
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bo He | -- | 2549 | 2022-11-19 06:17:12 | | | |
| 2 | Bo He | Meta information modification | 2549 | 2022-11-19 06:22:29 | | | | |
| 3 | Camila Xu | Meta information modification | 2549 | 2022-11-21 07:30:54 | | | | |
| 4 | Camila Xu | Meta information modification | 2549 | 2022-11-21 07:39:07 | | | | |
| 5 | Camila Xu | + 1 word(s) | 2550 | 2022-11-21 07:53:31 | | |
Video Upload Options
To date, effectively controlling resistant weeds has been a great challenge in modern agricultural production. Developing new modes of action of herbicides would be an efficient, convenient, and . In particular, new modes of herbicide action do not appear to have evolutionary resistance or cross-resistance with existing herbicides. However, a few successful herbicides with new modes of action (MoAs) have been marketed in the past 20 years. Researchers summarized the positive herbicide targets for the herbicides that have been discovered in recent years, such as Solanyl Diphosphate Synthase (SPS), Fatty Acid Thioesterase (FAT), Plastid Peptide Deformylase (PDEF), and Dihydroxy-Acid Dehydratase (DHAD). Some commercial herbicide varieties have been obtained based on novel herbicide targets, such as Homogentisate Solanesyltransferase (HST) and Dihydroorotate Dehydrogenase (DHODH). This provides a new reference and idea for herbicide molecular design in the future.
1. Introduction
2. Herbicide Resistance Risk
| Herbicide Group | Example Group | Dicots | Monocots | Total |
|---|---|---|---|---|
| Inhibition of Acetolactate Synthase | Chlorsulfuron | 105 | 66 | 171 |
| PSII inhibitors-Serine 264 Binders | Chlorotoluron | 53 | 34 | 87 |
| Inhibition of Enolpyruvyl Shikimate Phosphate Synthase |
Glyphosate | 27 | 29 | 56 |
| Inhibition of Acetyl CoA Carboxylase |
Sethoxydim | 0 | 50 | 50 |
| Auxin Mimics | 2,4-D | 34 | 8 | 42 |
| PS I Electron Diversion | Paraquat | 22 | 10 | 32 |
| Inhibition of Protoporphyrinogen Oxidase | Oxyfluorfen | 10 | 4 | 14 |
| Very Long-Chain Fatty Acid Synthesis inhibitors | Butachlor | 2 | 11 | 13 |
| Inhibition of Microtubule Assembly 2 |
Trifluralin | 2 | 10 | 12 |
| Inhibition of Lycopene Cyclase | Amitrole | 1 | 5 | 6 |
| Inhibition of Glutamine Synthetase | Glufosinate-ammonium | 1 | 5 | 6 |
| Phytoene Desaturase inhibitors | Diflufenican | 4 | 1 | 5 |
| PSII inhibitors-Histidine 215 Binders |
Bromoxynil | 3 | 1 | 4 |
| Inhibition of Cellulose Synthesis | Dichlobenil | 0 | 4 | 4 |
| Inhibition of Hydroxyphenyl Pyruvate Dioxygenase |
Isoxaflutole | 3 | 0 | 3 |
| Inhibition of Microtubule Assembly | Clomazone | 0 | 3 | 3 |
| Antimicrotubule mitotic disrupter | Flamprop-methyl | 0 | 3 | 3 |
| Inhibition of Microtubule Organization |
Propham | 0 | 1 | 1 |
| Nucleic acid inhibitors | MSMA | 1 | 0 | 1 |
| Unknown | Endothall | 0 | 1 | 1 |
| Cell elongation inhibitors | Difenzoquat | 0 | 1 | 1 |
3. Novel Potential Herbicide Targets
3.1. The Discovery of New Targets Based on Known Herbicides
3.1.1. Solanyl Diphosphate Synthase (SPS)
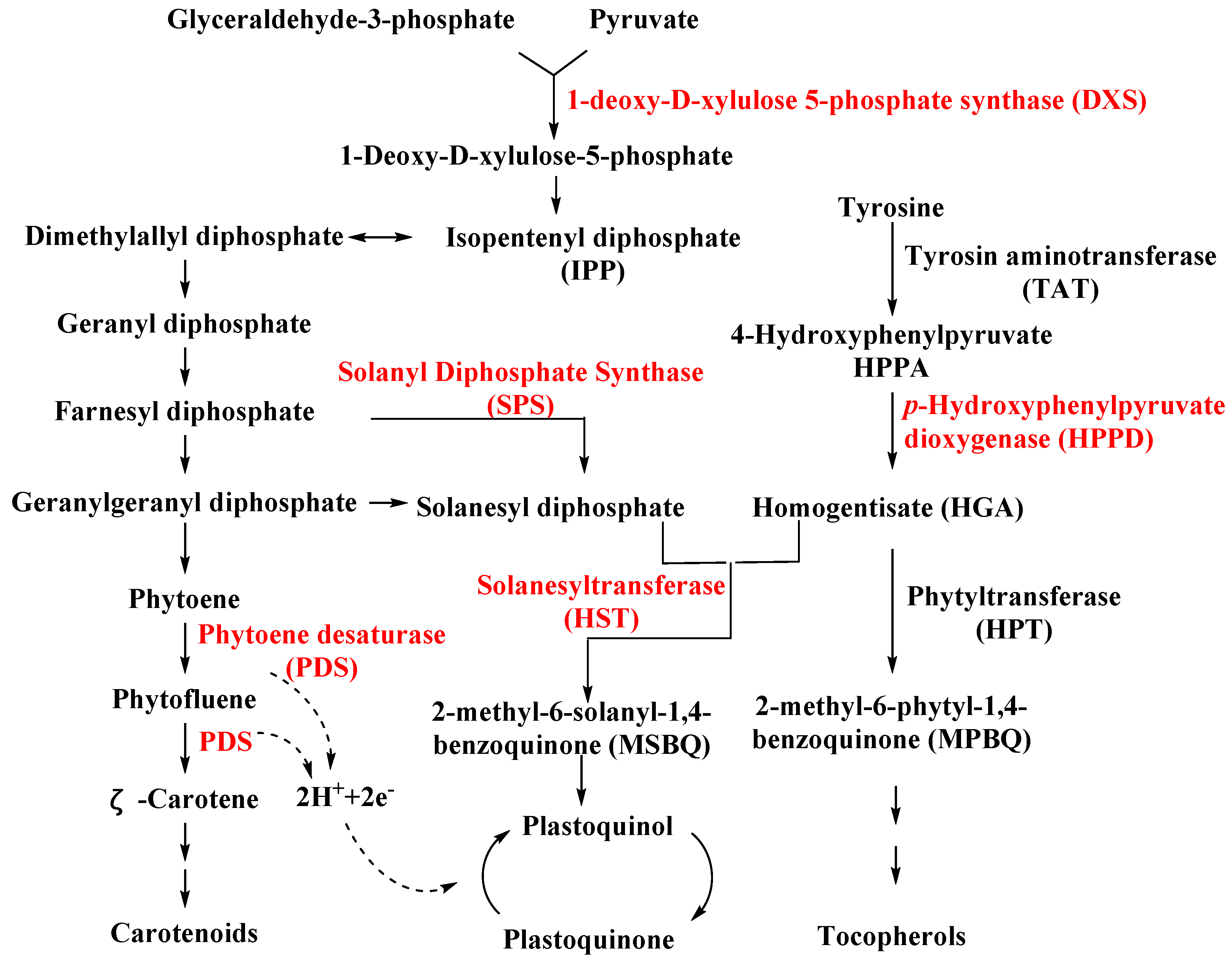
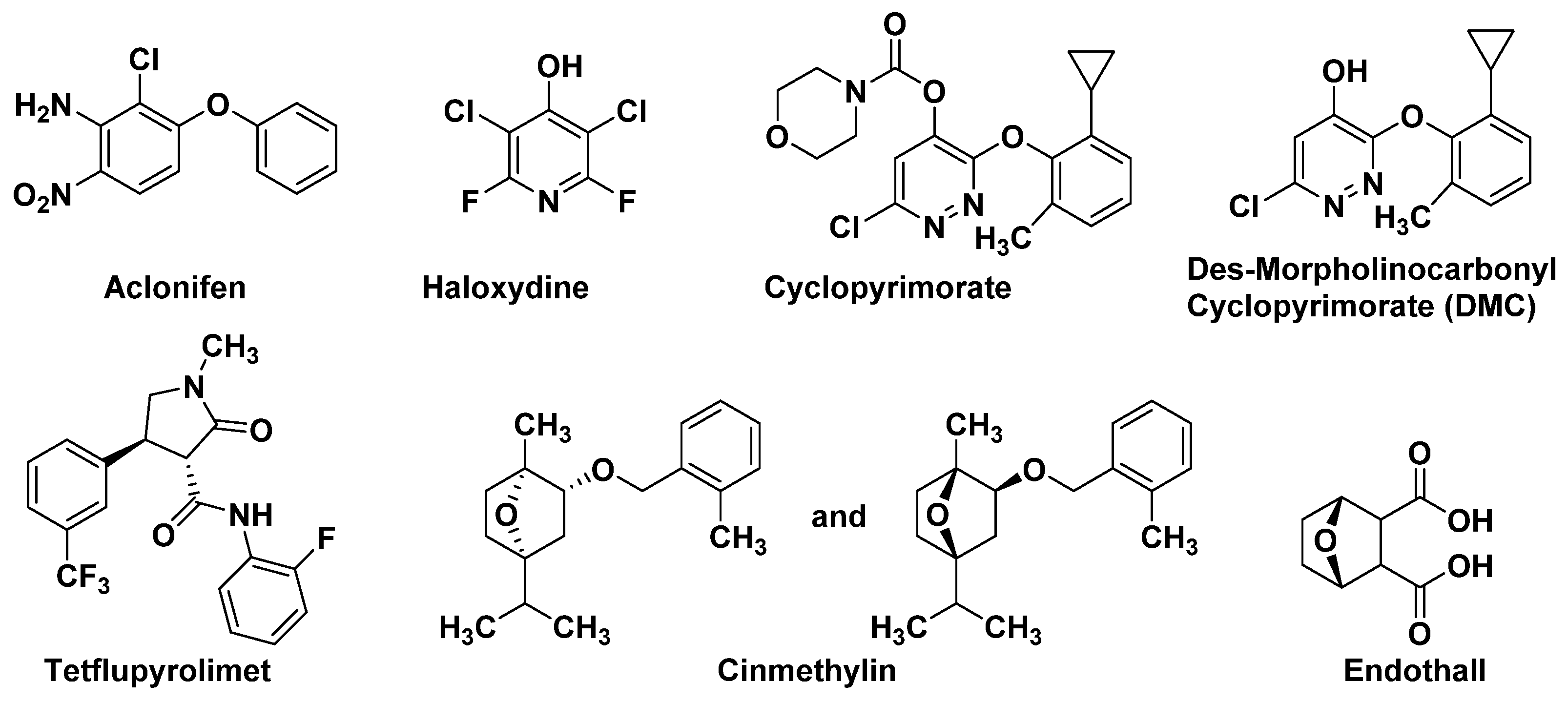
3.1.2. Homogentisate Solanesyltransferase (HST)
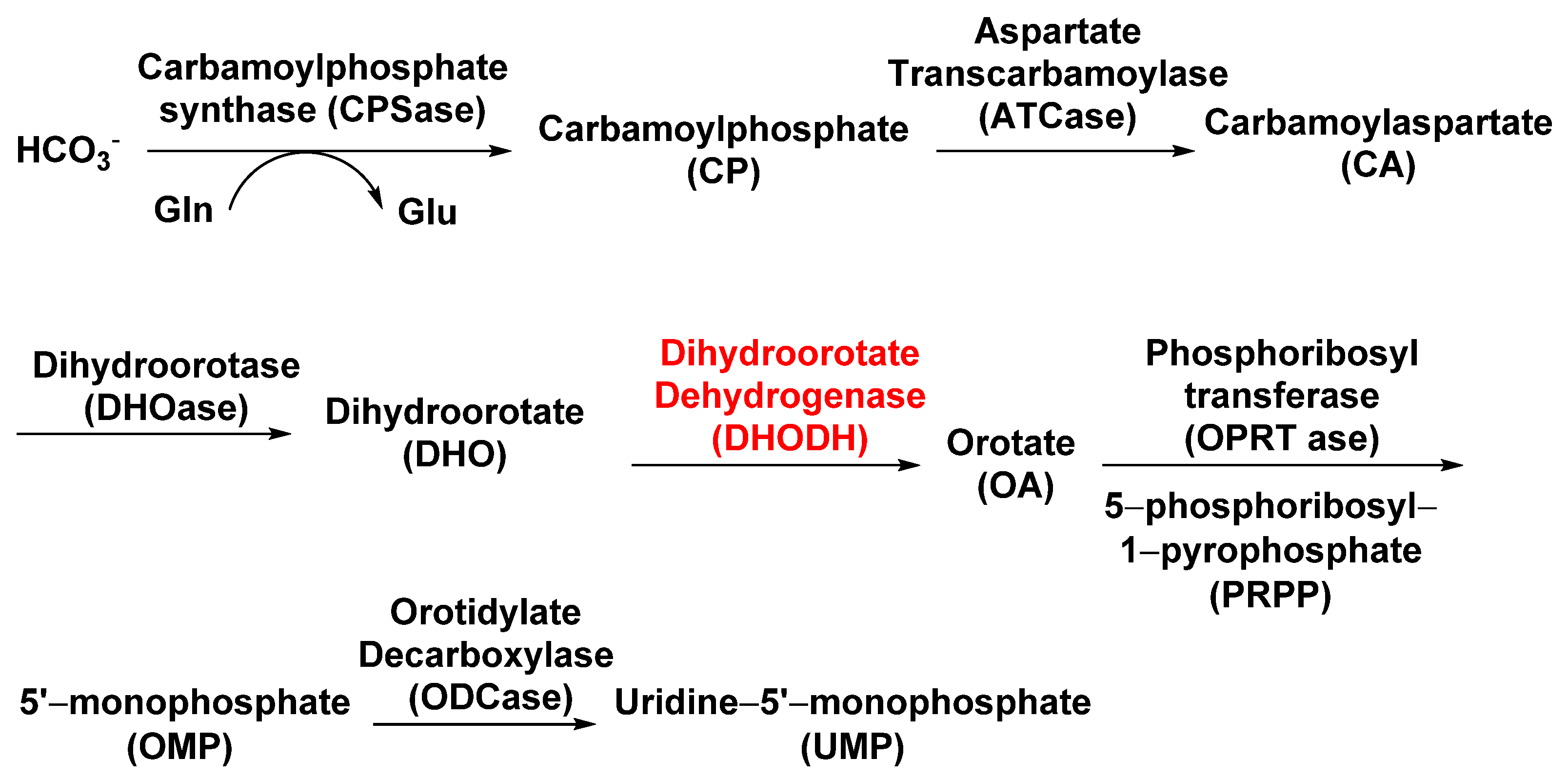
3.1.3. Dihydroorotate Dehydrogenase (DHODH)
3.1.4. Fatty Acid Thioesterase (FAT)
3.1.5. Serine/Threonine Protein Phosphatases (PPs)
3.2. Exploring New Herbicide MoAs Based on Natural Products
3.2.1. Dihydroxy-Acid Dehydratase (DHAD)
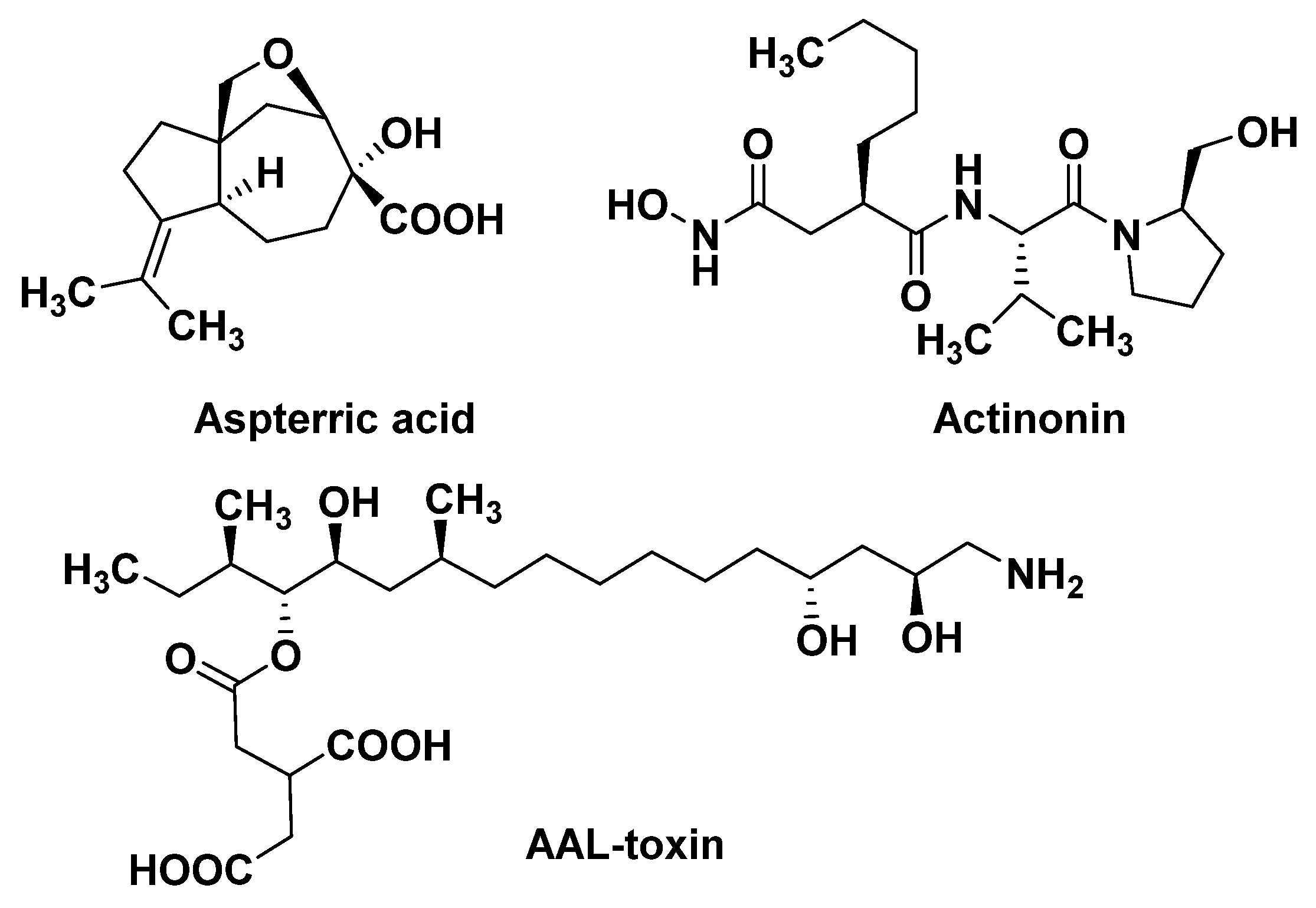
3.2.2. Plastid Peptide Deformylase (PDEF)
3.2.3. Ceramide Synthase
3.3. Exploration of Potential Herbicide MoAs Based on Their Biochemical Mechanisms
3.3.1. DNA Gyrase
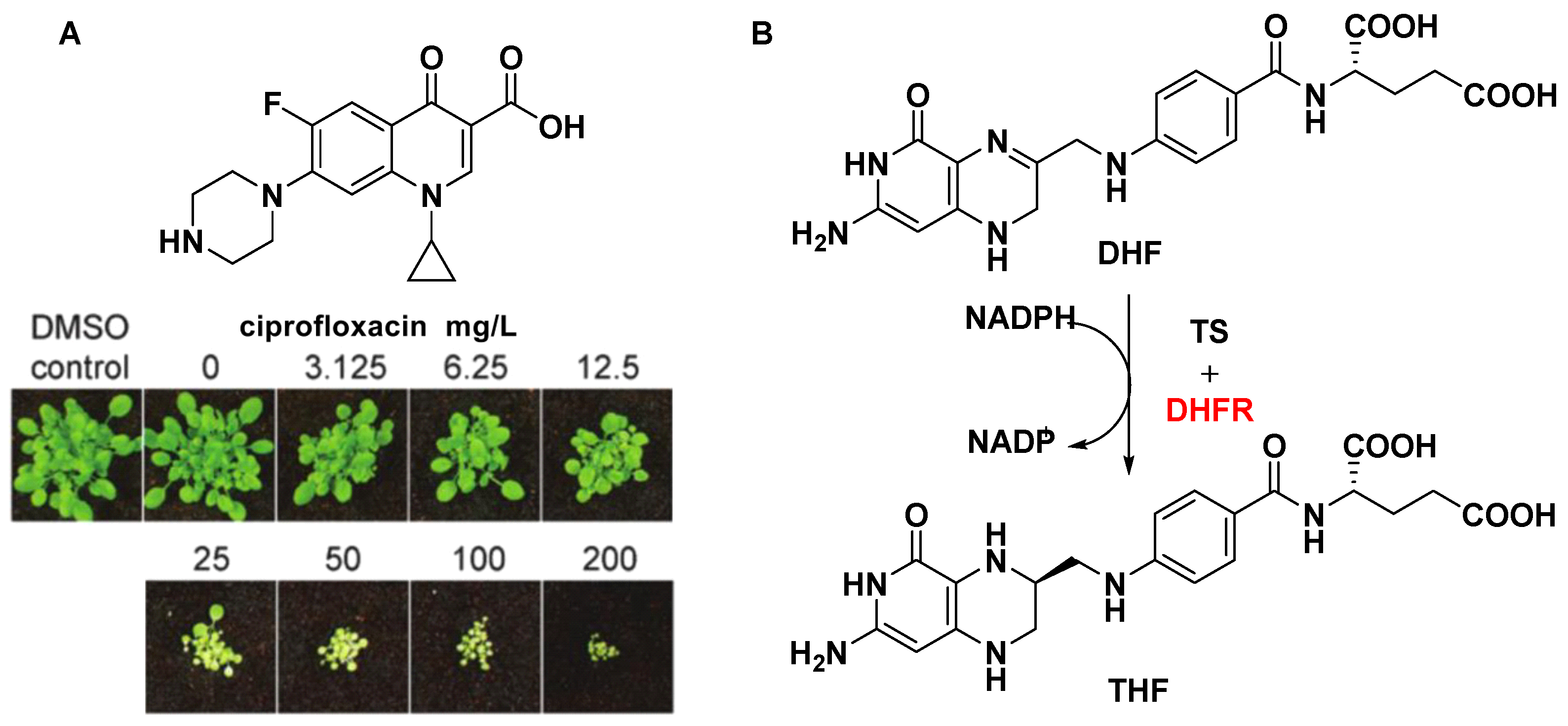
3.3.2. Dihydrofolate Reductase (DHFR)
References
- Mascarelli, A. Growing up with pesticides. Science 2013, 341, 740–741.
- Sparks, T.C.; Lorsbach, B.A. Perspectives on the agrochemical industry and agrochemical discovery. Pest Manag. Sci. 2017, 73, 672–677.
- Godfray, H.C.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818.
- Enserink, M.; Hines, P.J.; Vignieri, S.N.; Wigginton, N.S.; Yeston, J.S. Smarter pest control. The pesticide paradox. Introduction. Science 2013, 341, 728–729.
- He, B.; Dong, J.; Lin, H.Y.; Wang, M.Y.; Li, X.K.; Zheng, B.F.; Chen, Q.; Hao, G.F.; Yang, W.C.; Yang, G.F. Pyrazole-isoindoline-1,3-dione hybrid: A promising scaffold for 4-hydroxyphenylpyruvate dioxygenase inhibitors. J. Agric. Food Chem. 2019, 67, 10844–10852.
- Lamberth, C.; Jeanmart, S.; Luksch, T.; Plant, A. Current challenges and trends in the discovery of agrochemicals. Science 2013, 341, 742–746.
- Peterson, G.E. The discovery and development of 2,4-D. Agric. Hist. Soc. 1967, 41, 243–254.
- Sterling, T.M.; Hall, J.C. Mechanism of action of auxins and the kinetics of cellular growth. In Herbicide Activity: Biochemistry and Molecular Biology; Roe, R.M., Burton, J.D., Kuhr, R.J., Eds.; IOS Press: Amsterdam, The Netherlands, 1997; pp. 111–141.
- Service, R.F. Agriculture. What happens when weed killers stop killing? Science 2013, 341, 1329.
- Wang, Q.; Ge, L.; Zhao, N.; Zhang, L.; You, L.; Wang, D.; Liu, W.; Wang, J. A Trp-574-Leu mutation in the acetolactate synthase (ALS) gene of Lithospermum arvense L. confers broad-spectrum resistance to ALS inhibitors. Pestic. Biochem. Physiol. 2019, 158, 12–17.
- Beckie, H.J.; Tardif, F.J. Herbicide cross resistance in weeds. Crop Prot. 2012, 35, 15–28.
- Duke, S.O. Why have no new herbicide modes of action appeared in recent years? Pest Manag. Sci. 2012, 68, 505–512.
- Tresch, S.; Schmotz, J.; Grossmann, K. Probing mode of action in plant cell cycle by the herbicide endothall, a protein phosphatase inhibitor. Pestic. Biochem. Physiol. 2011, 99, 86–95.
- Kahlau, S.; Schroder, F.; Freigang, J.; Laber, B.; Lange, G.; Passon, D.; Kleessen, S.; Lohse, M.; Schulz, A.; von Koskull-Doring, P.; et al. Aclonifen targets solanesyl diphosphate synthase, representing a novel mode of action for herbicides. Pest Manag. Sci. 2020, 76, 3377–3388.
- Liu, M.; Lu, S. Plastoquinone and ubiquinone in plants: Biosynthesis, physiological function and metabolic engineering. Front. Plant Sci. 2016, 7, 1898.
- Ohara, K.; Sasaki, K.; Yazaki, K. Two solanesyl diphosphate synthases with different subcellular localizations and their respective physiological roles in Oryza sativa. J. Exp. Bot. 2010, 61, 2683–2692.
- Dayan, F.E.; Haesaert, G.; van Leeuwen, T.; Holden-Dye, L.; Crossthwaite, A.; Nauen, R. Pesticides modes of action and resistance: A perspective from the 2019 IUPAC congress. Outlooks Pest Manag. 2019, 30, 157–163.
- Jun, L.; Saiki, R.; Tatsumi, K.; Nakagawa, T.; Kawamukai, M. Identification and subcellular localization of two solanesyl diphosphate synthases from Arabidopsis thaliana. Plant Cell Physiol. 2004, 45, 1882–1888.
- Sadre, R.; Frentzen, M.; Saeed, M.; Hawkes, T. Catalytic reactions of the homogentisate prenyl transferase involved in plastoquinone-9 biosynthesis. J. Biol. Chem. 2010, 285, 18191–18198.
- Collakova, E.; DellaPenna, D. Homogentisate phytyltransferase activity is limiting for tocopherol biosynthesis in Arabidopsis. Plant Physiol. 2003, 131, 632–642.
- Collakova, E.; DellaPenna, D. Isolation and functional analysis of homogentisate phytyltransferase from Synechocystis sp. PCC 6803 and arabidopsis. Plant Physiol. 2001, 127, 1113–1124.
- Shino, M.; Hamada, T.; Shigematsu, Y.; Hirase, K.; Banba, S. Action mechanism of bleaching herbicide cyclopyrimorate, a novel homogentisate solanesyltransferase inhibitor. J. Pestic. Sci. 2018, 43, 233–239.
- Ullrich, A.; Knecht, W.; Piskur, J.; Löffler, M. Plant dihydroorotate dehydrogenase differs significantly in substrate specificity and inhibition from the animal enzymes. FEBS Lett. 2002, 529, 346–350.
- Zrenner, R.; Stitt, M.; Sonnewald, U.; Boldt, R. Pyrimidine and purine biosynthesis and degradation in plants. Annu. Rev. Plant Biol. 2006, 57, 805–836.
- Campe, R.; Hollenbach, E.; Kammerer, L.; Hendriks, J.; Hoffken, H.W.; Kraus, H.; Lerchl, J.; Mietzner, T.; Tresch, S.; Witschel, M.; et al. A new herbicidal site of action: Cinmethylin binds to acyl-ACP thioesterase and inhibits plant fatty acid biosynthesis. Pestic. Biochem. Physiol. 2018, 148, 116–125.
- Umetsu, N.; Shirai, Y. Development of novel pesticides in the 21st century. J. Pestic. Sci. 2020, 45, 54–74.
- Dayan, F.E.; Romagni, J.G.; Duke, S.O. Herbicides, cinmethylin. In Encyclopedia of Agrochemicals; Plimmer, J.R., Gammon, D.W., Ragsdale, N.N., Eds.; John Wiley & Sons: New York, NY, USA, 2003; pp. 754–757.
- Uhrig, R.G.; Labandera, A.M.; Moorhead, G.B. Arabidopsis PPP family of serine/threonine protein phosphatases: Many targets but few engines. Trends Plant Sci. 2013, 18, 505–513.
- Bajsa, J.; Pan, Z.; Dayan, F.E.; Owens, D.K.; Duke, S.O. Validation of serine/threonine protein phosphatase as the herbicide target site of endothall. Pestic. Biochem. Physiol. 2012, 102, 38–44.
- Duke, S.O.; Stidham, M.A.; Dayan, F.E. A novel genomic approach to herbicide and herbicide mode of action discovery. Pest Manag. Sci. 2019, 75, 314–317.
- Yan, Y.; Liu, Q.; Zang, X.; Yuan, S.; Bat-Erdene, U.; Nguyen, C.; Gan, J.; Zhou, J.; Jacobsen, S.E.; Tang, Y. Resistance-gene-directed discovery of a natural-product herbicide with a new mode of action. Nature 2018, 559, 415–418.
- Chen, D.Z.; Patel, D.V.; Hackbarth, C.J.; Wang, W.; Dreyer, G.; Young, D.C.; Margolis, P.S.; Wu, C.; Ni, Z.J.; Trias, J.; et al. Actinonin, a naturally occurring antibacterial agent, is a potent deformylase inhibitor. Biochemistry 2000, 39, 1256–1262.
- Bisson, C.; Britton, K.L.; Sedelnikova, S.E.; Rodgers, H.F.; Eadsforth, T.C.; Viner, R.C.; Hawkes, T.R.; Baker, P.J.; Rice, D.W. Crystal structures reveal that the reaction mechanism of imidazoleglycerol-phosphate dehydratase is controlled by switching Mn(II) coordination. Structure 2015, 23, 1236–1245.
- Dayan, F.E.; Duke, S.O. Natural compounds as next-generation herbicides. Plant Physiol. 2014, 166, 1090–1105.
- Adams, J.M. On the release of the formyl group from nascent protein. J. Mol. Biol. 1968, 33, 571–589.
- Serero, A.; Giglione, C.; Sardini, A.; Martinez-Sanz, J.; Meinnel, T. An unusual peptide deformylase features in the human mitochondrial N-terminal methionine excision pathway. J. Biol. Chem. 2003, 278, 52953–52963.
- Wang, E.; Merrill, A.H. Ceramide synthase. Methods Enzymol. 2000, 311, 15–21.
- Gechev, T.S.; Gadjev, I.Z.; Hille, J. An extensive microarray analysis of AAL-toxin-induced cell death in Arabidopsis thaliana brings new insights into the complexity of programmed cell death in plants. Cell. Mol. Life Sci. 2004, 61, 1185–1197.
- Duke, S.O.; Dayan, F.E. Clues to new herbicide mechanisms of action from natural sources. ACS Symp. Ser. 2013, 1141, 203–215.
- Hsiao, P.; Sanjaya; Su, R.C.; Teixeira da Silva, J.A.; Chan, M.T. Plant native tryptophan synthase beta 1 gene is a non-antibiotic selection marker for plant transformation. Planta 2007, 225, 897–906.
- Dayan, F.E.; Ferreira, D.; Wang, Y.H.; Khan, I.A.; McInroy, J.A.; Pan, Z. A pathogenic fungi diphenyl ether phytotoxin targets plant enoyl (acyl carrier protein) reductase. Plant Physiol. 2008, 147, 1062–1071.
- Templeton, M.D.; Reinhardt, L.A.; Collyer, C.A.; Mitchell, R.E.; Cleland, W.W. Kinetic analysis of the L-ornithine transcarbamoylase from Pseudomonas savastanoi pv. phaseolicola that is resistant to the transition state analogue (R)-N delta-(N′-sulfodiaminophosphinyl)-L-ornithine. Biochemistry 2005, 44, 4408–4415.
- Groth, G. Structure of spinach chloroplast F1-ATPase complexed with the phytopathogenic inhibitor tentoxin. Proc. Natl. Acad. Sci. USA 2002, 99, 3464–3468.
- Meiss, E.; Konno, H.; Groth, G.; Hisabori, T. Molecular processes of inhibition and stimulation of ATP synthase caused by the phytotoxin tentoxin. J. Biol. Chem. 2008, 283, 24594–24599.
- Hou, C.X.; Dirk, L.M.; Pattanaik, S.; Das, N.C.; Maiti, I.B.; Houtz, R.L.; Williams, M.A. Plant peptide deformylase: A novel selectable marker and herbicide target based on essential cotranslational chloroplast protein processing. Plant Biotechnol. J. 2007, 5, 275–281.
- Driouich, A.; Jauneau, A.; Staehelin, L.A. 7-Dehydrobrefeldin A, a naturally occurring brefeldin a derivative, inhibits secretion and causes a cis-to-trans breakdown of golgi stacks in plant cells. Plant Physiol. 1997, 113, 487–492.
- Hejli, A.M.; Koster, K.L. The allelochemical sorgoleone inhibits root H+-ATPase and water uptake. J. Chem. Ecol. 2004, 30, 2181–2191.
- Danielsen, E.M. Tagetitoxin inhibits RNA synthesis directed by RNA polymerases from chloroplasts and Escherichia coli. J. Biol. Chem. 1990, 29, 493–498.
- Wallace, M.D.; Waraich, N.F.; Debowski, A.W.; Corral, M.G.; Maxwell, A.; Mylne, J.S.; Stubbs, K.A. Developing ciprofloxacin analogues against plant DNA gyrase: A novel herbicide mode of action. Chem. Commun. 2018, 54, 1869–1872.
- Evans-Roberts, K.M.; Mitchenall, L.A.; Wall, M.K.; Leroux, J.; Mylne, J.S.; Maxwell, A. DNA gyrase is the target for the quinolone drug ciprofloxacin in Arabidopsis thaliana. J. Biol. Chem. 2016, 291, 3136–3144.
- Dayan, F.E. Current Status and Future Prospects in Herbicide Discovery. Plants 2019, 8, 341.
- Wilson, P.M.; Danenberg, P.V.; Johnston, P.G.; Lenz, H.J.; Ladner, R.D. Standing the test of time: Targeting thymidylate biosynthesis in cancer therapy. Nat. Rev. Clin. Oncol. 2014, 11, 282–298.
- Corral, M.G.; Haywood, J.; Stehl, L.H.; Stubbs, K.A.; Murcha, M.W.; Mylne, J.S. Targeting plant DIHYDROFOLATE REDUCTASE with antifolates and mechanisms for genetic resistance. Plant J. 2018, 95, 727–742.
- Peter, E.; Rothbart, M.; Oelze, M.L.; Shalygo, N.; Dietz, K.J.; Grimm, B. Mg protoporphyrin monomethylester cyclase deficiency and effects on tetrapyrrole metabolism in different light conditions. Plant Cell Physiol. 2010, 51, 1229–1241.
- Maimann, S.; Wagner, C.; Kreft, O.; Zeh, M.; Willmitzer, L.; Hofgen, R.; Hesse, H. Transgenic potato plants reveal the indispensable role of cystathionine beta-lyase in plant growth and development. Plant J. 2000, 23, 747–758.
- Silverman, D.N.; Lindskog, S. The catalytic mechanism of carbonic anhydrase: Implications of a rate-limiting protolysis of water. Acc. Chem. Res. 2002, 21, 30–36.
- Kochetov, G.A.; Solovjeva, O.N. Structure and functioning mechanism of transketolase. Biochim. Biophys. Acta 2014, 1844, 1608–1618.
- Zheng, Y.; Tian, L.; Liu, H.; Pan, Q.; Zhan, J.; Huang, W. Sugars induce anthocyanin accumulation and flavanone 3-hydroxylase expression in grape berries. Plant Growth Regul. 2009, 58, 251–260.




