
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Farid Menaa | + 2698 word(s) | 2698 | 2020-12-09 05:14:17 | | | |
| 2 | Peter Tang | -207 word(s) | 2491 | 2020-12-18 13:48:53 | | |
Video Upload Options
Polysaccharides such as hyaluronic acid (HA) which is omnipresent in the human body and exert pleiotropic biological functions such as tissue repair and tissue regeneration, may be exploited for cosmetics development, esthetic medicine, tissue engineering and regenerative medicine. In this work, the authors describe the excellent biocompatibility and biodegradability of HA-derived hydrogels with make them ideal materials for tissue engineering applications.
1. Introduction
Over the past three decades, tissue engineering (TE) and regenerative medicine have emerged in order to find alternative therapies to organ transplants, a life-threatening medical procedure. Indeed, tissue loss and organ failure alternatives represent one of the greatest challenges in human health-care avoiding (i) severe drawbacks due to the huge demand for organs, (ii) scarce number of donors and, (iii) life-term medication (e.g. immunosuppressive drugs).
TE is an interdisciplinary field which applies the principles of engineering and life sciences towards developing biological substitutes which restore, maintain, or improve tissue function [1]. One of the major approaches in TE is to deliver cells and/or bioactive substances (e.g. growth factors) to patients using three-dimensional scaffolds [2] (Figure 1). Cells and growth factors are chosen based on the type of tissue to be restored, and the scaffolds should function as temporary artificial extracellular matrices (ECM) which accommodate the cells and guides their growth in three dimensions to form new tissue [3].
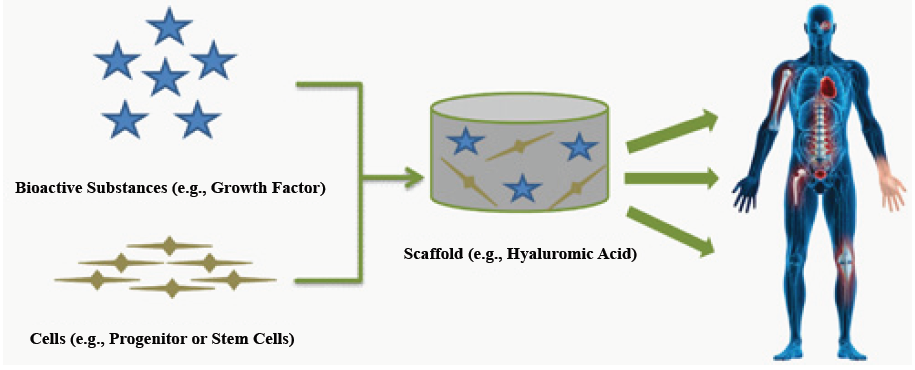
Figure 1: Principles of tissue engineering. A scaffold containing cells and bioactive substances can be used as a biocompatible and biodegradable bioreactor and implant with the purpose of restoring or improving the tissue defects.
Polymers are ideal candidates as scaffold materials for TE since they can be tailored to have desired properties (e.g. mechanical features, geometrical shapes, biocompatibility, minimal toxicity) and, be degraded in the same rate as new tissue is formed [4][5]. They are represented either by synthetic or natural molecules. For instance, synthetic polymers such as poly(-N-isopropylacrylamid) (PNIPAM) [6], poly(vinyl alcohol) (PVA) [7][8] and poly(ethylene glycol) (PEG) [9], have been widely explored because of their relatively simple modification to prepare gels with desired mechanical and physical properties. Natural polymers such as collagen type-I [10], fibrin [11], alginate [12], chitosan [13], chondroitin sulfate [14] and HA [15] have been used to prepare hydrogel scaffolds. Hydrogels of naturally derived polymers have the advantage of biodegradability and resemble to the natural ECM. Nevertheless, some of them have also their limitations. Thereby, collagen hydrogels can be immunogenic [10] while fibrin hydrogels can yield insoluble fibrin peptides aggregates and can be associated with a certain degree of shrinkage when used as matrices for cell encapsulation [11].
HA is a glycosaminoglycan (GAG), a polysaccharide that contains no protein backbone and which is receiving special attention in a wide range of biomedical and TE applications [15]. Indeed, this main component of ECM is naturally involved in tissue repair, and displays unique physical-chemical properties (e.g. viscoelasticity, biodegrability, biocompatibility), making it an ideal material for TE [16][17]. Over the years, HA has been isolated from rooster combs or, through microbial fermentation. Today the production and purification of HA has turned into an industry. Highly pure HA is available in a wide range of molecular weights at relatively low costs. However, due to the short turnover rate and limited mechanical properties of native HA solutions, chemical modifications are required to obtain suitable stable biomaterials (e.g. hydrogels for in vivo tissue repair). Thereby, methods of chemical crosslinking using different linkers have been investigated [18][19][20].
2. Physiological and Physical-Chemical Properties of Hyaluronic Acid (HA)
2.1. Structure and physical-chemical properties
HA was first isolated, in 1934, from the bovine vitreous humor where it was recognized as a muco-polysaccharide of high molecular weight (range of 102-104 kDa) [21]. The structure of this natural polymer is highly conserved between mammalian species and, is defined as a versatile, linear straight-chain, non-sulfated GAG, composed of 2000-25000 repeating alternative sequence of disaccharide units (β1,3 glucuronic acid (GlcA) and β1,4 N-acetylglucosamine (GlcNAc)) (Figure 2), with an extended length of 2-25 mm [22][23][24]. Its pseudorandom coil configuration in aqueous solvents occupies a large volume of solvent. It is thus considered as a space-filling molecule, loosening tissues, creating spaces for cell motility, decreasing cell-cell contacts, and impeding intercellular communication [25].
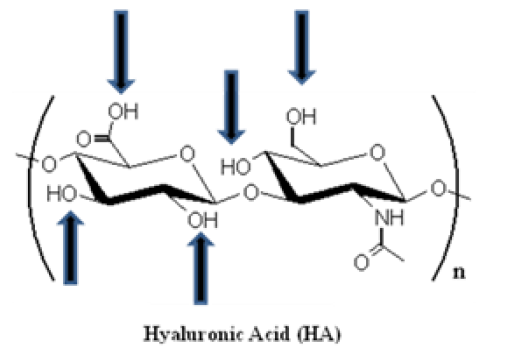
Figure 2: Spatial structure of hyaluronic acid (HA). HA is composed of alternating D-glucuronic acid and D-N-acetylglucosamine moieties, linked together via alternating β-1,4 and β-1,3 glycosidic bonds. The number of repeating disaccharide units (n) can be up to 25000, and each unit has a molecular weight of 401g/mol. The arrows schematize the functional groups that can be used for performing chemical modifications (e.g. addition of a linker).
Interestingly, HA displays viscoelastic properties [23][26], based on a mixture of intrinsic factors related to its structure (polymeric and polyelectrolyte) characteristics [16] such as (i) high molecular weight, (ii) electrostatic repulsions of the carboxylate ions (COO-), (iii) intramolecular hydrogen bonds and, (iv) double helical conformation.
2.2. Distribution and biological functions
HA is predominantly present in the ECM of some tissues (e.g. skin, umbilical cord, embryonic and malignant tissues) and body fluids (e.g. synovial fluid, vitreous humor) of all vertebrates as well as in some bacteria [22][27][28][29][30]. HA is also highly expressed in the glycocalyx, a pericellular coat of most cells, and is particularly prominent on the apical surface of endothelial cells [31].
Since its discovery, the biological functions of HA have been thoroughly investigated [23][25]. For instance, HA is involved in the (i) healing wound, tissue repair and regeneration [23][32][33][34], (ii) organization of the ECM [23][35], (iii) lubrication of the joints [23][28], (iv) regulation of the cell adhesion and motility through receptors that interact with the cytoskeleton [23][36][37], (v) angiogenesis by mediating cell proliferation, cell migration and cell differentiation [23][38][39]. Some of those functions (e.g. angiogenesis, tissue homeostasis, tissue remodeling) are exerted by binding to a family of cellular proteoglycan receptors, the hyaladhedrins (e.g. RHAMM aka CD168, CD44) [24][40].
Interestingly, the relevant role of HA in promoting cancer is actively investigated. Indeed, HA-HA receptor interaction is known to generate intracellular signaling, which, in turn, promotes tumor growth, metastasis, angiogenesis, trafficking of tumor-associated macrophages, and chemoresistance [41][42][43][44][45][46][47]. In fact, a HA-hyaluronidase (HAHAase) complex system would be involved in tumor angiogenesis. Indeed, HA synthase (Has) expression required HYAL-1, a HAase, to promote tumor growth and progression [48][49]. Concordantly, the use of 4-methylumbelliferone (4-MU), an HA synthesis (Has) inhibitor, displayed antitumor activity in cancer cells [50]. Besides, HYAL-1 is able to degrade HA into proangiogenic fragments that support tumor progression, invasion, and angiogenesis in some cancer models [51][52]. Therefore, HYAL-1 expression is potentially an independent predictor of metastasis [53][54][55][56] and, HYAL-1 can be considered as a valuable target for cancer therapy. Thereby, the use of sulfated HA (sHA), generated by O-sulfation of HA, was shown to inhibit HAases [57] and tumor growth [58]. Eventually, a recent safety assessment report showed that HA does not play a causal role in cancer metastasis and, that the widespread clinical use of HA has been shown free of significant adverse reactions [59].
Nevertheless, further mechanistic insights into the tumorassociated HA-HAase system and a preclinical proof-of-concepts of the safety and efficacy of inhibitors against HAases, are still required. Moreover, in our opinion, the risks of developing cancer using HAbased scaffolds in TE shall be rigorously evaluated in a time-dependent manner as HA biodegradation can generate pro-angiogenic fragments.
2.3. Metabolism
Circulating HA is mostly derived from lymph. Lymph nodes may nevertheless extract as much as 80-90% from peripheral lymph before it can reach the bloodstream [29]. HA can be taken up by cells [60] through receptors such as CD44 [61][62][63] and the receptor for HA-mediated motility (RHAMM) [64]. Nevertheless, HA is not immediately degraded by the cells since intact HA chains have been detected in cytoplasm, in nucleus, and even within the nucleolus [65][66][67]. Furthermore, it has been suggested that stromal cells (aka connective tissue cells such fibroblasts and endothelial cells) primarily synthesize HA, through specific synthases, before supplying it to the epithelial cells [68]. The general pattern of distribution suggested that HA is absorbed from plasma and tissue fluids by elements of the reticuloendothelial (RES) system [69].
Despite the increasing importance of HA in biology, little is still known about the degradation of HA in tissues. The catabolism scheme of endogenous HA did report an uptake of the lymphatic system followed by blood transportation to the liver where it is fully degraded [70]. Uptake and metabolism are thus primarily effected in liver and lymph node by endothelial cells lining the sinusoids of each [29]. A significant plasmatic flux of radio-labeled HA was also taken up in bone marrow and in lymph nodes [29][69]. In lymph nodes and in spleen, macrophage-like cells intertwined with the endothelial cells to take up HA [29].
HA can be then degraded by glycosylphosphatidylinositol (GPI)- anchored HAases, class of enzymes that cleave specifically HA glycosidic bonds [71], by oxidation due to reactive oxygen species (ROS) [72], heat [73], hydrolysis [74], among others [75]. The daily metabolic turnover of HA ended up to approximately one third of the total body content (e.g. 15g of HA for a 70 kg individual) [76][77]. Between different tissues, rates of turnover varied widely. In the bloodstream, the t1/2 for HA was rapid (i.e. 2-5 min) while in a tissue as relatively inert as cartilage, HA turnover occurs in 1-3 weeks [78]. Interestingly, normal turnover in the bloodstream has been estimated in the range of 0.3- 1.0 μg/min/kg body weight, and turnover in peripheral tissues may be effected by degradation in situ, or by transfer into lymph by diffusion or hydrodynamic forces [29].
Increasing evidence points to the existence of a mini-organelle, a hyaluronasome, a possible multi-protein membrane-associated complex, that has both HA synthetic and catabolic activities and, which possesses sensitive sensor mechanisms that can respond to various metabolic states [79][80]. Thereby, it has been suggested that high molecular HA is tethered to the plasma cell surface by HA receptors, combined possibly with an interaction with Hyal-2, a GPI-linked hyaluronidase anchored to the plasma membrane [80].
3. Some HA-based Scaffolds Applications in Bio-Medicine and Pharmacy
3.1. Use of HA-based scaffolds in regenerative medicine
When a tissue is largely damaged, tissue transplantation (e.g. bone) or prosthetic implants usually are considered as major medical solutions [81]. However, those therapeutic options are not without limitations and risks for the patient. Indeed, tissue transplantation may (i) be limited by tissue supply, (ii) cause discomfort to the patients, (iii) increase the risk of disease transmission and, (iv) cause host reactions [82]. Besides, prosthetic implants are not physiologically functional and, are too often accompanied by infection and structural failure [83].
As an alternative therapeutic option to tissue transplantation and prosthetic implantation, TE showed very promising results mainly for acute and relatively small lesions (e.g. cartilage reconstruction) and, it is expected that TE will have tremendous clinical applications if it can be applied to chronic lesions (e.g. large chondral lesions in patients with degenerative osteoarthritis) [84].
TE consists in inducing or accelerating the tissue forming process by in situ delivering of progenitor/stem cells, and/or growth factors using degradable biomaterial scaffolds (e.g. HA based-scaffolds designed according to the tissue defect) [85]. Importantly, the sitedirected injection of a growth factor without a scaffold was helpless [86], because the later was required to locally concentrate the growth factor [87].
Bone represents one of the most frequently transplanted tissues in spite of its capacity to self-regenerate [88]. Nevertheless, bone transplantation is limited and presents the risks previously evoked [82][83]. Then, bone TE often involved injectable polymer-based scaffolds (e.g. HA hydrogels), osteoprogenitor or osteo-stem cells (e.g. mesenchymal stem cells (MSCs)) which were able to get differentiated in bone-building cells (aka osteoblasts) in presence of growth factors (e.g. bone morphogenic proteins (BMPs), a group of proteins belonging to the transforming growth factor-b TGF-b) superfamily) [89][90][91]. Thereby, HA-based polymers were used as cell carriers for tissueengineered repair of bone and cartilage [92][93]. Interestingly, a new minimally invasive tissue-engineering approach, named Hyalograft®, consisting in the implantation of expanded autologous chondrocytes grown on a three-dimensional hyaluronan-based scaffold, was described as a safe, rapid, easy-handle, and viable therapeutic option for treatment of acute cartilage lesions over currently available autologous chondrocyte implantation techniques [94]. Besides, co-encapsulation of TGF-β3 containing nanofilm-coated alginate microspheres, along with human MSCs in HA hydrogels, has recently shown promising results in animal models towards the development of implantable constructs for cartilage reconstruction (aka chondrogenesis) [95]. Eventually, to establish medical use of TE technology for ligament and tendon injuries, a scaffold with sufficient ability for cell growth, cell differentiation, and mechanical properties, has been developed [96]. This scaffold made from chitosan and 0.1% HA exhibited adequate biodegradability, biocompatibility, joint stabilization, low toxicity and low inflammation in animal experiments [96].
3.2. Use of HA-based scaffolds as drug delivery systems
HA hydrogels, among others, are widely used as dermal and transdermal drug delivery systems. These innovative carrier systems were designed for the controlled release of drugs through the skin into the systemic circulation, in order to maintain consistent efficacy and reduce the dose and potential side-effects of the drug(s) [97].
For instance, a local delivery of DNA through a hydrogel scaffold would increase the applicability of gene therapy in tissue regeneration and cancer therapy [98]. Thereby, a novel process, termed caged nanoparticle encapsulation (aka CnE), has been developed for loading concentrated and unaggregated non-viral gene delivery nanoparticles into various HA hydrogels [98].
Furthermore, HA-bioconjugates have been developed to enhance selective entry of cytotoxic drugs into HA receptor-expressing cancerous cells (e.g. CD44 and RHAMM in ovarian cancer cells) [99][100]. Indeed, it was shown that (PEG)-conjugated HA nanoparticles (HA-NPs) were largely taken up by cancer cells over-expressing the HAreceptor CD44 comparatively to normal fibroblast cells, improving the tumor targetability in vivo [100]. Concordantly, a new HA-paclitaxel bioconjugate, Oncofid-P®, was more effective than the use of free paclitaxel for intraperitoneal treatment of ovarian cancer in mice [101].
However, HA and its degradation products, accumulated into the stroma of various human tumors, can modulate intracellular signaling pathways and, positively affect angiogenesis of malignant cells and multidrug resistance [102].
3.3. Use of HA-based scaffolds in anti-aging and esthetic medicine
HA reached prominence in cosmetic practice where it represents the injectable dermal filler of choice for most anti-aging, esthetic and plastic specialists [103].
For instance, HA is used for the correction of soft tissue defects (e.g. skin regeneration, wrinkle-treatment, wound healing) [103][104]. Thereby, HA material provided an effective, non invasive, non surgical alternative for correction of the contour defects of the face due to its enormous ability to bind water and easiness of implantation [105]. In the US, eight HA dermal fillers were approved for commercialization by the Food and Drug Administration (FDA) although severe adverse effects have been reported [106].
4. Conclusion
HA is a naturally occurring polysaccharide which is present in extracellular matrices of soft connective tissues and body fluids. With regards to its mechanism of synthesis, its size and its physical-chemical properties, HA is unique among other glycosaminoglycans. HA is able to interact with other macromolecules (e.g. proteins) and, participates in regulating the cell behavior during numerous morphogenic, restorative, and pathological processes in the body. The role of HA in diseases, such as in various forms of cancers, arthritis and osteoporosis, is leading to new impetus in research and development. The preparation of the safest and efficient HA-based biomaterials for theranostic medicine for any type of lesions, regardless their surface, remains an exciting challenge.
References
- Langer R, Vacanti JP (1993) Tissue engineering. Science260: 920-926.
- Vacanti JP, Langer R (1999) Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 354: SI32-34.
- Yang SF, Leong KF, Du ZH, Chua CK (2001) The design of scaffolds for use in tissue engineering. Part 1. Traditional factors. Tissue Eng7: 679-689.
- Levenberg S, Langer R, Gerald PS (2004) Advances in tissue engineering. Curr Top Dev Biol 61: 113-134.
- Sokolsky-Papkov M, Agashi K, Olaye A, Shakesheff K, Domb AJ (2007) Polymer carriers for drug delivery in tissue engineering. Adv Drug Deliv Rev 59: 187-206.
- Yasuda A, Kojima K,Tinsley KW,Yoshioka H,Mori Y,et al. (2006) In vitro culture of chondrocytes in a novel thermoreversible gelation polymer scaffold containing growth factors. Tissue Eng 12: 1237-1245.
- Schmedlen KH, Masters KS, West JL (2002) Photocrosslinkable polyvinyl alcohol hydrogels that can be modified with cell adhesion peptides for use in tissue engineering. Biomaterials 23: 4325-4332.
- Bryant SJ, Davis-Arehart KA, Luo N, Shoemaker RK, Arthur JA, et al. (2004) Synthesis and characterization of photopolymerized multifunctional hydrogels: Water-soluble poly(vinyl alcohol) and chondroitin sulfate macromers for chondrocyte encapsulation. Macromol 37: 6726-6733.
- Hubbell JA (1998) Synthetic biodegradable polymers for tissue engineering and drug delivery. Curr Opin Solid State & Mater Sci3: 246-251.
- Glowacki J, Mizuno S (2008) Collagen scaffolds for tissue engineering. Biopolymers 89: 338-344.
- Rowe SL, Lee S, Stegemann JP (2007) Influence of thrombin concentration on the mechanical and morphological properties of cell-seeded fibrin hydrogels. ActaBiomater 3: 59-67.
- Jay SM, Shepherd BR, Bertram JP, Pober JS, Saltzman WM (2008) Engineering of multifunctional gels integrating highly efficient growth factor delivery with endothelial cell transplantation. FASEB J22: 2949-2956.
- Feng Z, Chian KS, Ong WF, Mhaisalka PS, Chan V, et al. (2007) Dual requirements of extracellular matrix protein and chitosan for inducing adhesion contact evolution of esophageal epithelia. J Biomed Mater Res A 82: 788-801.
- Wang DA, Varghese S, Sharma B, Strehin I, Fermanian S, et al. (2007)Multifunctional chondroitin sulphate for cartilage tissue biomaterial integration. Nat Mater6: 385-392.
- Kogan G,Soltés L,Stern R,Gemeiner P (2007) Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol Lett 29: 17-25.
- Kuo JW (2005) Practical aspects of hyaluronan based medical products. CRC Press, Boca Raton.
- Solchaga LA,Gao J,Dennis JE,Awadallah A,Lundberg M,et al. (2002) Treatment of osteochondral defects with autologous bone marrow in a hyaluronan-based delivery vehicle. Tissue Eng 8: 333-347.
- Hartig M, Joos U, Wiesmann HP (2000) Capacitively coupled electric fields accelerate proliferation of osteoblast-like primary cells and increase bone extracellular matrix formation in vitro. Eur Biophys J29: 499-506.
- Zein I, Hutmacher DW, Tan KC, Teoh SH (2002) Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 23: 1169-1185.
- Shu XZ, Liu Y, Palumbo F, Prestwich GD (2003) Disulfide-crosslinked hyaluronan-gelatin hydrogel films: a covalent mimic of the extracellular matrix for in vitro cell growth. Biomaterials 24: 3825-3834.
- Meyer K, Palmer JW (1934) The polysaccharide of the vitreous humor. J BiolChem 107: 629-634.
- Fraser JRE, Laurent TC, Laurent UBG (1997) Hyaluronan: Its nature, distribution, functions and turnover. J Int Med242: 27-33.
- Volpi N,Schiller J,Stern R,Soltés L (2009) Role, metabolism, chemical modifications and applications of hyaluronan. Curr Med Chem 16: 1718-1745.
- Gaffney J, Matou-Nasri S, Grau-Olivares M, Slevin M (2010) Therapeutic applications of hyaluronan. Mol BioSyst 6: 437-443.
- Laurent TC (1998) The chemistry, biology and medical applications of hyaluronan and its derivatives, Portland Press, London.
- Wik HB, Wik O (1998) Rheology of Hyaluronan. In The chemistry, biology and medical applications of hyaluronan and its derivatives. Ed Laurent TC, Portland Press, London 25-32.
- Longaker MT, Chiu ES, Adzick NS, Stern M, Harrison MR, et al. (1991) Studies in fetal wound healing. V. A prolonged presence of hyaluronic acid characterizes fetal wound fluid. Ann Surg 213: 292-296.
- Kappler J, Kaminski TP, Gieselmann V, Kubitscheck U, Jerosch J (2010) Single-molecule imaging of hyaluronan in human synovial fluid. J Biomed Opt. 15: 060504.
- Fraser JR,Laurent TC (1989) Turnover andmetabolismofhyaluronan. Ciba Found Symp143: 41-53.
- Tammi RH, Kultti A, Kosma VM, Pirinen R, Auvinen P, et al. (2008) Hyaluronan in human tumors: pathobiological and prognostic messages from cell-associated and stromal hyaluronan.Semin Cancer Biol18: 288-295.
- Henry CB, Duling BR (1999) Permeation of the luminal capillary glycocalyx is determined by hyaluronan. Am J Physiol 277: H508-H514.
- Gall Y (2010) Hyaluronic acid: structure, metabolism and implication in cicatrisation. Ann Dermatol Venereol137: S30-39.
- Jiang D, Liang J, Noble PW (2007) Hyaluronan intissueinjury andrepair. Annu Rev Cell Dev Biol 23: 435-461.
- Galeano M, Polito F, Bitto A, Irrera N, Campo GM, et al. (2011) Systemic administration of high-molecular weight hyaluronan stimulates wound healing in genetically diabetic mice. Biochim Biophys Acta 1812: 752-759.
- Knudson CB (2003) Hyaluronan and CD44: strategic players for cell-matrix interactions during chondrogenesis and matrix assembly. Birth Defects Res C Embryo Today69: 174-196.
- Entwistle J, Hall CL, Turley EA (1996) HA receptors: regulators of signalling to the cytoskeleton. J Cell Biochem 61: 569-577.
- Bourguignon LY, Zhu H, Shao L, Chen YW (2001) CD44 interaction with c-Src kinase promotes cortactin-mediated cytoskeleton function and hyaluronic acid-dependent ovarian tumor cell migration. J Biol Chem 276: 7327-7336.
- Sironen RK, Tammi M, Tammi R, Auvinen PK, Anttila M, et al. (2011) Hyaluronan in human malignancies. Exp Cell Res 317: 383-391.
- SimpsonMA,LokeshwarVB (2008) Hyaluronan and hyaluronidase in genitourinary tumors.Front Biosci13: 5664-5680.
- Toole BP (2001) Hyaluronan in morphogenesis. Semin Cell Dev Biol 12: 79-87.
- Toole BP, Slomiany MG (2008) Hyaluronan: a constitutive regulator of chemoresistance and malignancy in cancer cells.Semin Cancer Biol18: 244-250.
- TooleBP(2004) Hyaluronan: from extracellular glue to pericellular cue.Nat Rev Cancer 4: 528-539.
- Kobayashi N, Miyoshi S,Mikami T, Koyama H, Kitazawa M, et al. (2010) Hyaluronan deficiency in tumor stroma impairs macrophage trafficking and tumor neovascularization.Cancer Res70: 7073-7083.
- Itano N, Zhuo L, Kimata K (2008) Impact of the hyaluronan-rich tumor microenvironment on cancer initiation and progression.Cancer Sci99: 1720-1725.
- Maxwell CA, McCarthy J, Turley E (2008) Cell-surface and mitotic-spindle RHAMM: moonlighting or dual oncogenic functions?J Cell Sci121: 925-932.
- Orian-Rousseau V (2010) CD44, a therapeutic target for metastasising tumours.Eur J Cancer46: 1271-1277.
- Toole BP (2009) Hyaluronan-CD44 interactions in cancer: paradoxes and possibilities. Clin Cancer Res15: 7462-7468.
- Bharadwaj AG, Kovar JL, Loughman E, Elowsky C, Oakley GG, et al. (2009) Spontaneous metastasis of prostate cancer is promoted by excess hyaluronan synthesis and processing.Am J Pathol174: 1027-1036.
- Kovar JL, Johnson MA, Volcheck WM, Chen J, Simpson MA (2006) Hyaluronidase expression induces prostate tumor metastasis in an orthotopic mouse model.Am J Pathol169: 1415-1426.
- Lokeshwar VB, Lopez LE, Munoz D, Chi A, Shirodkar SP, et al. (2010) Antitumor activity of hyaluronic acid synthesis inhibitor 4-methylumbelliferone in prostate cancer cells.Cancer Res70: 2613-2623.
- Lokeshwar VB,Cerwinka WH, Lokeshwar BL (2005) HYAL1 hyaluronidase: a molecular determinant of bladder tumor growth and invasion.Cancer Res65: 2243-2250.
- Lokeshwar VB, Cerwinka WH, Isoyama T, Lokeshwar BL (2005) HYAL1 hyaluronidase in prostate cancer: a tumor promoter and suppressor.Cancer Res65: 7782-7789.
- Ekici S, Cerwinka WH, Duncan R, Gomez P, Civantos F, et al. (2004) Comparison of the prognostic potential of hyaluronic acid, hyaluronidase (HYAL-1), CD44v6 and microvessel density for prostate cancer.Int J Cancer112:121-129.
- Gomez CS, Gomez P, Knapp J,Jorda M,Soloway MS, et al. (2009)Hyaluronic acid and HYAL-1 in prostate biopsy specimens: predictors of biochemical recurrence.J Urol182: 1350-1356.
- Kramer MW, Escudero DO, Lokeshwar SD, Golshani R, Ekwenna OO, et al. (2010) Association of hyaluronic acid family members (HAS1, HAS2, and HYAL-1) with bladder cancer diagnosis and prognosis.Cancer117: 1197-1209.
- Poola I,Abraham J, Marshalleck JJ, Yue Q, Lokeshwar VB, et al. (2008) Molecular risk assessment for breast cancer development in patients with ductal hyperplasias.Clin Cancer Res15: 1274-1280.
- Balazs EA, Hogberg B, Laurent TC (1951) The biological activity of hyaluron sulfuric acid. Acta Physiol Scand23: 168-178.
- Benitez A,Yates TJ,Lopez LE,Cerwinka WH,Bakkar A,et al. (2011)Targeting hyaluronidase for cancer therapy: antitumor activity of sulfated hyaluronic acid in prostate cancer cells. Cancer Res 71: 4085-4095.
- Becker LC,Bergfeld WF,Belsito DV,Klaassen CD,Marks JG Jr,et al. (2009)Final report of the safety assessment of hyaluronic acid, potassium hyaluronate, and sodium hyaluronate. Int J Toxicol 28: 5-67.
- Collis L, Hall C, Lange L, Ziebell M, Prestwich R, et al. (1998) Rapid hyaluronan uptake is associated with enhanced motility: implications for an intracellular mode of action. FEBS Lett 440: 444-449.
- Culty M, Nguyen HA, Underhill CB (1992) The hyaluronan receptor (CD44) participates in the uptake and degradation of hyaluronan. J Cell Biol 116: 1055-1062.
- Hua Q, Knudson CB, Knudson W (1993) Internalization of hyaluronan by chondrocytes occurs via receptor-mediated endocytosis. J Cell Sci 106: 365-375.
- Kaya G, Rodriguez I, Jorcano JL, Vassalli P, Stamenkovic I (1997) Selective suppression of CD44 in keratinocytes of mice bearing an antisense CD44 transgene driven by a tissue-specific promoter disrupts hyaluronate metabolism in the skin and impairs keratinocyte proliferation. Genes Dev 11: 996-1007.
- Cheung WF, Cruz TF, Turley EA (1999) Receptor for hyaluronan-mediated motility (RHAMM), a hyaladherin that regulates cell responses to growth factors. Biochem Soc Trans 27: 135-142.
- Evanko SP, Wight TN (1999) Intracellular localization of hyaluronan in proliferating cells. J Histochem Cytochem 47: 1331-1342.
- Evanko SP, Angello JC, Wight TN (1999) Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 19: 1004-1013.
- Tammi R, Rilla K, Pienimaki JP, MacCallum DK, Hogg M, et al. (2001) Hyaluronan enters keratinocytes by a novel endocytic route for catabolism. J Biol Chem 276: 35111-35122.
- Pasonen-Seppanen S, Karvinen S, Torronen K, Hyttinen JM, Jokela T, et al. (2003) EGF upregulates, whereas TGF-beta downregulates, the hyaluronan synthases Has2 and Has3 in organotypic keratinocyte cultures: correlations with epidermal proliferation and differentiation. J Invest Dermatol 120: 1038-1044.
- Fraser JR,Appelgren LE,Laurent TC (1983) Tissue uptake of circulatinghyaluronic acid. A whole body autoradiographic study. Cell Tissue Res233: 285-293.
- Laurent TC, Fraser JRE (1992) Hyaluronan. FASEB J6: 2397-2404.
- Kreil G (1995) Hyaluronidases-A group of neglected enzymes. Protein Sci4: 1666-1669.
- Soltés L, Mendichi R, Kogan G, Schiller J, Stankovská M, et al. (2006) Degradative action of reactive oxygen species on hyaluronan.Biomacromolecules 7: 659-668.
- Bothner H, Waaler T, Wik O (1988) Limiting viscosity number and weight average molecular weight of hyaluronate samples produced by heat degradation. Int J Biol Macromol10: 287-291.
- Inoue Y, Nagasawa K (1985) Preparation, by chemical degradation of hyaluronic acid, of a series of even- and odd-numbered oligosaccharides having a 2- acetamido-2-deoxy--glucose and a -glucuronic acid residue, respectively, at the reducing end. Carbohydr Res 141: 99-110.
- Stern R, Kogan G, Jedrzejas MJ, Soltes L (2007) The many ways to cleave hyaluronan. Biotechnol Adv 25: 537-557.
- Fraser JRE, Brown TJ, Laurent, TC (1998) Catabolism of hyaluronan. In The chemistry, biology and medical applications of hyaluronan and its derivatives. Ed Laurent TC, Portland Press, London 85-92.
- Stern R (2003) Devisingapathwayforhyaluronancatabolism: are we thereyet? Glycobiology 13: 105R-115R.
- Fraser JR, Laurent TC, Pertoft H, Baxter E (1981) Plasma clearance, tissue distribution and metabolism of hyaluronic acid injected intravenously in the rabbit. Biochem J 200: 415-424.
- Mian N (1986) Analysis of cell-growth-phase-related variations in hyaluronate synthase activity of isolated plasma-membrane fractions of cultured human skin fibroblasts. Biochem J 237: 333-342.
- Mian N (1986) Characterization of a high-Mr plasma-membrane-bound protein and assessment of its role as a constituent of hyaluronate synthase complex. Biochem J 237: 343-357.
- Khan Y, Yaszemski MJ, Mikos AG, Laurencin CT (2008) Tissue engineering of bone: Material and matrix considerations. J Bone Joint Surg Am 90A: 36-42.
- Silber JS,Anderson DG,Daffner SD,Brislin BT,Leland JM,et al. (2003) Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine28: 134-139.
- Suh H, Park JC, Han DW, Lee DH, Han CD (2001) A bone replaceable artificial bone substitute: cytotoxicity, cell adhesion, proliferation, and alkaline phosphatase activity. Artif Organs25: 14-21.
- Sittinger M,Burmester GR (2006) Canengineeredcartilagetransplantsbe used fortreatingrheumatic diseases? Nat Clin Pract Rheumatol2: 172-173.
- Cowan CM, Soo C, Ting K, Wu B (2005) Evolving concepts in bone tissue engineering. Curr Top Dev Biol 66: 239-285.
- Seeherman HJ, Bouxsein M,Kim H,Li R,Li XJ,et al. (2004)Recombinant human bone morphogenetic protein-2 delivered in an injectable calcium phosphate paste accelerates osteotomy-site healing in a nonhuman primate model. J Bone Joint Surg Am 86: 1961-1972.
- Geiger M, Li RH, Friess W (2003) Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev55: 1613-1629.
- Ma PX, Zhang R, Xiao G, Franceschi R (2001) Engineering new bone tissue in vitro on highly porous poly(alpha-hydroxyl acids)/hydroxyapatite composite scaffolds. J Biomed Mater Res54: 284-293.
- Wu X, Shi W, Cao X (2007) Multiplicity of BMP signaling in skeletal development.Ann N Y Acad Sci 1116: 29-49.
- Li RH, Wozney JM (2001) Delivering on the promise of bone morphogenetic proteins. Trends Biotechnol 19: 255-265.
- Shea CM, Edgar CM, Einhorn TA, Gerstenfeld LC (2003) BMP treatment of C3H10T1/2 mesenchymal stem cells induces both chondrogenesis and osteogenesis.J Cell Biochem90: 1112-1127.
- Elisseeff J, Puleo C, Yang F, Sharma B (2005) Advances in skeletal tissue engineering with hydrogels. Orthod Craniofac Res8: 150-161.
- Solchaga LA, Dennis JE, Goldberg VM, Caplan AI (1999) Hyaluronic acid-based polymers as cell carriers for tissue-engineered repair of bone and cartilage. J Orthop Res 17: 205-213.
- Tognana E, Borrione A, De Luca C, Pavesio A (2007)Hyalograft C: hyaluronan-based scaffolds in tissue-engineered cartilage. Cells Tissues Organs 186: 97-103.
- Bian L,Zhai DY,Tous E,Rai R,Mauck RL,et al. (2011) Enhanced MSC chondrogenesis following delivery of TGF-ß3 from alginate microspheres within hyaluronic acid hydrogels invitro and invivo. Biomaterials 32: 6425-6434.
- Majima T,Irie T,Sawaguchi N,Funakoshi T,Iwasaki N,et al. (2007) Chitosan-based hyaluronan hybrid polymer fibre scaffold for ligament and tendon tissue engineering. Proc Inst Mech Eng H 221: 537-546.
- Basavaraj KH,Johnsy G,Navya MA,Rashmi R (2010) Biopolymers as transdermal drug delivery systems in dermatology therapy. Crit Rev Ther Drug Carrier Syst 27: 155-185.
- Lei Y,Rahim M,Ng Q,Segura T (2011) Hyaluronic acid and fibrin hydrogels with concentrated DNA/PEI polyplexes for local gene delivery.J Controlled Release153: 255-261.
- Choi KY,Min KH,Yoon HY,Kim K,Park JH,et al. (2011) PEGylation of hyaluronic acid nanoparticles improves tumor targetability in vivo. Biomaterials 32: 1880-1889.
- Auzenne E, Ghosh SC, Khodadadian M, Rivera B, Farquhar D, et al. (2007) Hyaluronicacid-paclitaxel: antitumor efficacy against CD44(+) human ovarian carcinoma xenografts. Neoplasia 9: 479-486.
- De Stefano I,Battaglia A,Zannoni GF,Prisco MG,Fattorossi A,et al. (2011) Hyaluronic acid-paclitaxel: effects of intraperitoneal administration against CD44(+) human ovarian cancer xenografts. Cancer Chemother Pharmacol 68: 107-116.
- Sironen RK,Tammi M,Tammi R,Auvinen PK,Anttila M (2011) Hyaluronan in human malignancies. Exp Cell Res317: 383-391.
- Price RD,Berry MG,Navsaria HA (2007) Hyaluronic acid: the scientific and clinical evidence. J Plast Reconstr Aesthet Surg60: 1110-1119.
- Kingsley M,Dover J (2010) What's new in cosmetic procedures. G Ital Dermatol Venereol 145: 651-658.
- Brandt FS,Cazzaniga A (2008) Hyaluronic acid gel fillers in the management of facial aging. Clin Interv Aging 3: 153-159.
- Requena L,Requena C,Christensen L,Zimmermann US,Kutzner H,et al. (2011) Adverse reactions to injectable soft tissue fillers. J Am Acad Dermatol 64: 1-34.




