Polysaccharides such as hyaluronic acid (HA) which is omnipresent in the human body and exert pleiotropic biological functions such as tissue repair and tissue regeneration, may be exploited for cosmetics development, esthetic medicine, tissue engineering and regenerative medicine. In this work, the authors describe the excellent biocompatibility and biodegradability of HA-derived hydrogels with make them ideal materials for tissue engineering applications.
- hyaluronic acid
- tissue engineering
- regenerative medicine
- aesthetics
- tissue repair
- biomaterials
- polysaccharides
- cosmetics
Please note: Below is an entry draft based on your previous paper, which is wrirren tightly around the entry title. Since it may not be very comprehensive, we kindly invite you to modify it (both title and content can be replaced) according to your extensive expertise. We believe this entry would be beneficial to generate more views for your work. In addition, no worry about the entry format, we will correct it and add references after the entry is online (you can also send a word file to us, and we will help you with submitting).
Definition
hyaluronic acid (HA) is a natural occurring glycosaminoglycan, a polysaccharide of high molecular weight which displays interesting viscoelastic properties. Among other organisms, HA is omnipresent in the human body, occurring in almost all biological fluids and tissues, although the highest amounts of HA are found in the extracellular matrix of soft connective tissues. HA is synthesized in a unique manner by a family of hyaluronan synthases and degraded by hyaluronidases and, exerts pleiotropic biological functions such as tissue repair and tissue regeneration. The excellent biocompatibility and biodegradability of HA-derived hydrogels make them ideal materials for tissue engineering.
1. Introduction
Over the past three decades, tissue engineering (TE) and regenerative medicine have emerged in order to find alternative therapies to organ transplants, a life-threatening medical procedure. Indeed, tissue loss and organ failure alternatives represent one of the greatest challenges in human health-care avoiding (i) severe drawbacks due to the huge demand for organs, (ii) scarce number of donors and, (iii) life-term medication (e.g. immunosuppressive drugs).
TE is an interdisciplinary field which applies the principles of engineering and life sciences towards developing biological substitutes which restore, maintain, or improve tissue function [1]. One of the major approaches in TE is to deliver cells and/or bioactive substances (e.g. growth factors) to patients using three-dimensional scaffolds [2] (Figure 1). Cells and growth factors are chosen based on the type of tissue to be restored, and the scaffolds should function as temporary artificial extracellular matrices (ECM) which accommodate the cells and guides their growth in three dimensions to form new tissue [3].
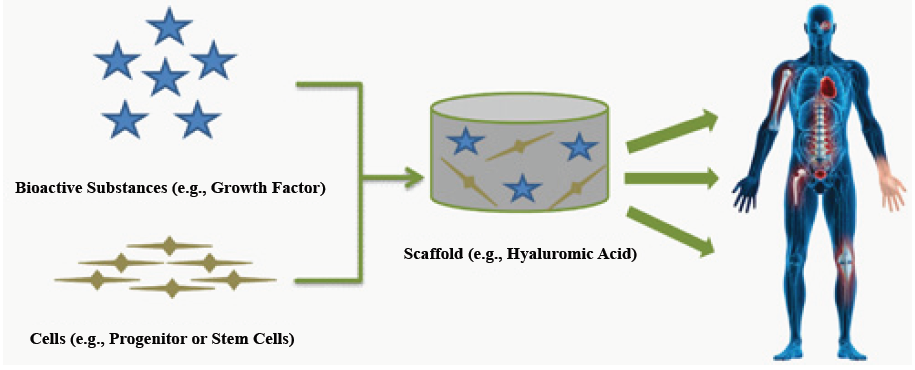
Figure 1: Principles of tissue engineering. A scaffold containing cells and bioactive substances can be used as a biocompatible and biodegradable bioreactor and implant with the purpose of restoring or improving the tissue defects.
Polymers are ideal candidates as scaffold materials for TE since they can be tailored to have desired properties (e.g. mechanical features, geometrical shapes, biocompatibility, minimal toxicity) and, be degraded in the same rate as new tissue is formed [4][5][4,5]. They are represented either by synthetic or natural molecules. For instance, synthetic polymers such as poly(-N-isopropylacrylamid) (PNIPAM) [6], poly(vinyl alcohol) (PVA) [7][8][7,8] and poly(ethylene glycol) (PEG) [9], have been widely explored because of their relatively simple modification to prepare gels with desired mechanical and physical properties. Natural polymers such as collagen type-I [10], fibrin [11], alginate [12], chitosan [13], chondroitin sulfate [14] and HA [15] have been used to prepare hydrogel scaffolds. Hydrogels of naturally derived polymers have the advantage of biodegradability and resemble to the natural ECM. Nevertheless, some of them have also their limitations. Thereby, collagen hydrogels can be immunogenic [10] while fibrin hydrogels can yield insoluble fibrin peptides aggregates and can be associated with a certain degree of shrinkage when used as matrices for cell encapsulation [11].
HA is a glycosaminoglycan (GAG), a polysaccharide that contains no protein backbone and which is receiving special attention in a wide range of biomedical and TE applications [15]. Indeed, this main component of ECM is naturally involved in tissue repair, and displays unique physical-chemical properties (e.g. viscoelasticity, biodegrability, biocompatibility), making it an ideal material for TE [16][17][16-17]. Over the years, HA has been isolated from rooster combs or, through microbial fermentation. Today the production and purification of HA has turned into an industry. Highly pure HA is available in a wide range of molecular weights at relatively low costs. However, due to the short turnover rate and limited mechanical properties of native HA solutions, chemical modifications are required to obtain suitable stable biomaterials (e.g. hydrogels for in vivo tissue repair). Thereby, methods of chemical crosslinking using different linkers have been investigated [18][19][20][18-20].
2. Physiological and Physical-Chemical Properties of Hyaluronic Acid (HA)
2.1. Structure and physical-chemical properties
HA was first isolated, in 1934, from the bovine vitreous humor where it was recognized as a muco-polysaccharide of high molecular weight (range of 102-104 kDa) [21]. The structure of this natural polymer is highly conserved between mammalian species and, is defined as a versatile, linear straight-chain, non-sulfated GAG, composed of 2000-25000 repeating alternative sequence of disaccharide units (β1,3 glucuronic acid (GlcA) and β1,4 N-acetylglucosamine (GlcNAc)) (Figure 2), with an extended length of 2-25 mm [22][23][24][22-24]. Its pseudorandom coil configuration in aqueous solvents occupies a large volume of solvent. It is thus considered as a space-filling molecule, loosening tissues, creating spaces for cell motility, decreasing cell-cell contacts, and impeding intercellular communication [25].
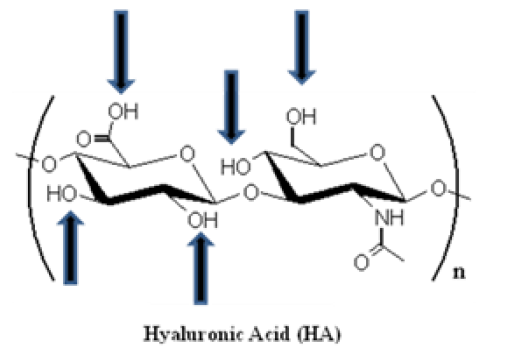
Figure 2: Spatial structure of hyaluronic acid (HA). HA is composed of alternating D-glucuronic acid and D-N-acetylglucosamine moieties, linked together via alternating β-1,4 and β-1,3 glycosidic bonds. The number of repeating disaccharide units (n) can be up to 25000, and each unit has a molecular weight of 401g/mol. The arrows schematize the functional groups that can be used for performing chemical modifications (e.g. addition of a linker).
Interestingly, HA displays viscoelastic properties [23][26][23,26], based on a mixture of intrinsic factors related to its structure (polymeric and polyelectrolyte) characteristics [16] such as (i) high molecular weight, (ii) electrostatic repulsions of the carboxylate ions (COO-), (iii) intramolecular hydrogen bonds and, (iv) double helical conformation.
2.2. Distribution and biological functions
HA is predominantly present in the ECM of some tissues (e.g. skin, umbilical cord, embryonic and malignant tissues) and body fluids (e.g. synovial fluid, vitreous humor) of all vertebrates as well as in some bacteria [22][27][28][29][30][22,27-30]. HA is also highly expressed in the glycocalyx, a pericellular coat of most cells, and is particularly prominent on the apical surface of endothelial cells [31].
Since its discovery, the biological functions of HA have been thoroughly investigated [23][25][23,25]. For instance, HA is involved in the (i) healing wound, tissue repair and regeneration [23][32][33][34][23,32-34], (ii) organization of the ECM [23][35][23,35], (iii) lubrication of the joints [23][28][23,28], (iv) regulation of the cell adhesion and motility through receptors that interact with the cytoskeleton [23][36][37][23,36,37], (v) angiogenesis by mediating cell proliferation, cell migration and cell differentiation [23][38][39][23,38,39]. Some of those functions (e.g. angiogenesis, tissue homeostasis, tissue remodeling) are exerted by binding to a family of cellular proteoglycan receptors, the hyaladhedrins (e.g. RHAMM aka CD168, CD44) [24][40][24,40].
Interestingly, the relevant role of HA in promoting cancer is actively investigated. Indeed, HA-HA receptor interaction is known to generate intracellular signaling, which, in turn, promotes tumor growth, metastasis, angiogenesis, trafficking of tumor-associated macrophages, and chemoresistance [41][42][43][44][45][46][47][41-47]. In fact, a HA-hyaluronidase (HAHAase) complex system would be involved in tumor angiogenesis. Indeed, HA synthase (Has) expression required HYAL-1, a HAase, to promote tumor growth and progression [48][49][48-49]. Concordantly, the use of 4-methylumbelliferone (4-MU), an HA synthesis (Has) inhibitor, displayed antitumor activity in cancer cells [50]. Besides, HYAL-1 is able to degrade HA into proangiogenic fragments that support tumor progression, invasion, and angiogenesis in some cancer models [51][52][51-52]. Therefore, HYAL-1 expression is potentially an independent predictor of metastasis [53][54][55][56][53-56] and, HYAL-1 can be considered as a valuable target for cancer therapy. Thereby, the use of sulfated HA (sHA), generated by O-sulfation of HA, was shown to inhibit HAases [57] and tumor growth [58]. Eventually, a recent safety assessment report showed that HA does not play a causal role in cancer metastasis and, that the widespread clinical use of HA has been shown free of significant adverse reactions [59].
Nevertheless, further mechanistic insights into the tumorassociated HA-HAase system and a preclinical proof-of-concepts of the safety and efficacy of inhibitors against HAases, are still required. Moreover, in our opinion, the risks of developing cancer using HAbased scaffolds in TE shall be rigorously evaluated in a time-dependent manner as HA biodegradation can generate pro-angiogenic fragments.
2.3. Metabolism
Circulating HA is mostly derived from lymph. Lymph nodes may nevertheless extract as much as 80-90% from peripheral lymph before it can reach the bloodstream [29]. HA can be taken up by cells [60] through receptors such as CD44 [61][62][63][61-63] and the receptor for HA-mediated motility (RHAMM) [64]. Nevertheless, HA is not immediately degraded by the cells since intact HA chains have been detected in cytoplasm, in nucleus, and even within the nucleolus [65][66][67][65-67]. Furthermore, it has been suggested that stromal cells (aka connective tissue cells such fibroblasts and endothelial cells) primarily synthesize HA, through specific synthases, before supplying it to the epithelial cells [68]. The general pattern of distribution suggested that HA is absorbed from plasma and tissue fluids by elements of the reticuloendothelial (RES) system [69].
Despite the increasing importance of HA in biology, little is still known about the degradation of HA in tissues. The catabolism scheme of endogenous HA did report an uptake of the lymphatic system followed by blood transportation to the liver where it is fully degraded [70]. Uptake and metabolism are thus primarily effected in liver and lymph node by endothelial cells lining the sinusoids of each [29]. A significant plasmatic flux of radio-labeled HA was also taken up in bone marrow and in lymph nodes [29][69][29,69]. In lymph nodes and in spleen, macrophage-like cells intertwined with the endothelial cells to take up HA [29].
HA can be then degraded by glycosylphosphatidylinositol (GPI)- anchored HAases, class of enzymes that cleave specifically HA glycosidic bonds [71], by oxidation due to reactive oxygen species (ROS) [72], heat [73], hydrolysis [74], among others [75]. The daily metabolic turnover of HA ended up to approximately one third of the total body content (e.g. 15g of HA for a 70 kg individual) [76][77][76,77]. Between different tissues, rates of turnover varied widely. In the bloodstream, the t1/2 for HA was rapid (i.e. 2-5 min) while in a tissue as relatively inert as cartilage, HA turnover occurs in 1-3 weeks [78]. Interestingly, normal turnover in the bloodstream has been estimated in the range of 0.3- 1.0 μg/min/kg body weight, and turnover in peripheral tissues may be effected by degradation in situ, or by transfer into lymph by diffusion or hydrodynamic forces [29].
Increasing evidence points to the existence of a mini-organelle, a hyaluronasome, a possible multi-protein membrane-associated complex, that has both HA synthetic and catabolic activities and, which possesses sensitive sensor mechanisms that can respond to various metabolic states [79][80][79,80]. Thereby, it has been suggested that high molecular HA is tethered to the plasma cell surface by HA receptors, combined possibly with an interaction with Hyal-2, a GPI-linked hyaluronidase anchored to the plasma membrane [80].
3. Some HA-based Scaffolds Applications in Bio-Medicine and Pharmacy
3.1. Use of HA-based scaffolds in regenerative medicine
When a tissue is largely damaged, tissue transplantation (e.g. bone) or prosthetic implants usually are considered as major medical solutions [81][103]. However, those therapeutic options are not without limitations and risks for the patient. Indeed, tissue transplantation may (i) be limited by tissue supply, (ii) cause discomfort to the patients, (iii) increase the risk of disease transmission and, (iv) cause host reactions [82][104]. Besides, prosthetic implants are not physiologically functional and, are too often accompanied by infection and structural failure [83][105].
As an alternative therapeutic option to tissue transplantation and prosthetic implantation, TE showed very promising results mainly for acute and relatively small lesions (e.g. cartilage reconstruction) and, it is expected that TE will have tremendous clinical applications if it can be applied to chronic lesions (e.g. large chondral lesions in patients with degenerative osteoarthritis) [84][106].
TE consists in inducing or accelerating the tissue forming process by in situ delivering of progenitor/stem cells, and/or growth factors using degradable biomaterial scaffolds (e.g. HA based-scaffolds designed according to the tissue defect) [85][107]. Importantly, the sitedirected injection of a growth factor without a scaffold was helpless [86][108], because the later was required to locally concentrate the growth factor [87][109].
Bone represents one of the most frequently transplanted tissues in spite of its capacity to self-regenerate [88][110]. Nevertheless, bone transplantation is limited and presents the risks previously evoked [82][83][104,105]. Then, bone TE often involved injectable polymer-based scaffolds (e.g. HA hydrogels), osteoprogenitor or osteo-stem cells (e.g. mesenchymal stem cells (MSCs)) which were able to get differentiated in bone-building cells (aka osteoblasts) in presence of growth factors (e.g. bone morphogenic proteins (BMPs), a group of proteins belonging to the transforming growth factor-b TGF-b) superfamily) [89][90][91][111-113]. Thereby, HA-based polymers were used as cell carriers for tissueengineered repair of bone and cartilage [92][93][114,115]. Interestingly, a new minimally invasive tissue-engineering approach, named Hyalograft®, consisting in the implantation of expanded autologous chondrocytes grown on a three-dimensional hyaluronan-based scaffold, was described as a safe, rapid, easy-handle, and viable therapeutic option for treatment of acute cartilage lesions over currently available autologous chondrocyte implantation techniques [94][116]. Besides, co-encapsulation of TGF-β3 containing nanofilm-coated alginate microspheres, along with human MSCs in HA hydrogels, has recently shown promising results in animal models towards the development of implantable constructs for cartilage reconstruction (aka chondrogenesis) [95][117]. Eventually, to establish medical use of TE technology for ligament and tendon injuries, a scaffold with sufficient ability for cell growth, cell differentiation, and mechanical properties, has been developed [96][118]. This scaffold made from chitosan and 0.1% HA exhibited adequate biodegradability, biocompatibility, joint stabilization, low toxicity and low inflammation in animal experiments [96][118].
3.2. Use of HA-based scaffolds as drug delivery systems
HA hydrogels, among others, are widely used as dermal and transdermal drug delivery systems. These innovative carrier systems were designed for the controlled release of drugs through the skin into the systemic circulation, in order to maintain consistent efficacy and reduce the dose and potential side-effects of the drug(s) [97][119].
For instance, a local delivery of DNA through a hydrogel scaffold would increase the applicability of gene therapy in tissue regeneration and cancer therapy [98][120]. Thereby, a novel process, termed caged nanoparticle encapsulation (aka CnE), has been developed for loading concentrated and unaggregated non-viral gene delivery nanoparticles into various HA hydrogels [98][120].
Furthermore, HA-bioconjugates have been developed to enhance selective entry of cytotoxic drugs into HA receptor-expressing cancerous cells (e.g. CD44 and RHAMM in ovarian cancer cells) [99][100][121-122]. Indeed, it was shown that (PEG)-conjugated HA nanoparticles (HA-NPs) were largely taken up by cancer cells over-expressing the HAreceptor CD44 comparatively to normal fibroblast cells, improving the tumor targetability in vivo [100][122]. Concordantly, a new HA-paclitaxel bioconjugate, Oncofid-P®, was more effective than the use of free paclitaxel for intraperitoneal treatment of ovarian cancer in mice [101][123].
However, HA and its degradation products, accumulated into the stroma of various human tumors, can modulate intracellular signaling pathways and, positively affect angiogenesis of malignant cells and multidrug resistance [102][124].
3.3. Use of HA-based scaffolds in anti-aging and esthetic medicine
HA reached prominence in cosmetic practice where it represents the injectable dermal filler of choice for most anti-aging, esthetic and plastic specialists [103][125].
For instance, HA is used for the correction of soft tissue defects (e.g. skin regeneration, wrinkle-treatment, wound healing) [103][104][125,126]. Thereby, HA material provided an effective, non invasive, non surgical alternative for correction of the contour defects of the face due to its enormous ability to bind water and easiness of implantation [105][127]. In the US, eight HA dermal fillers were approved for commercialization by the Food and Drug Administration (FDA) although severe adverse effects have been reported [106][128].
4. Conclusion
HA is a naturally occurring polysaccharide which is present in extracellular matrices of soft connective tissues and body fluids. With regards to its mechanism of synthesis, its size and its physical-chemical properties, HA is unique among other glycosaminoglycans. HA is able to interact with other macromolecules (e.g. proteins) and, participates in regulating the cell behavior during numerous morphogenic, restorative, and pathological processes in the body. The role of HA in diseases, such as in various forms of cancers, arthritis and osteoporosis, is leading to new impetus in research and development. The preparation of the safest and efficient HA-based biomaterials for theranostic medicine for any type of lesions, regardless their surface, remains an exciting challenge.
