
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gloria Benítez-King | -- | 3076 | 2022-11-18 19:58:00 | | | |
| 2 | Peter Tang | + 1 word(s) | 3077 | 2022-11-19 05:20:58 | | |
Video Upload Options
Melatonin, N-acetyl-5-hydroxytryptamine, is a hormone that synchronizes the internal environment with the photoperiod. It is synthesized in the pineal gland and greatly depends on the endogenous circadian clock located in the suprachiasmatic nucleus and the retina’s exposure to different light intensities. Among its most studied functions are the regulation of the waking-sleep rhythm and body temperature. Furthermore, melatonin has pleiotropic actions, which affect, for instance, the modulation of the immune and the cardiovascular systems, as well as the neuroprotection achieved by scavenging free radicals.
1. Introduction
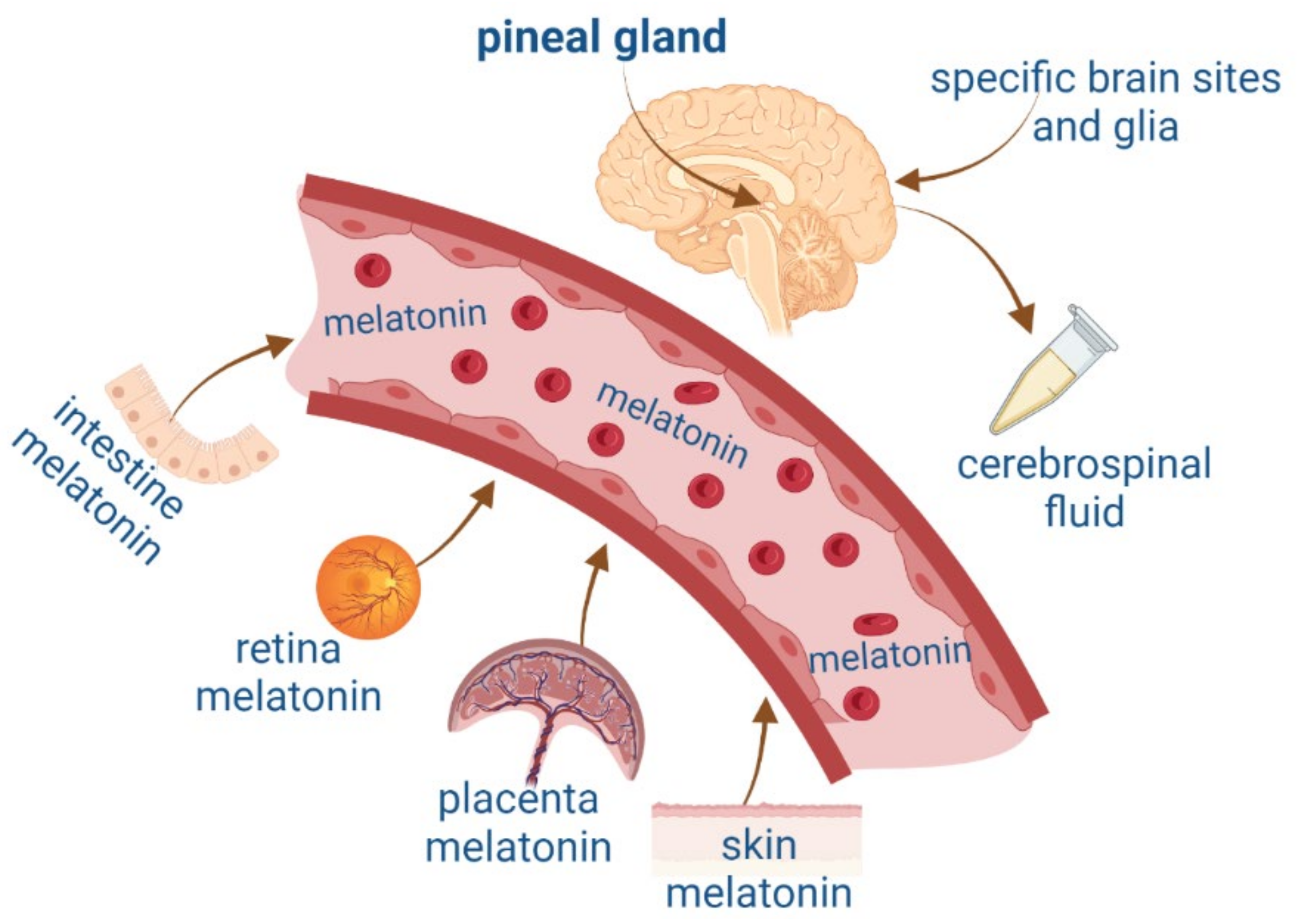
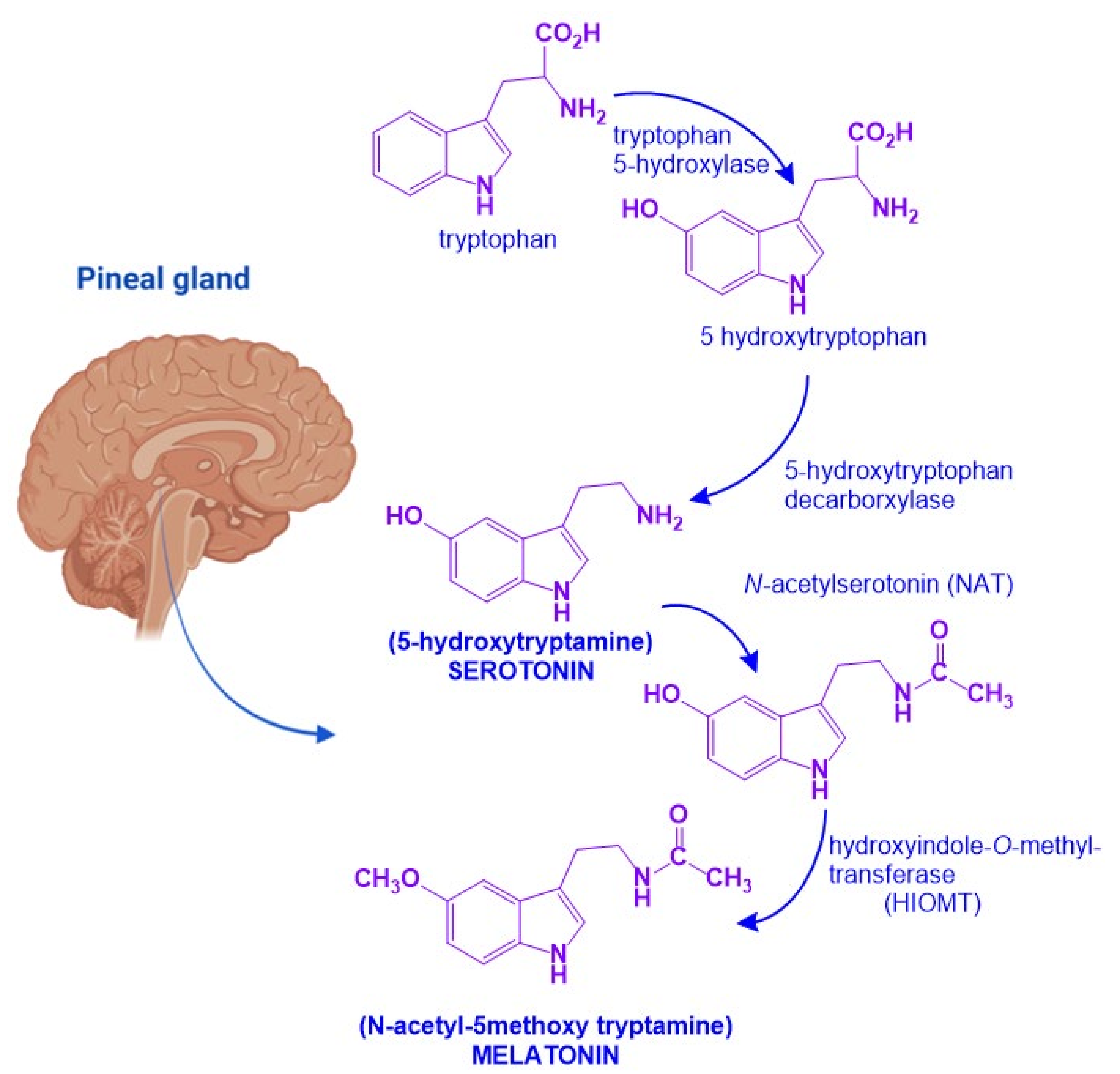
2. Melatonin and Neurotrophic Factors Synthesis
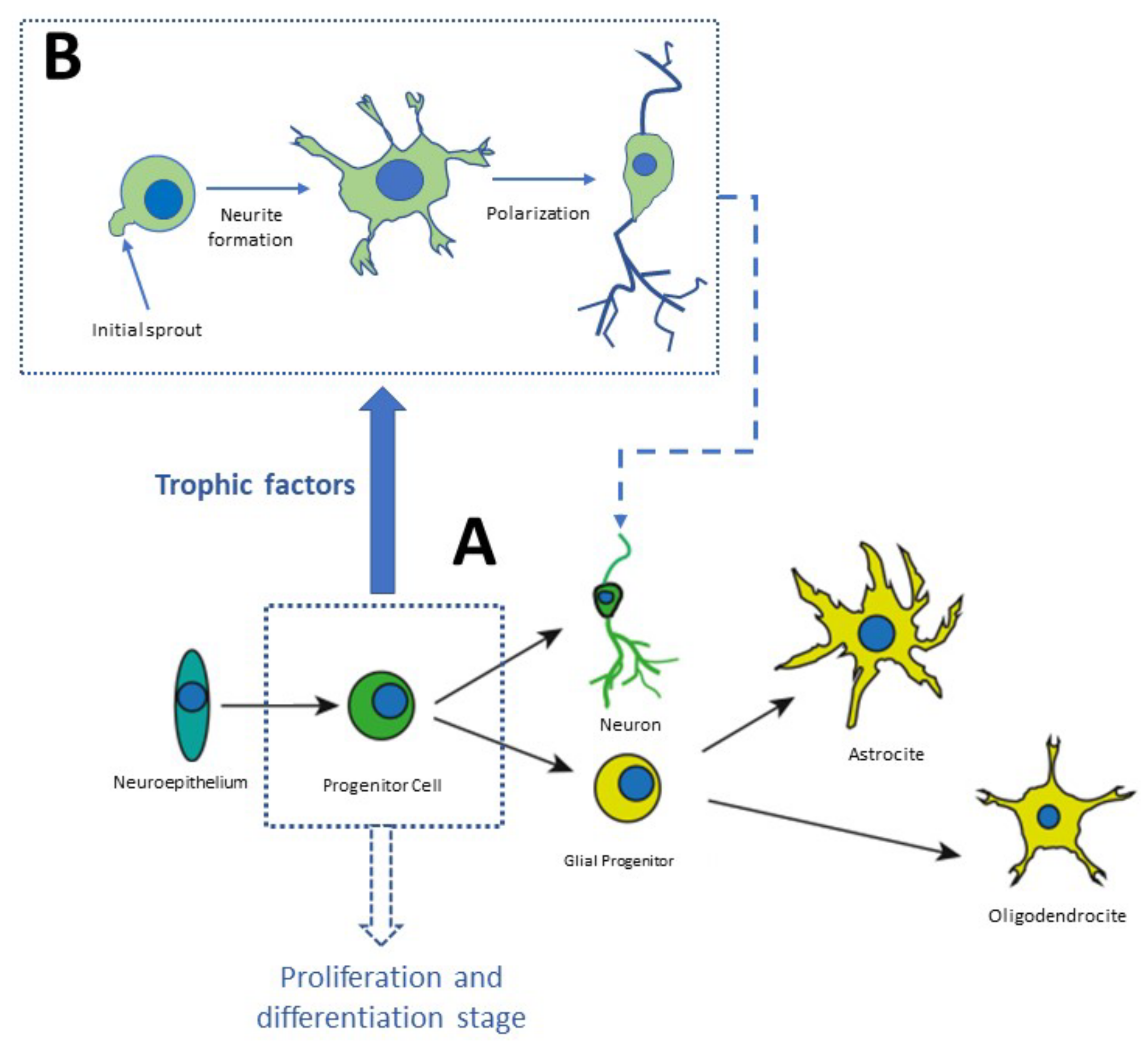
3. The Neurodevelopment in the Adult Brain
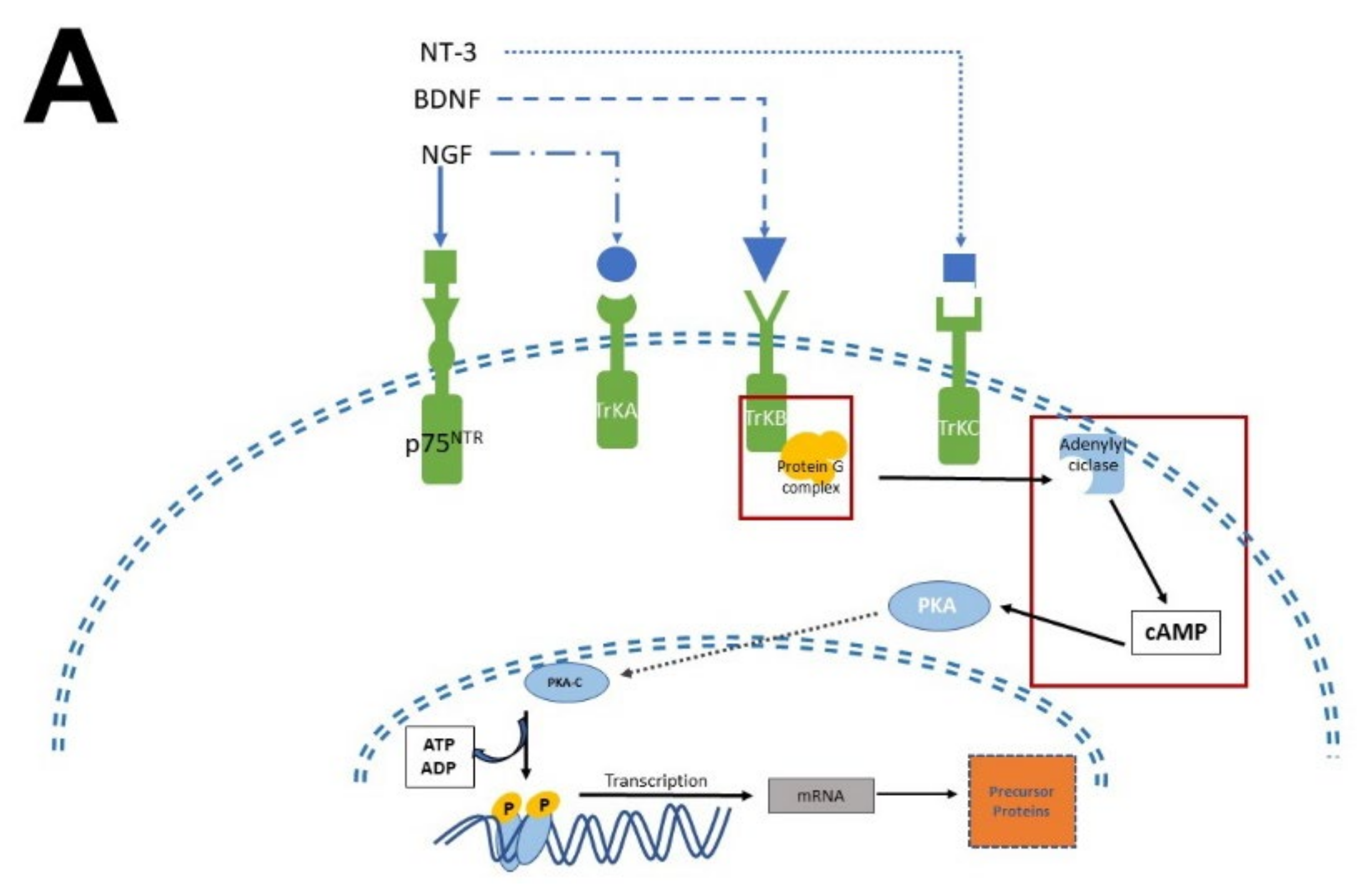
4. Evidence That Supports Melatonin Acts as a Neurotrophic Factor
4.1. Mechanism of Action Involved in Neurogenesis and Neural Differentiation: Neurotrophic Factors and Melatonin
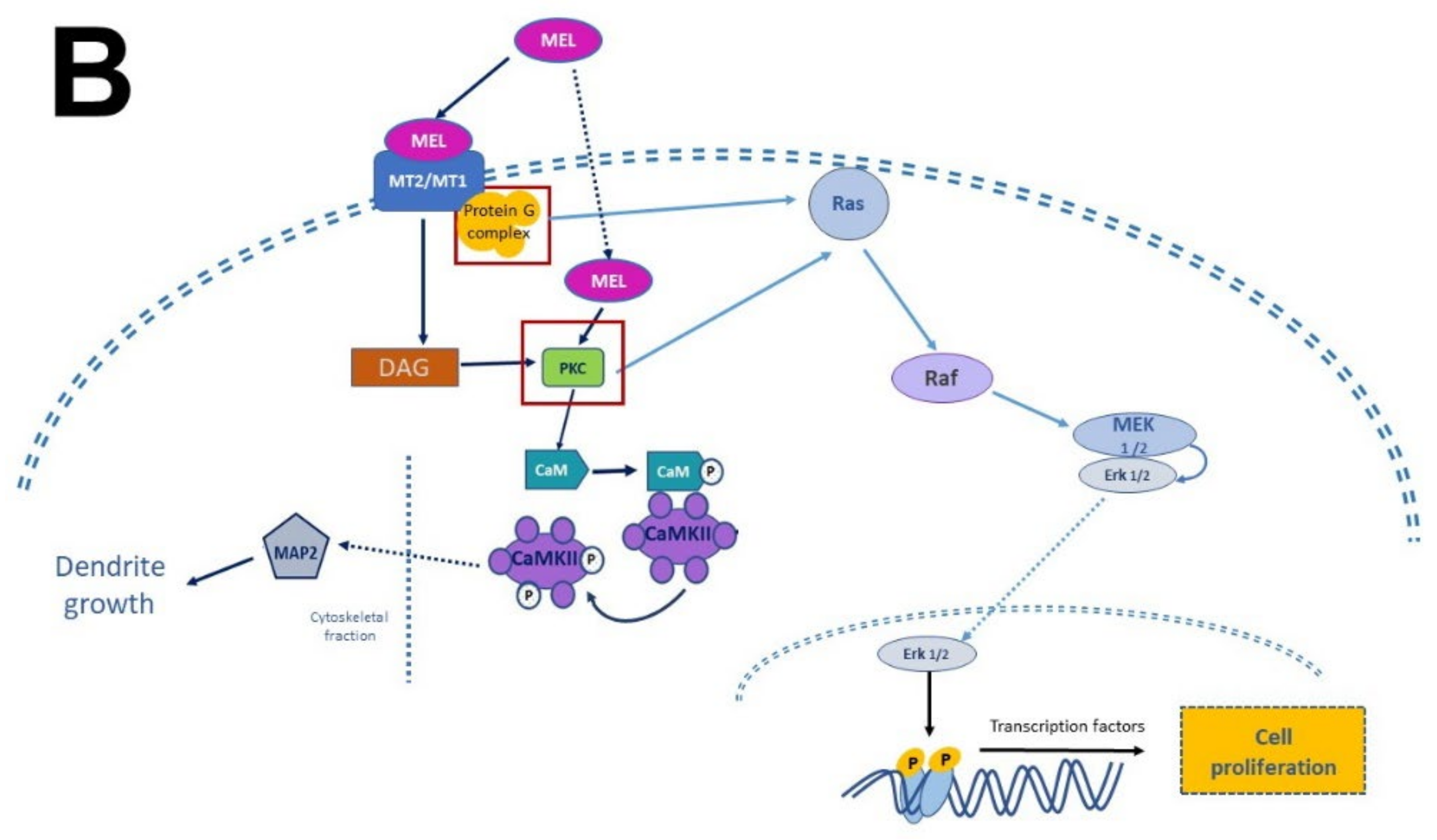
In this regard, evidence accumulated in the last decade shows that MEL antidepressant-like effects in rodents are associated with increased neurogenesis in the dentate gyrus of the hippocampus [11][69][70][71]. Administration of MEL reduced the immobility time in the forced swimming test (FST) and in the tail suspension test (TST) paradigms, providing evidence for the antidepressant effects of MEL [72][73]. In this respect, one must add that the effect of MEL was potentiated depending on the time of administration [71].
In summary, the researchers collected evidence indicating that MEL is a factor that modulates neuronal survival, proliferation, differentiation, apoptosis, and the structural polarization of neurons. Remarkably, NTFs and MEL act similarly although they are chemically distinct. The former are peptides in nature while the latter is an indolamine with a low molecular mass. In addition, MEL is endogenously produced like neurotrophins and its actions can be mediated by specific receptors that activate signaling pathways promoting these processes. In addition, and similarly to NTFs, MEL stimulates neurogenesis in organisms at the early stages of their development and in the adult brain, promoting dendritogenesis and the extension of dendritic trees, particularly in the hippocampus.
Despite considerable efforts made in this field by specialists, MEL’s mechanisms of action have not been thoroughly understood and more knowledge gaps arise when it is used in combination with other substances. For this reason, it is necessary to conduct more research at molecular level to determine, firstly, whether these types of interactions exist, secondly, to understand these mechanisms in depth together with the signaling pathways that are activated to conduct their functions, and thirdly to study the utility of melatonin in the treatment of neurodegerative and neuropsychiatric diseses.
References
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of Melatonin, Pineal Factor That Lightens Melanocytes. J. Am. Chem. Soc. 1958, 80, 2587.
- Reiter, R.J.; Tan, D.X.; Zhou, Z.; Cruz, M.H.C.; Fuentes-Broto, L.; Galano, A. Phytomelatonin: Assisting Plants to Survive and Thrive. Molecules 2015, 20, 7396–7437.
- Kim, T.K.; Atigadda, V.R.; Brzeminski, P.; Fabisiak, A.; Tang, E.K.Y.; Tuckey, R.C.; Reiter, R.J.; Slominski, A.T. Detection of Serotonin, Melatonin, and Their Metabolites in Honey. ACS Food Sci. Technol. 2021, 1, 1228–1235.
- Kim, T.K.; Fabisiak, A.; Brzeminski, P.; Reiter, R.J.; Slominski, A.T. Serotonin, Melatonin and Their Precursors and Metabolites and Vitamin D3 Derivatives in Honey. Melatonin Res. 2022, 5, 374–380.
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249.
- Tricoire, H.; Møller, M.; Chemineau, P.; Malpaux, B. Origin of Cerebrospinal Fluid Melatonin and Possible Function in the Integration of Photoperiod. Reprod. Suppl. 2003, 61, 311–321.
- Hardeland, R.; Pandi-Perumal, S.R.; Cardinali, D.P. Melatonin. Int. J. Biochem. Cell Biol. 2006, 38, 313–316.
- Reiter, R.J. Melatonin: The Chemical Expression of Darkness. Mol. Cell. Endocrinol. 1991, 79, C153–C158.
- Prakash, Y.; Thompson, M.A.; Meuchel, L.; Pabelick, C.M.; Mantilla, C.B.; Zaidi, S.; Martin, R.J. Neurotrophins in Lung Health and Disease. Expert Rev. Respir. Med. 2010, 4, 395–411.
- Thoenen, H. Neurotrophins and Neuronal Plasticity. Science 1995, 270, 593–598.
- Duman, R.S.; Nakagawa, S.; Malberg, J. Regulation of Adult Neurogenesis by Antidepressant Treatment. Neuropsychopharmacology 2001, 25, 836–844.
- Landreth, G.E. Growth Factors. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects; Siegel, G.J., Albers, R.W., Brady, S.T., Price, D.L., Eds.; Elsevier: Oxford, UK, 2006; pp. 471–484. ISBN 0-12-088397-X.
- Duman, R.S.; Monteggia, L.M. A Neurotrophic Model for Stress-Related Mood Disorders. Biol. Psychiatry 2006, 59, 1116–1127.
- Sariola, H. The Neurotrophic Factors in Non-Neuronal Tissues. Cell. Mol. Life Sci. 2001, 58, 1061–1066.
- Chang, H.M.; Wu, H.C.; Sun, Z.G.; Lian, F.; Leung, P.C.K. Neurotrophins and Glial Cell Linederived Neurotrophic Factor in the Ovary: Physiological and Pathophysiological Implications. Hum. Reprod. Update 2019, 25, 224–242.
- Skaper, S.D. Nerve Growth Factor: A Neuroimmune Crosstalk Mediator for All Seasons. Immunology 2017, 151, 1–15.
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.-X.X.; Reiter, R.J. Extrapineal Melatonin: Sources, Regulation, and Potential Functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025.
- Slominski, A.T.; Zmijewski, M.A.; Semak, I.; Kim, T.K.; Janjetovic, Z.; Slominski, R.M.; Zmijewski, J.W. Melatonin, Mitochondria, and the Skin. Cell. Mol. Life Sci. 2017, 74, 3913–3925.
- Tan, D.X.; Manchester, L.C.; Hardeland, R.; Lopez-Burillo, S.; Mayo, J.C.; Sainz, R.M.; Reiter, R.J. Melatonin: A Hormone, a Tissue Factor, an Autocoid, a Paracoid, and an Antioxidant Vitamin. J. Pineal Res. 2003, 34, 75–78.
- Mannino, G.; Pernici, C.; Serio, G.; Gentile, C.; Bertea, C.M. Melatonin and Phytomelatonin: Chemistry, Biosynthesis, Metabolism, Distribution and Bioactivity in Plants and Animals-An Overview. Int. J. Mol. Sci. 2021, 22, 9996.
- Schomerus, C.; Korf, H.W. Mechanisms Regulating Melatonin Synthesis in the Mammalian Pineal Organ. Ann. N. Y. Acad. Sci. 2005, 1057, 372–383.
- Leone, A.M.; Francis, P.L.; McKenzie-Gray, B. Rapid and Simple Synthesis for the Sulphate Esters of 6-Hydroxy-Melatonin and N-Acetyl-Serotonin. J. Pineal Res. 1988, 5, 367–371.
- Skene, D.J.; Papagiannidou, E.; Hashemi, E.; Snelling, J.; Lewis, D.F.V.; Fernandez, M.; Ioannides, C. Contribution of CYP1A2 in the Hepatic Metabolism of Melatonin: Studies with Isolated Microsomal Preparations and Liver Slices. J. Pineal Res. 2001, 31, 333–342.
- Hardeland, R. Melatonin Metabolism in the Central Nervous System. Curr. Neuropharmacol. 2010, 8, 168.
- Francis, P.L.; Leone, A.M.; Young, I.M.; Stovell, P.; Silman, R.E. Gas Chromatographic-Mass Spectrometric Assay for 6-Hydroxymelatonin Sulfate and 6-Hydroxymelatonin Glucuronide in Urine. Clin. Chem. 1987, 33, 453–457.
- Keefe, K.; Sheikh, I.; Smith, G. Targeting Neurotrophins to Specific Populations of Neurons: NGF, BDNF, and NT-3 and Their Relevance for Treatment of Spinal Cord Injury. Int. J. Mol. Sci. 2017, 18, 548.
- Skaper, S.D. Neurotrophic Factors: An Overview. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2018; Volume 1727, pp. 1–17.
- Jackman, S.L.; Regehr, W.G. The Mechanisms and Functions of Synaptic Facilitation. Neuron 2017, 94, 447–464.
- Song, M.; Martinowich, K.; Lee, F.S. BDNF at the Synapse: Why Location Matters. Mol. Psychiatry 2017, 22, 1370–1375.
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 2001, 24, 677–736.
- Levi-Montalcini, R. The Nerve Growth Factor 35 Years Later. Science 1987, 237, 1154–1162.
- Alvarez-Buylla, A.; García-Verdugo, J.M.; Tramontin, A.D. A Unified Hypothesis on the Lineage of Neural Stem Cells. Nat. Rev. Neurosci. 2001, 2, 287–293.
- Nualart, F. Unconventional Neurogenic Niches and Neurogenesis Modulation by Vitamins. J. Stem Cell Res. Ther. 2014, 4, 3.
- Alberts, B.; Bray, D.; Lewis, J.; Raff, M.; Roberts, K.; Watson, D.J. Biología Molecular de La Célula, 3rd ed.; Durfort, M., Llobera, M., Eds.; Ediciones Omega: Barcelona, Spain, 2002; ISBN 84-282-1011-X.
- Da Silva, J.S.; Dotti, C.G. Breaking the Neuronal Sphere: Regulation of the Actin Cytoskeleton in Neuritogenesis. Nat. Rev. Neurosci. 2002, 3, 694–704.
- Shu, T.; Wu, T.; Pang, M.; Liu, C.; Wang, X.; Wang, J.; Liu, B.; Rong, L. Effects and Mechanisms of Melatonin on Neural Differentiation of Induced Pluripotent Stem Cells. Biochem. Biophys. Res. Commun. 2016, 474, 566–571.
- Galván-Arrieta, T.; Trueta, C.; Cercós, M.G.; Valdés-Tovar, M.; Alarcón, S.; Oikawa, J.; Zamudio-Meza, H.; Benítez-King, G. The Role of Melatonin in the Neurodevelopmental Etiology of Schizophrenia: A Study in Human Olfactory Neuronal Precursors. J. Pineal Res. 2017, 63, e12421.
- Valdés-Tovar, M.; Estrada-Reyes, R.; Solís-Chagoyán, H.; Argueta, J.; Dorantes-Barrón, A.M.; Quero-Chávez, D.; Cruz-Garduño, R.; Cercós, M.G.; Trueta, C.; Oikawa-Sala, J.; et al. Circadian Modulation of Neuroplasticity by Melatonin: A Target in the Treatment of Depression. Br. J. Pharmacol. 2018, 175, 3200–3208.
- Moriya, T.; Horie, N.; Mitome, M.; Shinohara, K. Melatonin Influences the Proliferative and Differentiative Activity of Neural Stem Cells. J. Pineal Res. 2007, 42, 411–418.
- Ramírez-Rodríguez, G.; Klempin, F.; Babu, H.; Benítez-King, G.; Kempermann, G. Melatonin Modulates Cell Survival of New Neurons in the Hippocampus of Adult Mice. Neuropsychopharmacology 2009, 34, 2180–2191.
- Sotthibundhu, A.; Phansuwan-Pujito, P.; Govitrapong, P. Melatonin Increases Proliferation of Cultured Neural Stem Cells Obtained from Adult Mouse Subventricular Zone. J. Pineal Res. 2010, 49, 291–300.
- Liu, J.; Somera-Molina, K.C.; Hudson, R.L.; Dubocovich, M.L. Melatonin Potentiates Running Wheel-Induced Neurogenesis in the Dentate Gyrus of Adult C3H/HeN Mice Hippocampus. J. Pineal Res. 2013, 54, 222–231.
- Estrada-Reyes, R.; Quero-Chávez, D.B.; Trueta, C.; Miranda, A.; Valdés-Tovar, M.; Alarcón-Elizalde, S.; Oikawa-Sala, J.; Argueta, J.; Constantino-Jonapa, L.A.; Muñoz-Estrada, J.; et al. Low Doses of Ketamine and Melatonin in Combination Produce Additive Antidepressant-like Effects in Mice. Int. J. Mol. Sci. 2021, 22, 9225.
- Estrada-Reyes, R.; Quero-Chávez, D.B.; Alarcón-Elizalde, S.; Cercós, M.G.; Trueta, C.; Constantino-Jonapa, L.A.; Oikawa-Sala, J.; Argueta, J.; Cruz-Garduño, R.; Dubocovich, M.L.; et al. Antidepressant Low Doses of Ketamine and Melatonin in Combination Produce Additive Neurogenesis in Human Olfactory Neuronal Precursors. Molecules 2022, 27, 5650.
- Domínguez-Alonso, A.; Ramírez-Rodríguez, G.; Benítez-King, G. Melatonin Increases Dendritogenesis in the Hilus of Hippocampal Organotypic Cultures. J. Pineal Res. 2012, 52, 427–436.
- Benítez-King, G. Melatonin as a Cytoskeletal Modulator: Implications for Cell Physiology and Disease. J. Pineal Res. 2006, 40, 1–9.
- Bellon, A.; Ortíz-López, L.; Ramírez-Rodríguez, G.; Antón-Tay, F.; Benítez-King, G. Melatonin Induces Neuritogenesis at Early Stages in N1E-115 Cells through Actin Rearrangements via Activation of Protein Kinase C and Rho-Associated Kinase. J. Pineal Res. 2007, 42, 214–221.
- Ramirez-Rodriguez, G.; Ortíz-López, L.; Domínguez-Alonso, A.; Benítez-King, G.A.; Kempermann, G. Chronic Treatment with Melatonin Stimulates Dendrite Maturation and Complexity in Adult Hippocampal Neurogenesis of Mice. J. Pineal Res. 2011, 50, 29–37.
- Domínguez-Alonso, A.; Valdés-Tovar, M.; Solís-Chagoyán, H.; Benítez-King, G. Melatonin Stimulates Dendrite Formation and Complexity in the Hilar Zone of the Rat Hippocampus: Participation of the Ca++/Calmodulin Complex. Int. J. Mol. Sci. 2015, 16, 1907–1927.
- Ramírez-Rodríguez, G.B.; Palacios-Cabriales, D.M.; Ortiz-López, L.; Estrada-Camarena, E.M.; Vega-Rivera, N.M. Melatonin Modulates Dendrite Maturation and Complexity in the Dorsal-and Ventral-Dentate Gyrus Concomitantly with Its Antidepressant-like Effect in Male Balb/c Mice. Int. J. Mol. Sci. 2020, 21, 1724.
- González-Burgos, I.; Letechipía-Vallejo, G.; López-Loeza, E.; Moralí, G.; Cervantes, M. Long-Term Study of Dendritic Spines from Hippocampal CA1 Pyramidal Cells, after Neuroprotective Melatonin Treatment Following Global Cerebral Ischemia in Rats. Neurosci. Lett. 2007, 423, 162–166.
- Solís-Chagoyán, H.; Domínguez-Alonso, A.; Valdés-Tovar, M.; Argueta, J.; Sánchez-Florentino, Z.A.; Calixto, E.; Benítez-King, G. Melatonin Rescues the Dendrite Collapse Induced by the Pro-Oxidant Toxin Okadaic Acid in Organotypic Cultures of Rat Hilar Hippocampus. Molecules 2020, 25, 5508.
- Jockers, R.; Delagrange, P.; Dubocovich, M.L.; Markus, R.P.; Renault, N.; Tosini, G.; Cecon, E.; Zlotos, D.P. Update on Melatonin Receptors: IUPHAR Review 20. Br. J. Pharmacol. 2016, 173, 2702–2725.
- Delcourt, N.; Bockaert, J.; Marin, P. GPCR-Jacking: From a New Route in RTK Signalling to a New Concept in GPCR Activation. Trends Pharmacol. Sci. 2007, 28, 602–607.
- Lemmon, M.A.; Schlessinger, J. Cell Signaling by Receptor Tyrosine Kinases. Cell 2010, 141, 1117–1134.
- Deinhardt, K.; Kim, T.; Spellman, D.S.; Mains, R.E.; Eipper, B.A.; Neubert, T.A.; Chao, M.V.; Hempstead, B.L. Neuronal Growth Cone Retraction Relies on Proneurotrophin Receptor Signaling through Rac. Sci. Signal. 2011, 4, ra82.
- Barford, K.; Deppmann, C.; Winckler, B. The Neurotrophin Receptor Signaling Endosome: Where Trafficking Meets Signaling. Dev. Neurobiol. 2017, 77, 405–418.
- Caldeira, M.V.; Melo, C.V.; Pereira, D.B.; Carvalho, R.F.; Carvalho, A.L.; Duarte, C.B. BDNF Regulates the Expression and Traffic of NMDA Receptors in Cultured Hippocampal Neurons. Mol. Cell. Neurosci. 2007, 35, 208–219.
- Schinder, A.F.; Berninger, B.; Poo, M. ming Postsynaptic Target Specificity of Neurotrophin-Induced Presynaptic Potentiation. Neuron 2000, 25, 151–163.
- Dubocovich, M.L.; Delagrange, P.; Krause, D.N.; Sugden, D.; Cardinali, D.P.; Olcese, J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, Classification, and Pharmacology of G Protein-Coupled Melatonin Receptors. Pharmacol. Rev. 2010, 63, 343–380.
- Hardeland, R. Melatonin: Signaling Mechanisms of a Pleiotropic Agent. Biofactors 2009, 35, 183–192.
- Assimakopoulou, M.; Kondyli, M.; Gatzounis, G.; Maraziotis, T.; Varakis, J. Neurotrophin Receptors Expression and JNK Pathway Activation in Human Astrocytomas. BMC Cancer 2007, 7, 202.
- Wang, J.; Xiao, X.; Zhang, Y.; Shi, D.; Chen, W.; Fu, L.; Liu, L.; Xie, F.; Kang, T.; Huang, W.; et al. Simultaneous Modulation of COX-2, P300, Akt, and Apaf-1 Signaling by Melatonin to Inhibit Proliferation and Induce Apoptosis in Breast Cancer Cells. J. Pineal Res. 2012, 53, 77–90.
- Santarelli, L.; Saxe, M.; Gross, C.; Surget, A.; Battaglia, F.; Dulawa, S.; Weisstaub, N.; Lee, J.; Duman, R.; Arancio, O.; et al. Requirement of Hippocampal Neurogenesis for the Behavioral Effects of Antidepressants. Science 2003, 301, 805–809.
- Hare, B.D.; Duman, R.S. Prefrontal Cortex Circuits in Depression and Anxiety: Contribution of Discrete Neuronal Populations and Target Regions. Mol. Psychiatry 2020, 25, 2742–2758.
- Phillips, C.; Baktir, M.A.; Srivatsan, M.; Salehi, A. Neuroprotective Effects of Physical Activity on the Brain: A Closer Look at Trophic Factor Signaling. Front. Cell. Neurosci. 2014, 8, 170.
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593.
- Numakawa, T.; Odaka, H.; Adachi, N. Actions of Brain-Derived Neurotrophin Factor in the Neurogenesis and Neuronal Function, and Its Involvement in the Pathophysiology of Brain Diseases. Int. J. Mol. Sci. 2018, 19, 3650.
- Labban, S.; Alshehri, F.S.; Kurdi, M.; Alatawi, Y.; Alghamdi, B.S. Melatonin Improves Short-Term Spatial Memory in a Mouse Model of Alzheimer’s Disease. Degener. Neurol. Neuromuscul. Dis. 2021, 11, 15–27.
- Labban, S.; Alghamdi, B.S.; Alshehri, F.S.; Kurdi, M. Effects of Melatonin and Resveratrol on Recognition Memory and Passive Avoidance Performance in a Mouse Model of Alzheimer’s Disease. Behav. Brain Res. 2021, 402, 113100.
- Guaiana, G.; Gupta, S.; Chiodo, D.; Davies, S.J.; Haederle, K.; Koesters, M. Agomelatine versus Other Antidepressive Agents for Major Depression. Cochrane Database Syst. Rev. 2013, 2013, CD008851.
- Taniguti, E.H.; Ferreira, Y.S.; Stupp, I.J.V.; Fraga-Junior, E.B.; Mendonça, C.B.; Rossi, F.L.; Ynoue, H.N.; Doneda, D.L.; Lopes, L.; Lima, E.; et al. Neuroprotective Effect of Melatonin against Lipopolysaccharide-Induced Depressive-like Behavior in Mice. Physiol. Behav. 2018, 188, 270–275.
- Estrada-Reyes, R.; Valdés-Tovar, M.; Arrieta-Baez, D.; Dorantes-Barrón, A.M.; Quero-Chávez, D.; Solís-Chagoyán, H.; Argueta, J.; Dubocovich, M.L.; Benítez-King, G. The Timing of Melatonin Administration Is Crucial for Its Antidepressant-like Effect in Mice. Int. J. Mol. Sci. 2018, 19, 2278.





