To date, effectively controlling resistant weeds has been a great challenge in modern agricultural production. Developing new modes of action of herbicides would be an efficient, convenient, and . In particular, new modes of herbicide action do not appear to have evolutionary resistance or cross-resistance with existing herbicides. However, a few successful herbicides with new modes of action (MoAs) have been marketed in the past 20 years. We summarized the positive herbicide targets for the herbicides that have been discovered in recent years, such as Solanyl Diphosphate Synthase (SPS), Fatty Acid Thioesterase (FAT), Plastid Peptide Deformylase (PDEF), and Dihydroxy-Acid Dehydratase (DHAD). Some commercial herbicide varieties have been obtained based on novel herbicide targets, such as Homogentisate Solanesyltransferase (HST) and Dihydroorotate Dehydrogenase (DHODH). This provides a new reference and idea for herbicide molecular design in the future.
- herbicide target
- mode of action (MoA)
- resistant weeds control
1. Introduction
2. Herbicide Resistance Risk
The first synthetic auxin herbicide (2,4-D) was commercially released in the 1940s, which opened a new era of weed control in modern crop production and was used for the selective control of broadleaf weeds in cereal grain crops [7][8]. Subsequently, a series of highly effective selective herbicides were developed based on specific herbicide targets. Herbicides are becoming most widely used in agriculture. Although the existing herbicides play an important role in agricultural production, the herbicide-resistant weeds are still at risk of getting out of control. The rapid development of weed resistance due to the long-term use of a single type of herbicide resulted in the control effect of herbicides, that is, a significant reduction or even loss of control effectiveness [9]. Since 1956, when the first resistant weed Daucus carota L. was identified, the number of resistant weeds has increased by leaps and bounds. Statistics from the International Survey of Herbicide Resistance Weeds show that more than 500 species of weeds worldwide have evolved resistance to one or two known herbicides. Of all the herbicides, weeds have developed severe resistance to targeted acetolactate synthase (ALS) herbicides, and the number of resistant weeds has reached 171 (Table 1). Existing research shows that resistance to the herbicides that target ALS is mainly caused by amino acid mutations on the target [10].| Herbicide Group | Example Group | Dicots | Monocots | Total |
|---|---|---|---|---|
| Inhibition of Acetolactate Synthase | Chlorsulfuron | 105 | 66 | 171 |
| PSII inhibitors-Serine 264 Binders | Chlorotoluron | 53 | 34 | 87 |
| Inhibition of Enolpyruvyl Shikimate Phosphate Synthase |
Glyphosate | 27 | 29 | 56 |
| Inhibition of Acetyl CoA Carboxylase |
Sethoxydim | 0 | 50 | 50 |
| Auxin Mimics | 2,4-D | 34 | 8 | 42 |
| PS I Electron Diversion | Paraquat | 22 | 10 | 32 |
| Inhibition of Protoporphyrinogen Oxidase | Oxyfluorfen | 10 | 4 | 14 |
| Very Long-Chain Fatty Acid Synthesis inhibitors | Butachlor | 2 | 11 | 13 |
| Inhibition of Microtubule Assembly 2 |
Trifluralin | 2 | 10 | 12 |
| Inhibition of Lycopene Cyclase | Amitrole | 1 | 5 | 6 |
| Inhibition of Glutamine Synthetase | Glufosinate-ammonium | 1 | 5 | 6 |
| Phytoene Desaturase inhibitors | Diflufenican | 4 | 1 | 5 |
| PSII inhibitors-Histidine 215 Binders |
Bromoxynil | 3 | 1 | 4 |
| Inhibition of Cellulose Synthesis | Dichlobenil | 0 | 4 | 4 |
| Inhibition of Hydroxyphenyl Pyruvate Dioxygenase |
Isoxaflutole | 3 | 0 | 3 |
| Inhibition of Microtubule Assembly | Clomazone | 0 | 3 | 3 |
| Antimicrotubule mitotic disrupter | Flamprop-methyl | 0 | 3 | 3 |
| Inhibition of Microtubule Organization |
Propham | 0 | 1 | 1 |
| Nucleic acid inhibitors | MSMA | 1 | 0 | 1 |
| Unknown | Endothall | 0 | 1 | 1 |
| Cell elongation inhibitors | Difenzoquat | 0 | 1 | 1 |
3. Novel Potential Herbicide Targets
Research into herbicide targets began in the middle of the last century. There is no clear definition to describe a good herbicide target site. As Duke, S. O. mentioned [12], the crucial consideration is that blocking the function of a target is fatal and, as a result, plant survival is unrecoverable. Inhibition of a relatively small percentage of the target will cause serious injury to plants, followed by death. The biological function of the target is irreplaceable in the organism. Last cntury, using physiological and biochemical technology, many herbicide targets were determined. Later, high-throughput in vitro screening was used to discover potential herbicide targets. More recently, ‘omics’ approaches have been used to determine the targets of natural products or other bioactive molecules [12]. Using a molecular probe to study plant physiological and biochemical processes is becoming a more and more popular method to confirm potential herbicide targets [13].3.1. The Discovery of New Targets Based on Known Herbicides
3.1.1. Solanyl Diphosphate Synthase (SPS)
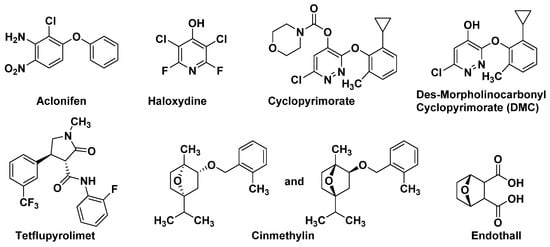
SPS is an important enzyme for plastoquinone synthesis, which catalyzes the multi-step condensation reaction of isopentenyl diphosphate and farnesyl diphosphate to form solanesyl diphosphate (Figure 1) [14]. Aclonifen (Figure 2), a relatively old diphenylether herbicide, caused the bleaching of treated plants. Recently, the binding site of aclonifen was confirmed via the inhibition of SPS and by blocking the biosynthesis of geranyl diphosphate [15][16]. A plant possesses three subtypes of SPS encoded by three genes: SPS1 and SPS2, which are localized in the chloroplast and involved in plastoquinone synthesis, and SPS3, which is involved in ubiquinone synthesis [17][18]. Existing research results show that aclonifen only inhibits SPS1 and SPS2, and has no response to SPS3. Herbicides that target HPPD have been used for many years and have achieved remarkable results in terms of herbicidal efficacy and safety. However, there has been not a significant breakthrough over the years for herbicides that target SPS. The main reason for this is that, for a long time in the past, the mechanism of action of SPS was not clear, and it was uncertain whether SPS would become a new herbicide target. Now that these problems have been solved, there will be more novel inhibitors targeting SPS based on the Aclonifen compound.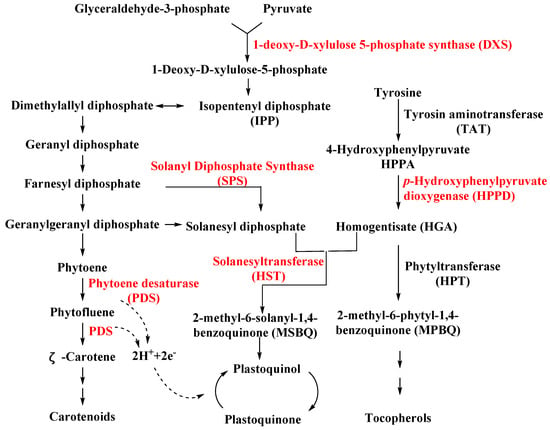
3.1.2. Homogentisate Solanesyltransferase (HST)
HST is another novel bleaching herbicide target that catalyzes the prenylation and decarboxylation of homogentisate (HGA) to form 2-methyl-6-solanesyl-1,4-benzoquinol (MSBQ) in the plastoquinone biosynthesis pathway (Figure 1) [19][20][21]. Plastoquinone, as the important cofactor, takes part in the biosynthesis process of carotenoids. Blocking the biosynthesis of carotenoids will interrupt the normal photosynthesis of plants, which leads to bleaching and death. Haloxydine (Figure 2) is a well-known HST inhibitor. Nevertheless, it has not yet been used commercially. Another HST inhibitor, cyclopyrimorate (Figure 2) as a rice herbicide, was invented by Mitsui Chemicals Agro Inc. and was launched in 2019 in Japan. Its metabolite product, cyclopyrimorate, strongly inhibited HST in crude E. coli extracts [22]. As a downstream protein of SPS and HPPD, with the successful launch of cyclopyrimorate, more efficient targeting of the HTS inhibitor can be quickly obtained using targeted herbicide molecular rational design methods.3.1.3. Dihydroorotate Dehydrogenase (DHODH)
Dihydroorotate dehydrogenase (DHODH; EC 1.3.99.11), located on the outer surface of the inner mitochondrial membrane, catalyzes the fourth step of pyrimidine biosynthesis [23]. De novo pyrimidine nucleotide biosynthesis consists of six enzymatic steps (Figure 3), which are evolutionarily conserved in all the species examined [24]. Inhibition of this pathway is lethal to most organisms. Tetflupyrolimet (Figure 2) is a novel targeting DHODH inhibitor that can interfere with de novo pyrimidine biosynthesis, and is expected to be commercialized in 2024. The target site of tetflupyrolimet was identified by adopting a combination of forwarding genetic screens and metabolomics approaches, and by testing the intrinsic affinities of analogs with the enzyme in vitro using biochemical methods [17]. The successful development of DHODH-targeted drugs will bring more selectivity to the management of resistant weeds.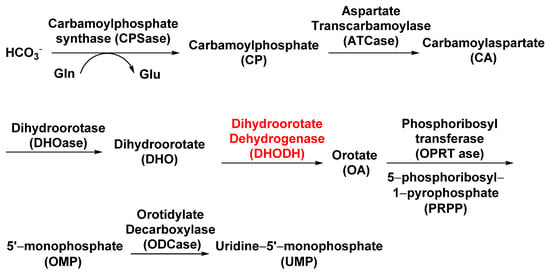
3.1.4. Fatty Acid Thioesterase (FAT)
In plants, fatty acid thioesterase (FAT) acts on the lipid biosynthesis pathway by mediating the release of fatty acids (FA) from its acyl carrier protein (ACP) and transferring them to the endoplasmic reticulum [25][26]. Cinmethylin (Figure 2), a natural product-like benzyl ether derivative of 1,4-cineole, is an old herbicide that was commercialized in 1989 [27]. The mode action of cinmethylin was not clear at first. Later, Campe R., et.al. confirmed that cinmethylin binds to FAT proteins from Lemna and Arabidopsis. using a fluorescence-based thermal shift assay. They also demonstrated the co-crystallization of cinmethylin within the FAT enzyme [25].3.1.5. Serine/Threonine Protein Phosphatases (PPs)
Phosphorylation plays a vital role in almost every aspect of cell life, and is one of the dominant means of controlling protein function and regulating most biological processes. PPs were divided into three subclasses—phosphoprotein phosphatases, metal-dependent protein phosphatases, and aspartate-based phosphatases. In plants, serine/threonine protein phosphatase belongs to the phosphoprotein phosphatase sub-class [28]. Endothall (Figure 2) was developed in the 1950s. Endothall inhibits the activity of alfalfa serine/threonine PP2A, and to a lesser extent, inhibits serine/threonine PP1, which further affects the coordination of chromosomal and microtubule events during plant cell mitosis [13][29].3.2. Exploring New Herbicide MoAs Based on Natural Products
3.2.1. Dihydroxy-Acid Dehydratase (DHAD)
The branched chain amino acid (BCAA) biosynthetic pathway consists of three common enzymes: acetolactate synthase (ALS), acetohydroxy acid isoreductase (KARI), and dihydroxy acid dehydratase (DHAD). DHAD is involved in the synthesis of essential amino acids in plants. It catalyzes the dehydration reaction of α, β-dihydroxy acid to generate the precursor α-keto acid of leucine, isoleucine, and valine in the BCAA pathway. DHAD is highly conserved in different plant species and is not present in animals. Therefore, DHAD is considered to be an ideal broad-spectrum herbicide target. Further work has demonstrated that aspterric acid (Figure 4) targets DHAD by an innovative resistant-gene-directed discovery approach [30][31].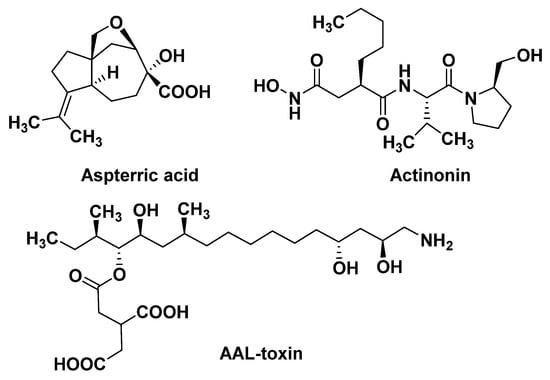
3.2.2. Plastid Peptide Deformylase (PDEF)
Peptide deformylase mainly exists in prokaryotes and higher plants, but is absent in mammalian cells. PDEF acts on the translation process of new proteins, and its function is to catalyze the hydrolysis of the N-formyl group from the initiating methionine. Actinonin (Figure 4), a target PDEF inhibitor, leads to stunting, bleaching, and necrosis of the treated weed [32][33][34]. Since the Adams group first discovered PDEF from the initial extracts of Escherichia coli and Bacillus stearothermophilus in 1968 [35], it has been widely studied by drug molecular designers, and has also been used as a target for crop antimicrobial drugs, which have been thoroughly studied. However, the recent report that the natural product Actinonin can act as a herbicide to inhibit plant growth clearly pushes the study of PDEF into new areas. Of course, studying it as a weed control target, as opposed to an antibiotic target, raises a lot of different questions. For example, it has been reported that the isoenzyme of PDEF is found in human mitochondria [36], and PDEF inhibitors may have inhibitory effects on it, which indicates the potential safety risk of targeting PDEF inhibitors.3.2.3. Ceramide Synthase
Ceramide synthase not only forms a complex lipid skeleton for sphingolipid biosynthesis, but also serves as a target for many microbial toxins [37]. Ceramide synthase catalyzes the acylation of sphinganine, sphingosine, and other long chain sphingoids to form their N-acyl derivatives. The AAL-toxin (Figure 4) can inhibit ceramide synthase, leading to the rapid accumulation of sphingosine and its substrate derivative phytosphingosine. Sphinganine and phytosphingosine are both highly phytotoxic. They cause similar symptoms to the AAL-toxin, and also affect plasma membrane integrity [38]. In addition, at lower concentrations, AAL-toxin may induce cell apoptosis caused by plasma membrane dysfunction. However, AAL-toxin, as an analogue of the fumonisins, is highly toxic to mammals [39]. To date, more than 200,000 secondary metabolites have been identified. Only a small number of compounds have determined modes of action, and many potential herbicide targets have been found. In this short chapter, we provide some brief examples of natural phytotoxins with novel MoAs, such as Trp synthase [40], Enoyl reductase [41], Orn carbamoyl transferase [42], CF1 ATPase [43][44], Peptide deformylase [45], Golgi assembly [46], H+-ATPase [47], and RNA polymerase [48]. Natural products are an important source of herbicides with new MoAs, and we believe that there will be more efficient and safer herbicides developed based on the natural products soon.3.3. Exploration of Potential Herbicide MoAs Based on Their Biochemical Mechanisms
3.3.1. DNA Gyrase
DNA topoisomerases, a key target for anticancer drugs and antibiotics, are classified as type I and II according to whether their reactions proceed via transient single (I)- or double (II)-stranded breaks in DNA. DNA gyrase is an indispensable type II topoisomerase, and affects proper plant growth. Michael D. W. [49] and Anthony M. [50] have shown that ciprofloxacin and its analogues inhibited DNA gyrase and displayed post-emergent herbicidal activity (Figure 5A). Although DNA topoisomerases are present in both animals and plants, and the concentration of the drug required to achieve plant death is high, there is no denying that DNA topoisomerases are attractive targets for the design of novel drug molecules based on biochemical mechanisms.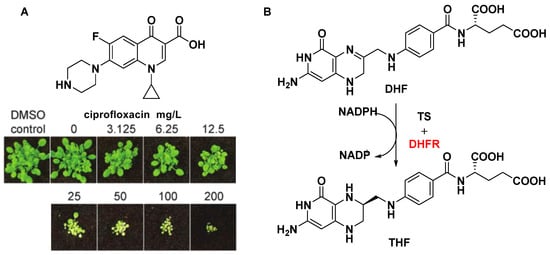
3.3.2. Dihydrofolate Reductase (DHFR)
Dihydrofolate reductase (DHFR) is another key enzyme in the folate biosynthetic pathway, relating to the synthesis of DNA precursors and some amino acids [51]. Dihydrofolate reductase (DHFR) catalyzes the reduction of dihydrofolate (DHF) into THF using NADPH as the reducing agent (Figure 5B). Unlike most prokaryotes and eukaryotes, DHFRs encodes a gene for a bifunctional protein composed in protozoa and plants. Another domain is thymidylate synthase (TS), which also plays an important role in the folate pathway [52]. Using a genetic knockout analysis, a recent study showed that DHFR can be regarded as a potential herbicide target due to the necessity of two isoforms of DHFR for seed development [53]. Since the rapid development of functional genomics, proteomics, structural biology, and gene-editing technology, many potential herbicide targets have been discovered, such as Mg protoporphyrin monomethylester [54], imadazoleglycerol phosphate dehydratase [33], cystathionine β-lyase [55], carbonic anhydrase [56], transketolase [57], and flavanone 3-hydroxylase [58].References
- Mascarelli, A. Growing up with pesticides. Science 2013, 341, 740–741.
- Sparks, T.C.; Lorsbach, B.A. Perspectives on the agrochemical industry and agrochemical discovery. Pest Manag. Sci. 2017, 73, 672–677.
- Godfray, H.C.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818.
- Enserink, M.; Hines, P.J.; Vignieri, S.N.; Wigginton, N.S.; Yeston, J.S. Smarter pest control. The pesticide paradox. Introduction. Science 2013, 341, 728–729.
- He, B.; Dong, J.; Lin, H.Y.; Wang, M.Y.; Li, X.K.; Zheng, B.F.; Chen, Q.; Hao, G.F.; Yang, W.C.; Yang, G.F. Pyrazole-isoindoline-1,3-dione hybrid: A promising scaffold for 4-hydroxyphenylpyruvate dioxygenase inhibitors. J. Agric. Food Chem. 2019, 67, 10844–10852.
- Lamberth, C.; Jeanmart, S.; Luksch, T.; Plant, A. Current challenges and trends in the discovery of agrochemicals. Science 2013, 341, 742–746.
- Peterson, G.E. The discovery and development of 2,4-D. Agric. Hist. Soc. 1967, 41, 243–254.
- Sterling, T.M.; Hall, J.C. Mechanism of action of auxins and the kinetics of cellular growth. In Herbicide Activity: Biochemistry and Molecular Biology; Roe, R.M., Burton, J.D., Kuhr, R.J., Eds.; IOS Press: Amsterdam, The Netherlands, 1997; pp. 111–141.
- Service, R.F. Agriculture. What happens when weed killers stop killing? Science 2013, 341, 1329.
- Wang, Q.; Ge, L.; Zhao, N.; Zhang, L.; You, L.; Wang, D.; Liu, W.; Wang, J. A Trp-574-Leu mutation in the acetolactate synthase (ALS) gene of Lithospermum arvense L. confers broad-spectrum resistance to ALS inhibitors. Pestic. Biochem. Physiol. 2019, 158, 12–17.
- Beckie, H.J.; Tardif, F.J. Herbicide cross resistance in weeds. Crop Prot. 2012, 35, 15–28.
- Duke, S.O. Why have no new herbicide modes of action appeared in recent years? Pest Manag. Sci. 2012, 68, 505–512.
- Tresch, S.; Schmotz, J.; Grossmann, K. Probing mode of action in plant cell cycle by the herbicide endothall, a protein phosphatase inhibitor. Pestic. Biochem. Physiol. 2011, 99, 86–95.
- Kahlau, S.; Schroder, F.; Freigang, J.; Laber, B.; Lange, G.; Passon, D.; Kleessen, S.; Lohse, M.; Schulz, A.; von Koskull-Doring, P.; et al. Aclonifen targets solanesyl diphosphate synthase, representing a novel mode of action for herbicides. Pest Manag. Sci. 2020, 76, 3377–3388.
- Liu, M.; Lu, S. Plastoquinone and ubiquinone in plants: Biosynthesis, physiological function and metabolic engineering. Front. Plant Sci. 2016, 7, 1898.
- Ohara, K.; Sasaki, K.; Yazaki, K. Two solanesyl diphosphate synthases with different subcellular localizations and their respective physiological roles in Oryza sativa. J. Exp. Bot. 2010, 61, 2683–2692.
- Dayan, F.E.; Haesaert, G.; van Leeuwen, T.; Holden-Dye, L.; Crossthwaite, A.; Nauen, R. Pesticides modes of action and resistance: A perspective from the 2019 IUPAC congress. Outlooks Pest Manag. 2019, 30, 157–163.
- Jun, L.; Saiki, R.; Tatsumi, K.; Nakagawa, T.; Kawamukai, M. Identification and subcellular localization of two solanesyl diphosphate synthases from Arabidopsis thaliana. Plant Cell Physiol. 2004, 45, 1882–1888.
- Sadre, R.; Frentzen, M.; Saeed, M.; Hawkes, T. Catalytic reactions of the homogentisate prenyl transferase involved in plastoquinone-9 biosynthesis. J. Biol. Chem. 2010, 285, 18191–18198.
- Collakova, E.; DellaPenna, D. Homogentisate phytyltransferase activity is limiting for tocopherol biosynthesis in Arabidopsis. Plant Physiol. 2003, 131, 632–642.
- Collakova, E.; DellaPenna, D. Isolation and functional analysis of homogentisate phytyltransferase from Synechocystis sp. PCC 6803 and arabidopsis. Plant Physiol. 2001, 127, 1113–1124.
- Shino, M.; Hamada, T.; Shigematsu, Y.; Hirase, K.; Banba, S. Action mechanism of bleaching herbicide cyclopyrimorate, a novel homogentisate solanesyltransferase inhibitor. J. Pestic. Sci. 2018, 43, 233–239.
- Ullrich, A.; Knecht, W.; Piskur, J.; Löffler, M. Plant dihydroorotate dehydrogenase differs significantly in substrate specificity and inhibition from the animal enzymes. FEBS Lett. 2002, 529, 346–350.
- Zrenner, R.; Stitt, M.; Sonnewald, U.; Boldt, R. Pyrimidine and purine biosynthesis and degradation in plants. Annu. Rev. Plant Biol. 2006, 57, 805–836.
- Campe, R.; Hollenbach, E.; Kammerer, L.; Hendriks, J.; Hoffken, H.W.; Kraus, H.; Lerchl, J.; Mietzner, T.; Tresch, S.; Witschel, M.; et al. A new herbicidal site of action: Cinmethylin binds to acyl-ACP thioesterase and inhibits plant fatty acid biosynthesis. Pestic. Biochem. Physiol. 2018, 148, 116–125.
- Umetsu, N.; Shirai, Y. Development of novel pesticides in the 21st century. J. Pestic. Sci. 2020, 45, 54–74.
- Dayan, F.E.; Romagni, J.G.; Duke, S.O. Herbicides, cinmethylin. In Encyclopedia of Agrochemicals; Plimmer, J.R., Gammon, D.W., Ragsdale, N.N., Eds.; John Wiley & Sons: New York, NY, USA, 2003; pp. 754–757.
- Uhrig, R.G.; Labandera, A.M.; Moorhead, G.B. Arabidopsis PPP family of serine/threonine protein phosphatases: Many targets but few engines. Trends Plant Sci. 2013, 18, 505–513.
- Bajsa, J.; Pan, Z.; Dayan, F.E.; Owens, D.K.; Duke, S.O. Validation of serine/threonine protein phosphatase as the herbicide target site of endothall. Pestic. Biochem. Physiol. 2012, 102, 38–44.
- Duke, S.O.; Stidham, M.A.; Dayan, F.E. A novel genomic approach to herbicide and herbicide mode of action discovery. Pest Manag. Sci. 2019, 75, 314–317.
- Yan, Y.; Liu, Q.; Zang, X.; Yuan, S.; Bat-Erdene, U.; Nguyen, C.; Gan, J.; Zhou, J.; Jacobsen, S.E.; Tang, Y. Resistance-gene-directed discovery of a natural-product herbicide with a new mode of action. Nature 2018, 559, 415–418.
- Chen, D.Z.; Patel, D.V.; Hackbarth, C.J.; Wang, W.; Dreyer, G.; Young, D.C.; Margolis, P.S.; Wu, C.; Ni, Z.J.; Trias, J.; et al. Actinonin, a naturally occurring antibacterial agent, is a potent deformylase inhibitor. Biochemistry 2000, 39, 1256–1262.
- Bisson, C.; Britton, K.L.; Sedelnikova, S.E.; Rodgers, H.F.; Eadsforth, T.C.; Viner, R.C.; Hawkes, T.R.; Baker, P.J.; Rice, D.W. Crystal structures reveal that the reaction mechanism of imidazoleglycerol-phosphate dehydratase is controlled by switching Mn(II) coordination. Structure 2015, 23, 1236–1245.
- Dayan, F.E.; Duke, S.O. Natural compounds as next-generation herbicides. Plant Physiol. 2014, 166, 1090–1105.
- Adams, J.M. On the release of the formyl group from nascent protein. J. Mol. Biol. 1968, 33, 571–589.
- Serero, A.; Giglione, C.; Sardini, A.; Martinez-Sanz, J.; Meinnel, T. An unusual peptide deformylase features in the human mitochondrial N-terminal methionine excision pathway. J. Biol. Chem. 2003, 278, 52953–52963.
- Wang, E.; Merrill, A.H. Ceramide synthase. Methods Enzymol. 2000, 311, 15–21.
- Gechev, T.S.; Gadjev, I.Z.; Hille, J. An extensive microarray analysis of AAL-toxin-induced cell death in Arabidopsis thaliana brings new insights into the complexity of programmed cell death in plants. Cell. Mol. Life Sci. 2004, 61, 1185–1197.
- Duke, S.O.; Dayan, F.E. Clues to new herbicide mechanisms of action from natural sources. ACS Symp. Ser. 2013, 1141, 203–215.
- Hsiao, P.; Sanjaya; Su, R.C.; Teixeira da Silva, J.A.; Chan, M.T. Plant native tryptophan synthase beta 1 gene is a non-antibiotic selection marker for plant transformation. Planta 2007, 225, 897–906.
- Dayan, F.E.; Ferreira, D.; Wang, Y.H.; Khan, I.A.; McInroy, J.A.; Pan, Z. A pathogenic fungi diphenyl ether phytotoxin targets plant enoyl (acyl carrier protein) reductase. Plant Physiol. 2008, 147, 1062–1071.
- Templeton, M.D.; Reinhardt, L.A.; Collyer, C.A.; Mitchell, R.E.; Cleland, W.W. Kinetic analysis of the L-ornithine transcarbamoylase from Pseudomonas savastanoi pv. phaseolicola that is resistant to the transition state analogue (R)-N delta-(N′-sulfodiaminophosphinyl)-L-ornithine. Biochemistry 2005, 44, 4408–4415.
- Groth, G. Structure of spinach chloroplast F1-ATPase complexed with the phytopathogenic inhibitor tentoxin. Proc. Natl. Acad. Sci. USA 2002, 99, 3464–3468.
- Meiss, E.; Konno, H.; Groth, G.; Hisabori, T. Molecular processes of inhibition and stimulation of ATP synthase caused by the phytotoxin tentoxin. J. Biol. Chem. 2008, 283, 24594–24599.
- Hou, C.X.; Dirk, L.M.; Pattanaik, S.; Das, N.C.; Maiti, I.B.; Houtz, R.L.; Williams, M.A. Plant peptide deformylase: A novel selectable marker and herbicide target based on essential cotranslational chloroplast protein processing. Plant Biotechnol. J. 2007, 5, 275–281.
- Driouich, A.; Jauneau, A.; Staehelin, L.A. 7-Dehydrobrefeldin A, a naturally occurring brefeldin a derivative, inhibits secretion and causes a cis-to-trans breakdown of golgi stacks in plant cells. Plant Physiol. 1997, 113, 487–492.
- Hejli, A.M.; Koster, K.L. The allelochemical sorgoleone inhibits root H+-ATPase and water uptake. J. Chem. Ecol. 2004, 30, 2181–2191.
- Danielsen, E.M. Tagetitoxin inhibits RNA synthesis directed by RNA polymerases from chloroplasts and Escherichia coli. J. Biol. Chem. 1990, 29, 493–498.
- Wallace, M.D.; Waraich, N.F.; Debowski, A.W.; Corral, M.G.; Maxwell, A.; Mylne, J.S.; Stubbs, K.A. Developing ciprofloxacin analogues against plant DNA gyrase: A novel herbicide mode of action. Chem. Commun. 2018, 54, 1869–1872.
- Evans-Roberts, K.M.; Mitchenall, L.A.; Wall, M.K.; Leroux, J.; Mylne, J.S.; Maxwell, A. DNA gyrase is the target for the quinolone drug ciprofloxacin in Arabidopsis thaliana. J. Biol. Chem. 2016, 291, 3136–3144.
- Dayan, F.E. Current Status and Future Prospects in Herbicide Discovery. Plants 2019, 8, 341.
- Wilson, P.M.; Danenberg, P.V.; Johnston, P.G.; Lenz, H.J.; Ladner, R.D. Standing the test of time: Targeting thymidylate biosynthesis in cancer therapy. Nat. Rev. Clin. Oncol. 2014, 11, 282–298.
- Corral, M.G.; Haywood, J.; Stehl, L.H.; Stubbs, K.A.; Murcha, M.W.; Mylne, J.S. Targeting plant DIHYDROFOLATE REDUCTASE with antifolates and mechanisms for genetic resistance. Plant J. 2018, 95, 727–742.
- Peter, E.; Rothbart, M.; Oelze, M.L.; Shalygo, N.; Dietz, K.J.; Grimm, B. Mg protoporphyrin monomethylester cyclase deficiency and effects on tetrapyrrole metabolism in different light conditions. Plant Cell Physiol. 2010, 51, 1229–1241.
- Maimann, S.; Wagner, C.; Kreft, O.; Zeh, M.; Willmitzer, L.; Hofgen, R.; Hesse, H. Transgenic potato plants reveal the indispensable role of cystathionine beta-lyase in plant growth and development. Plant J. 2000, 23, 747–758.
- Silverman, D.N.; Lindskog, S. The catalytic mechanism of carbonic anhydrase: Implications of a rate-limiting protolysis of water. Acc. Chem. Res. 2002, 21, 30–36.
- Kochetov, G.A.; Solovjeva, O.N. Structure and functioning mechanism of transketolase. Biochim. Biophys. Acta 2014, 1844, 1608–1618.
- Zheng, Y.; Tian, L.; Liu, H.; Pan, Q.; Zhan, J.; Huang, W. Sugars induce anthocyanin accumulation and flavanone 3-hydroxylase expression in grape berries. Plant Growth Regul. 2009, 58, 251–260.
