case, AD-MSCs will be delivered together with the other SVF components. The reparative success of SVF is also based on contained endothelial progenitor cells, M2-macrophages, smooth muscle cells, preadipocytes, etc. [25][26]. Those cells provide an essential microenvironment for MSCs and regulate their differentiation in an osteogenic direction via cell-to-cell cross-talks [27]. Also, known regenerative effects of SVF cells include improvement of angiogenesis and secretion of extracellular matrix. The chain of effects results in the ability of SVF to mineralize decellularized bone matrix and polycaprolactone and to increase new bone formation in vivo [9][28][29]. An experimental study in vitro showed that bone marrow-derived MSCs have even more osteogenic potential than adipose-derived cells [30]. However, multiple animal studies have shown no statistical difference between BM-MSCs and AD-MSCs in terms of bone regeneration [31][32]. At the same time, cell yield (incl. stem cell yield) is generally higher in adipose tissue than in bone marrow aspirate [33].
One of the promising directions is the isolation of MSCs from gingival tissues [34][35]. Some studies showed that gingiva-derived MSCs had better results in terms of staining for ALP; mineralized nodule formation; and APL, OSX, and RUNX2 gene expression compared to BM-MSCs in mice [36]. Among the advantages of isolating MSCs from the gingiva is minimal surgical intervention, as well as fast scarless healing of the gingival donor site [37]. Enzymatic treatment of gingival tissue takes around 2 h allowing the scaffold to be intraoperatively enriched with resulting suspension that contains gingiva-derived MSCs [37].
Sufficient vascularization is crucial for the regeneration of bone defects [38]. Endothelial progenitor cells (EPCs) have been shown to enhance vascularization in several studies [39][40]. EPC is included in the mononuclear fraction of bone marrow and also are found in SVF and peripheral blood.
3. Materials for Bone Tissue Engineering In Situ
The type of implantable materials is a key factor in maintaining the shape and mechanical properties of the bone graft after implantation [41]. All materials for bone tissue engineering are mainly divided into non-resorbable (e.g., ceramic and polymeric grafts) and bioresorbable materials [42][43][44]. Various osteoinductive materials, such as beta-tricalcium phosphate (β-TCP) [45], hydroxyapatite [46], and xenografts [47] are commonly used as scaffolds in preclinical studies and clinical cases due to their commercial availability. Recent evidence also suggested bioactive glass ceramics as a suitable material for bone tissue engineering, it being capable of the promotion of osteogenesis [48].
Notably, hydroxyapatite can also be in a resorbable form. The effectiveness of resorbable high-porous acellular hydroxyapatite scaffolds is generally based on the enhanced local regenerative potential [49][50]. Resorbable scaffolds could be a suitable solution for cartilage and bone tissue engineering [51][52][53]. While the mobilization of patients’ own osteoblasts leads to the revitalization of the graft, the scaffold could be completely replaced by a newly formed extracellular matrix.
Despite numerous types of scaffolds, bone graft autotransplantation is still considered a ‘gold-standard’ approach to repairing bone defects (Figure 2a). However, a well-known method of autologous osteoplasty is commonly limited by the harvested bone volume and associated with an additional risk of complications in the donor site [20][54].
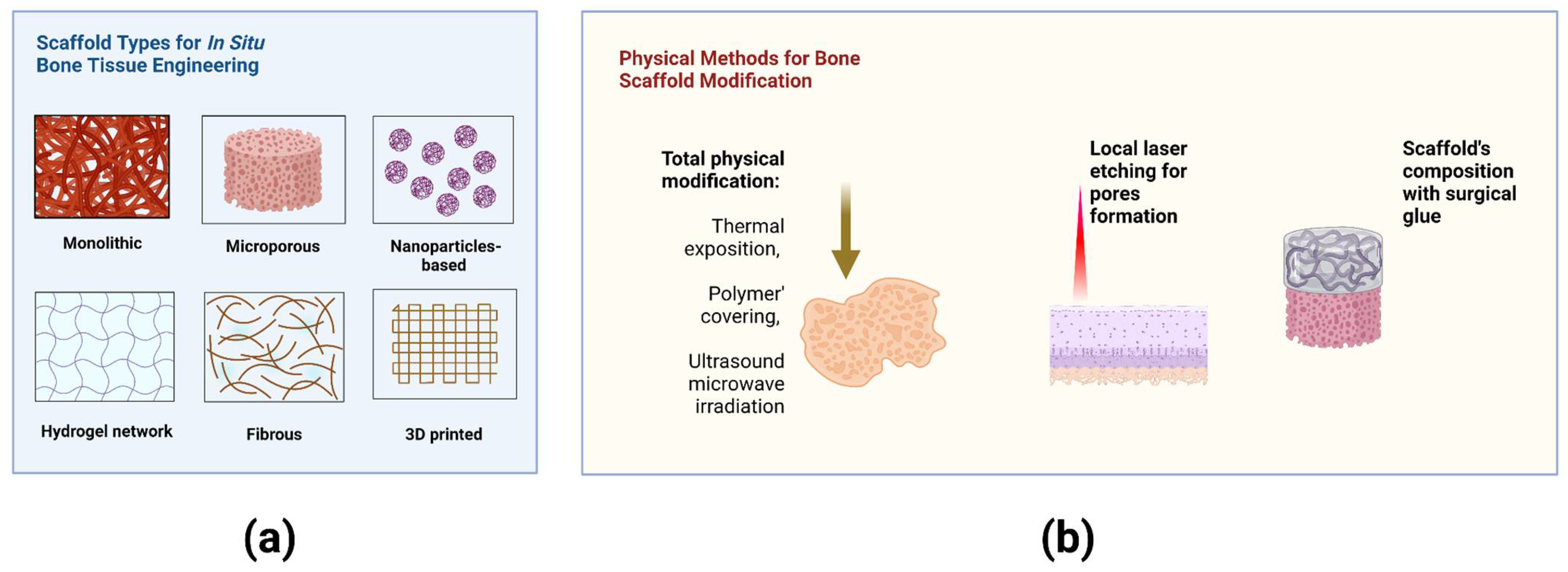
Figure 2. (a) Scaffold types for bone tissue engineering in situ. (b) Methods for physical modification of scaffolds immediately in the Operating Room. Created with BioRender.com.
The material-related complications remain to pose a sufficient risk due to individual response after implantation [55][56]. Insufficient vascularization and inflammatory response are often observed in the bone regeneration zone [57]. Bone tissue engineering in situ through BM-MSCs osteogenic differentiation may be performed using immobilized gene delivery nanocomplexes [58]. Bioactive scaffolds with modified surface properties or gene-activated materials have promising applications as scaffolds for cell seeding [59][60].
Promising physical approaches allow the modification of biomaterials and cells immediately in the Operating Room (O.R.). Laser engraving of cartilage led to improved deep chondrocyte migration into the scaffold after implantation [52][61]. The nonselective damaged cartilage also stimulated cell migration via cell secretion activity [62][63]. The methods for modification of bone scaffolds and their properties are summarized in Figure 2b.
Various materials are used in bone tissue engineering, and, notably, the adhesion capacity of BM-MNCs differs depending on the type of material. Henrich et al. (2015) showed that coating of β-TCP with fibronectin and human plasma does not increase the adhesion capacity of human BM-MNCs isolated by density gradient centrifugation, at the same time the percentage of attached cells was higher in β-TCP and demineralized bone matrix compared to bovine cancellous bone [64]. The comparative effectiveness of implantation of various materials for the treatment of large bone defects in rats showed that the fibrous demineralized bone matrix seeded with centrifugation-isolated BM-MNCs led to promising results in comparison with syngeneic cancellous bone implantation [65]. Centrifugation-isolated BM-MNCs were seeded on human demineralized bone matrix, bovine cancellous bone hydroxyapatite ceramic, and β-tricalcium phosphate scaffolds. The study showed that the effectiveness of BMC-supported therapy could be influenced by the type of scaffold. Although the demineralized bone matrix was superior in comparison to β-tricalcium phosphate and bovine cancellous bone hydroxyapatite ceramic, the level of autologous bone could not be attained [66].
References
- Gardner, J.; Faulkner, A.; Mahalatchimy, A.; Webster, A. Are there specific translational challenges in regenerative medicine? Lessons from other fields. Regen. Med. 2015, 10, 885–895.
- Qiu, T.; Hanna, E.; Dabbous, M.; Borislav, B.; Toumi, M. Regenerative medicine regulatory policies: A systematic review and international comparison. Health Policy 2020, 124, 701–713.
- Yamada, S.; Behfar, A.; Terzic, A. Regenerative medicine clinical readiness. Regen. Med. 2021, 16, 309–322.
- Du, F.; Wu, H.; Li, H.; Cai, L.; Wang, Q.; Liu, X.; Xiao, R.; Yin, N.; Cao, Y. Bone marrow mononuclear cells combined with beta-tricalcium phosphate granules for alveolar cleft repair: A 12-month clinical study. Sci. Rep. 2017, 7, 13773.
- Sengupta, D.; Waldman, S.D.; Li, S. From in vitro to in situ tissue engineering. Ann. Biomed. Eng. 2014, 42, 1537–1545.
- Umemura, T.; Nishioka, K.; Igarashi, A.; Kato, Y.; Ochi, M.; Chayama, K.; Yoshizumi, M.; Higashi, Y. Autologous bone marrow mononuclear cell implantation induces angiogenesis and bone regeneration in a patient with compartment syndrome. Circ. J. 2006, 70, 1362–1364.
- Müller, P.; Bulnheim, U.; Diener, A.; Lüthen, F.; Teller, M.; Klinkenberg, E.D.; Neumann, H.-G.; Nebe, B.; Liebold, A.; Steinhoff, G.; et al. Calcium phosphate surfaces promote osteogenic differentiation of mesenchymal stem cells. J. Cell. Mol. Med. 2008, 12, 281–291.
- Wen, C.; Kang, H.; Shih, Y.R.V.; Hwang, Y.; Varghese, S. In vivo comparison of biomineralized scaffold-directed osteogenic differentiation of human embryonic and mesenchymal stem cells. Drug Deliv. Transl. Res. 2016, 6, 121–131.
- Nyberg, E.; Farris, A.; O’Sullivan, A.; Rodriguez, R.; Grayson, W. Comparison of stromal vascular fraction and passaged adipose-derived stromal/stem cells as point-of-care agents for bone regeneration. Tissue Eng. Part A 2019, 25, 1459–1469.
- Krasilnikova, O.A.; Klabukov, I.D.; Baranovskii, D.S.; Shegay, P.V.; Kaprin, A.D. The new legal framework for minimally manipulated cells expands the possibilities for cell therapy in Russia. Cytotherapy 2021, 23, 754–755.
- Chu, W.; Wang, X.; Gan, Y.; Zhuang, Y.; Shi, D.; Liu, F.; Sun, Y.; Zhao, J.; Tang, T.; Dai, K. Screen-enrich-combine circulating system to prepare MSC/β-TCP for bone repair in fractures with depressed tibial plateau. Regen. Med. 2019, 14, 555–569.
- Hanna, E.; Rémuzat, C.; Auquier, P.; Toumi, M. Advanced therapy medicinal products: Current and future perspectives. J. Mark. Access Health Policy 2016, 4, 31036.
- Coelho, M.B.; Cabral, J.M.; Karp, J.M. Intraoperative stem cell therapy. Annu. Rev. Biomed. Eng. 2012, 14, 325.
- Ismail, H.D.; Phedy, P.; Kholinne, E.; Djaja, Y.P.; Kusnadi, Y.; Merlina, M.; Yulisa, N.D. Mesenchymal stem cell implantation in atrophic nonunion of the long bones: A translational study. Bone Jt. Res. 2016, 5, 287–293.
- Toosi, S.; Behravan, N.; Behravan, J. Nonunion fractures, mesenchymal stem cells and bone tissue engineering. J Biomed Mater Res A. 2018, 106, 2552–2562.
- Jäger, M.; Herten, M.; Fochtmann, U.; Fischer, J.; Hernigou, P.; Zilkens, C.; Hendrich, C.; Krauspe, R. Bridging the gap: Bone marrow aspiration concentrate reduces autologous bone grafting in osseous defects. J. Orthop. Res. 2011, 29, 173–180.
- Schlund, M.; Nicot, R.; Depeyre, A.; Alkasbi, J.; Ferri, J. Reconstruction of a large posttraumatic mandibular defect using bone tissue engineering with fresh-frozen humeral allograft seeded with autologous bone marrow aspirate and vascularized with a radial forearm flap. J. Craniofacial Surg. 2019, 30, 2085–2087.
- Verboket, R.; Leiblein, M.; Seebach, C.; Nau, C.; Janko, M.; Bellen, M.; Bönig, H.; Henrich, D.; Marzi, I. Autologous cell-based therapy for treatment of large bone defects: From bench to bedside. Eur. J. Trauma Emerg. Surg. 2018, 44, 649–665.
- Du, F.; Wang, Q.; Ouyang, L.; Wu, H.; Yang, Z.; Fu, X.; Liu, X.; Yan, L.; Cao, Y.; Xiao, R. Comparison of concentrated fresh mononuclear cells and cultured mesenchymal stem cells from bone marrow for bone regeneration. Stem Cells Transl. Med. 2021, 10, 598–609.
- Baranovskii, D.S.; Akhmedov, B.G.; Demchenko, A.G.; Krasheninnikov, M.E.; Balyasin, M.V.; Pavlova, O.Y.; Serova, N.S.; Krasil’nikova, O.A.; Shegai, P.V.; Kaprin, A.D.; et al. Minimally Manipulated Bone Marrow-Derived Cells Can Be Used for Tissue Engineering In Situ and Simultaneous Formation of Personalized Tissue Models. Bull. Exp. Biol. Med. 2022, 173, 139–145.
- Arthur, A.; Gronthos, S. Clinical application of bone marrow mesenchymal stem/stromal cells to repair skeletal tissue. Int. J. Mol. Sci. 2020, 21, 9759.
- Zhang, H.; Beilfuss, N.; Zabarylo, U.; Raum, K.; Puts, R. A Tissue Engineering Acoustophoretic (TEA) Set-up for the Enhanced Osteogenic Differentiation of Murine Mesenchymal Stromal Cells (mMSCs). Int. J. Mol. Sci. 2022, 23, 11473.
- Dilogo, I.H.; Phedy, P.; Kholinne, E.; Djaja, Y.P.; Fiolin, J.; Kusnadi, Y.; Yulisa, N.D. Autologous mesenchymal stem cell implantation, hydroxyapatite, bone morphogenetic protein-2, and internal fixation for treating critical-sized defects: A translational study. Int. Orthop. 2019, 43, 1509–1519.
- Gjerde, C.; Mustafa, K.; Hellem, S.; Rojewski, M.; Gjengedal, H.; Yassin, M.A.; Feng, X.; Skaale, S.; Berge, T.; Rosen, A.; et al. Cell therapy induced regeneration of severely atrophied mandibular bone in a clinical trial. Stem Cell Res. Ther. 2018, 9, 213.
- Nguyen, A.; Guo, J.; Banyard, D.A.; Fadavi, D.; Toranto, J.D.; Wirth, G.A.; Paydar, K.Z.; Evans, G.R.; Widgerow, A.D. Stromal vascular fraction: A regenerative reality? Part 1: Current concepts and review of the literature. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 170–179.
- Guo, J.; Nguyen, A.; Banyard, D.A.; Fadavi, D.; Toranto, J.D.; Wirth, G.A.; Paydar, K.Z.; Evans, G.R.D.; Widgerow, A.D. Stromal vascular fraction: A regenerative reality? Part 2: Mechanisms of regenerative action. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 180–188.
- Bouland, C.; Philippart, P.; Dequanter, D.; Corrillon, F.; Loeb, I.; Bron, D.; Lagneaux, L.; Meuleman, N. Cross-Talk Between Mesenchymal Stromal Cells (MSCs) and Endothelial Progenitor Cells (EPCs) in Bone Regeneration. Front. Cell Dev. Biol. 2021, 9, 674084.
- Rhee, S.C.; Ji, Y.H.; Gharibjanian, N.A.; Dhong, E.S.; Park, S.H.; Yoon, E.S. In vivo evaluation of mixtures of uncultured freshly isolated adipose-derived stem cells and demineralized bone matrix for bone regeneration in a rat critically sized calvarial defect model. Stem Cells Dev. 2011, 20, 233–242.
- Toplu, G.; Ozcelik, D.; Serin, M.; Erdem, H.; Topacoglu, A.T. Adipose tissue-derived stromal vascular fraction increases osteogenesis in an experimental design zygomatic bone defect model. J Craniofac Surg. 2017, 28, 2179–2182.
- Im, G.I.; Shin, Y.W.; Lee, K.B. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthr. Cartil. 2005, 13, 845–853.
- Kang, B.J.; Ryu, H.H.; Park, S.S.; Koyama, Y.; Kikuchi, M.; Woo, H.M.; Kweon, O.K. Comparing the osteogenic potential of canine mesenchymal stem cells derived from adipose tissues, bone marrow, umbilical cord blood, and Wharton’s jelly for treating bone defects. J. Vet. Sci. 2012, 13, 299–310.
- Stockmann, P.; Park, J.; von Wilmowsky, C.; Nkenke, E.; Felszeghy, E.; Dehner, J.F.; Schmitt, C.; Tudor, C.; Schlegel, K.A. Guided bone regeneration in pig calvarial bone defects using autologous mesenchymal stem/progenitor cells–a comparison of different tissue sources. J. Cranio-Maxillofac. Surg. 2012, 40, 310–320.
- Liao, H.T.; Chen, C.T. Osteogenic potential: Comparison between bone marrow and adipose-derived mesenchymal stem cells. World J. Stem Cells 2014, 6, 288.
- Soudi, A.; Yazdanian, M.; Ranjbar, R.; Tebyanian, H.; Yazdanian, A.; Tahmasebi, E.; Keshvad, A.; Seifalian, A. Role and application of stem cells in dental regeneration: A comprehensive overview. Excli J. 2021, 20, 454.
- Hussain, A.; Tebyaniyan, H.; Khayatan, D. The Role of Epigenetic in Dental and Oral Regenerative Medicine by Different Types of Dental Stem Cells: A Comprehensive Overview. Stem Cells Int. 2022, 2022, 5304860.
- Sun, Q.; Nakata, H.; Yamamoto, M.; Kasugai, S.; Kuroda, S. Comparison of gingiva-derived and bone marrow mesenchymal stem cells for osteogenesis. J. Cell. Mol. Med. 2019, 23, 7592–7601.
- Du, L.; Yang, P.; Ge, S. Isolation and characterization of human gingiva-derived mesenchymal stem cells using limiting dilution method. J. Dent. Sci. 2016, 11, 304–314.
- Makarevich, P.I.; Parfyonova, Y.V. Therapeutic angiogenesis: Foundations and practical application. In Physiologic and Pathologic Angiogenesis—Signaling Mechanisms and Targeted Therapy; Simionescu, D., Simionescu, A., Eds.; IntechOpen: London, UK, 2017; pp. 343–364.
- Keighron, C.; Lyons, C.J.; Creane, M.; O’Brien, T.; Liew, A. Recent advances in endothelial progenitor cells toward their use in clinical translation. Front. Med. 2018, 5, 354.
- Kaushik, K.; Das, A. Endothelial progenitor cell therapy for chronic wound tissue regeneration. Cytotherapy 2019, 21, 1137–1150.
- Lee, S.H.; Lee, K.G.; Hwang, J.H.; Cho, Y.S.; Lee, K.S.; Jeong, H.-J.; Park, S.-H.; Park, Y.; Cho, Y.-S.; Lee, B.-K. Evaluation of mechanical strength and bone regeneration ability of 3D printed kagome-structure scaffold using rabbit calvarial defect model. Mater. Sci. Eng. C 2019, 98, 949–959.
- Neto, A.S.; Ferreira, J.M. Synthetic and marine-derived porous scaffolds for bone tissue engineering. Materials 2018, 11, 1702.
- Dean, D.; Mott, E.; Luo, X.; Busso, M.; Wang, M.O.; Vorwald, C.; Siblani, A.; Fisher, J.P. Multiple initiators and dyes for continuous Digital Light Processing (cDLP) additive manufacture of resorbable bone tissue engineering scaffolds: A new method and new material to fabricate resorbable scaffold for bone tissue engineering via continuous Digital Light Processing. Virtual Phys. Prototyp. 2014, 9, 3–9.
- Jameson, J.F.; Pacheco, M.O.; Nguyen, H.H.; Phelps, E.A.; Stoppel, W.L. Recent Advances in Natural Materials for Corneal Tissue Engineering. Bioengineering 2021, 8, 161.
- Gao, P.; Zhang, H.; Liu, Y.; Fan, B.; Li, X.; Xiaokang, L.; Lan, P.; Li, M.; Geng, L.; Liu, D.; et al. Beta-tricalcium phosphate granules improve osteogenesis in vitro and establish innovative osteo-regenerators for bone tissue engineering in vivo. Sci. Rep. 2016, 6, 23367.
- Resende, R.F.; Sartoretto, S.C.; Uzeda, M.J.; Alves, A.T.; Calasans-Maia, J.A.; Rossi, A.M.; Granjeiro, J.M.; Calasans-Maia, M.D. Randomized controlled clinical trial of nanostructured carbonated hydroxyapatite for alveolar bone repair. Materials 2019, 12, 3645.
- Kasuya, S.; Kato-Kogoe, N.; Omori, M.; Yamamoto, K.; Taguchi, S.; Fujita, H.; Imagawa, N.; Sunano, A.; Inoue, K.; Ito, Y.; et al. New bone formation process using bio-oss and collagen membrane for rat calvarial bone defect: Histological observation. Implant. Dent. 2018, 27, 158–164.
- Abdal-hay, A.; Sheikh, F.A.; Shmroukh, A.N.; Mousa, H.M.; Kim, Y.K.; Ivanovski, S. Immobilization of bioactive glass 2D and 3D polyamide polymer substrates for bone tissue regeneration. Mater. Des. 2021, 210, 110094.
- Degli Esposti, M.; Chiellini, F.; Bondioli, F.; Morselli, D.; Fabbri, P. Highly porous PHB-based bioactive scaffolds for bone tissue engineering by in situ synthesis of hydroxyapatite. Mater. Sci. Eng. C 2019, 100, 286–296.
- Bu, S.; Yan, S.; Wang, R.; Xia, P.; Zhang, K.; Li, G.; Yin, J. In situ precipitation of cluster and acicular hydroxyapatite onto porous poly (γ-benzyl-l-glutamate) microcarriers for bone tissue engineering. ACS Appl. Mater. Interfaces 2020, 12, 12468–12477.
- Pamula, E.; Filová, E.; Bačáková, L.; Lisá, V.; Adamczyk, D. Resorbable polymeric scaffolds for bone tissue engineering: The influence of their microstructure on the growth of human osteoblast-like MG 63 cells. J. Biomed. Mater. Res. Part A 2009, 89, 432–443.
- Baranovskii, D.; Demner, J.; Nürnberger, S.; Lyundup, A.; Redl, H.; Hilpert, M.; Pigeot, S.; Krasheninnikov, M.; Krasilnikova, O.; Klabukov, I.; et al. Engineering of Tracheal Grafts Based on Recellularization of Laser-Engraved Human Airway Cartilage Substrates. Cartilage 2022, 13, 19476035221075951.
- Balyasin, M.V.; Baranovsky, D.S.; Demchenko, A.G.; Fayzullin, A.L.; Krasilnikova, O.A.; Klabukov, I.D.; Krasheninnikov, M.E.; Lyundup, A.V.; Parshin, V.D. Experimental orthotopic implantation of the tissue-engineered graft of trachea based on devitalized scaffold seeded with mesenchymal and epithelial cells. Russ. J. Transpl. Artif. Organs 2019, 21, 96–107.
- Moy, P.K.; Aghaloo, T. Risk factors in bone augmentation procedures. Periodontol. 2000 2019, 81, 76–90.
- Li, J.; Wang, H.L. Common implant-related advanced bone grafting complications: Classification, etiology, and management. Implant. Dent. 2008, 17, 389–401.
- Rolvien, T.; Barbeck, M.; Wenisch, S.; Amling, M.; Krause, M. Cellular mechanisms responsible for success and failure of bone substitute materials. Int. J. Mol. Sci. 2018, 19, 2893.
- Schmidt-Bleek, K.; Kwee, B.J.; Mooney, D.J.; Duda, G.N. Boon and bane of inflammation in bone tissue regeneration and its link with angiogenesis. Tissue Eng. Part B Rev. 2015, 21, 354–364.
- Malek-Khatabi, A.; Javar, H.A.; Dashtimoghadam, E.; Ansari, S.; Hasani-Sadrabadi, M.M.; Moshaverinia, A. In situ bone tissue engineering using gene delivery nanocomplexes. Acta Biomater. 2020, 108, 326–336.
- Raisin, S.; Belamie, E.; Morille, M. Non-viral gene activated matrices for mesenchymal stem cells based tissue engineering of bone and cartilage. Biomaterials 2016, 104, 223–237.
- Khvorostina, M.A.; Mironov, A.V.; Nedorubova, I.A.; Bukharova, T.B.; Vasilyev, A.V.; Goldshtein, D.V.; Komlev, V.S.; Popov, V.K. 3D Printed Gene-Activated Sodium Alginate Hydrogel Scaffolds. Gels 2022, 8, 421.
- Nürnberger, S.; Schneider, C.; Keibl, C.; Schädl, B.; Heimel, P.; Monforte, X.; Teuschl, A.H.; Nalbach, M.; Thurner, P.J.; Grillari, J.; et al. Repopulation of decellularised articular cartilage by laser-based matrix engraving. EBioMedicine 2021, 64, 103196.
- Baranovsky, D.; Lyundup, A.; Balyasin, M.; Klabukov, I.; Krasilnikova, O.; Krasheninnikov, M.; Parshin, V. Interleukin IL-1β stimulates cartilage scaffold revitalization in vitro with human nasal chondrocytes. Russ. J. Transpl. Artif. Organs 2019, 21, 88–95.
- Mumme, M.; Scotti, C.; Papadimitropoulos, A.; Todorov, A.; Hoffmann, W.; Bocelli-Tyndall, C.; Jakob, M.; Wendt, D.; Martin IBarbero, A. Interleukin-1beta modulates endochondral ossification by human adult bone marrow stromal cells. Eur. Cell Mater. 2012, 24, 224–236.
- Henrich, D.; Verboket, R.; Schaible, A.; Kontradowitz, K.; Oppermann, E.; Brune, J.C.; Nau, C.; Meier, S.; Bonig, H.; Marzi, I.; et al. Characterization of bone marrow mononuclear cells on biomaterials for bone tissue engineering in vitro. BioMed Res. Int. 2015, 2015, 762407.
- Verboket, R.D.; Irrle, T.; Busche, Y.; Schaible, A.; Schröder, K.; Brune, J.C.; Marzi, I.; Nau, C.; Henrich, D. Fibrous Demineralized Bone Matrix (DBM) Improves Bone Marrow Mononuclear Cell (BMC)-Supported Bone Healing in Large Femoral Bone Defects in Rats. Cells 2021, 10, 1249.
- Janko, M.; Sahm, J.; Schaible, A.; Brune, J.C.; Bellen, M.; Schroder, K.; Seebach, C.; Marzi, I.; Henrich, D. Comparison of three different types of scaffolds preseeded with human bone marrow mononuclear cells on the bone healing in a femoral critical size defect model of the athymic rat. J. Tissue Eng. Regen. Med. 2018, 12, 653–666.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
812
Revisions:
2 times
(View History)
Update Date:
22 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





