
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Elzbieta Radzyminska-Lenarcik | + 1937 word(s) | 1937 | 2020-11-16 06:38:44 | | | |
| 2 | Nicole Yin | -2 word(s) | 1935 | 2020-12-14 06:39:51 | | | | |
| 3 | Nicole Yin | Meta information modification | 1935 | 2020-12-16 04:54:08 | | |
Video Upload Options
Alkylimidazoles have good complexing properties, also they are cheap so can be successfully used in the separation of metal ions from aqueous solutions.
1. Introduction
An imidazole (1,3-diazole) ring is a building unit of compounds occurring in living organisms. The most common ones include histidine, histamine, pilocarpine, adenine, guanine, purine as well as vitamin H (biotine); the one named last was discovered by Utter in 1960[1]. Some derivatives of imidazole, for instance pilocarpine, have been used as medicines[2]. Imidazole, discovered in the 19th century by the Polish chemist Radziszewski[3], belongs to the family of five-membered heterocyclic bases called azoles. It is an aromatic compound with a planar ring structure. Owing to the intermolecular hydrogen bonds, imidazole is a solid compound. In an aqueous solution it is a base. All hydrogen atoms in the ring can be substituted, amongst others, by alkyls to give a homologous series of alkylimidazoles.
[1] Stryer, L.; Berg, J.M.; Tymoczko, J.L.; Gatto, G.J.Jr. Biochemistry, WH Freeman & Co 2019
[2] red. Chruściel T.: Leksykon leków. PZWL Warszawa, 1991
[3] Radziszewski B.: Über Glyoxalin und seine Homologe, Dtsch. Chem. Ges. 1882, 15, 2706-2708
2. Properties of Alkylimidazoles
In Pearson’s theory[4] (HSAB), imidazole (Figure 1) is an intermediate base (pKa = 7.14[5]) forming stable complexes with metal ions, a feature which is used for their separation[6][7][8][9][10][11][12]:
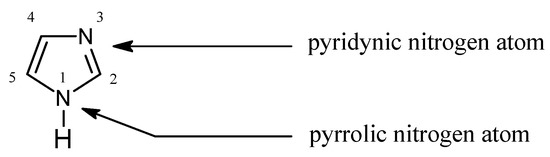
Figure 1. The imidazole molecule.
The alkaline, hydrophobic and complexing properties of imidazole can be affected by the introduction of various substituents into the ring, including alkyl ones. Alkylimidazole derivatives constitute a group of stable bases, whose properties (hydrophobic and alkaline) can be modified by changing the size of the alkyl group and its position in the imidazole ring[13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31]. The ability to separate metal ions was tested with the use of the following homologous series of alkylimidazoles: 1-alkylimidazoles[13][14][15][16][17], 2-alkylimidazoles[29][30][31], 1,2-dialkylimidazoles[15][18][19][22][23][24][25][26][27] and 1,4-dialkylimidazoles[19][20][21][22][28]. It has been proven[32] that, for individual homologous series, the basicity increases linearly as the number of carbon atoms in alkyl groups becomes larger. The primary parameter deciding about the usefulness of alkylimidazoles for the separation of metals in the process of (solvent, membrane, etc.) extraction involves their basicity.
Table 1 lists the values of dissociation constants (pKa) of the protonated forms (HL+) of the homologous series alkyl imidazole (L). The value of the constant term (the “b” constant) in linear equations may be a basis for comparing the basicity of homologous series of alkylimidazoles. The basicity of these homologues decreases in the following sequence: 1,2,4-trialkylimidazoles > 1,2-dialkylimidazoles > 2-alkylimidazoles > 1,4-dialkylimidazoles > 1-alkylimidazoles.
Table 1. Changes in the basicity of alkylimidazoles depending on the position and number of alkyl groups according to[33], in aqueous solutions at 25 °C, ionic strength 0.5 mol/dm3 (KNO3).
|
Homologous Series |
Linear Equation for Basicity pKa = an +b |
|
1-alkylimidazole |
pKa = 0.0222 n + 7.165 |
|
1,2-dialkylimidazole |
pKa = 0.0432 n + 8.01 |
|
1,2,4-trialkylimidazole |
pKa = 0.0503 n + 8.46 |
|
1,4-dialkylimidazole |
pKa = 0.0279 n + 7.79 |
|
2-alkylimidazole |
pKa = 0.0349 n + 7.87 |
n – is the sum of carbon atoms in the alkyl substituents.
2.1. The 1-Alkylimidazole Moiety as a Carrier
The placement of an alkyl substituent in the 1 position of imidazole causes a significant increase in the hydrophobic properties of the particle and a slight increase in its basicity (Table 1). 1‑Alkylimidazoles are used as extractants of numerous metal ions, such as, e.g., Cu(II), Hg(II), Co(II), Pb(II) and Zn(II)[33][34]. Cuprey[33] and Shakers et al.[34] have suggested complex imidazole derivatives, having in their substituents in position 1,2 or 1,2,4,5 large alkyl groups with 6 to 40 carbon atoms, or cycloalkyl groups consisting of 5–12 carbon atoms.
The processes of transport through the PIMs used 1-alkylimidazoles poorly soluble in water (Figure 2), having small alkyl substituents (from 6 to 10 carbon atoms).
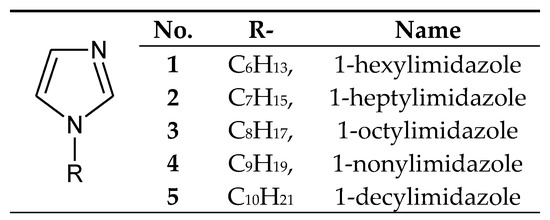
Figure 2. The 1-alkylimidazole molecule.
1-Alkylimidazoles 1–5 (Figure 2) were used to separate Cu(II) from a Cu–Zn–Co–Ni mixture from nitrate[35][36] or chloride solutions[37], as well as Zn(II) from Zn–Co–Ni[38], Zn–Cd–Ni[39] and Zn–Mn mixtures[40]. Ajji and Ali [12] used 1-vinylimidazole to separate Cu(II) and Fe(III) ions in the process of transport across polymer membranes made of polyvinyl acetate.
2.2. The 1-Alkyl-2-Methylimidazole and 1-Alkyl-4-Methylimidazole as a Carriers
The introduction of even a small group (e.g., methyl) into the 2 or 4 positions of the imidazole ring highly increases the basicity of the molecule (Table 1), with a simultaneous increase in the hardness of these bases (the HSAB theory[4]) compared to imidazole and 1-alkylimidazoles. 1-Alkyl-2-methylimidazoles 6–9 (Figure 3) were used to separate Cu(II) from a Cu–Zn–Co–Ni[35][41][42][43] mixture, as well as Zn(II) from a Zn–Cd–Ni mixture[39].
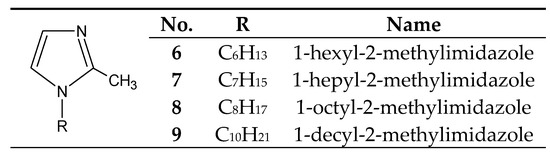
Figure 3. The 1-alkyl-2-metylimidazole molecule.
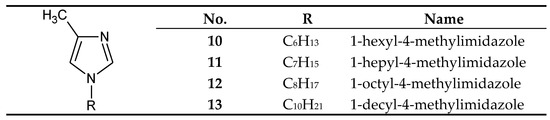
Figure 4. The 1-alkyl-4-metylimidazole molecule.
On the other hand, 1-alkyl-4-methylimidazoles 10-13 (Figure 4) were used to separate Cu(II) from a Cu–Zn–Co–Ni mixture[36] and Zn(II) from a Zn–Cd–Co–Ni[44][45] and a Zn–Cd–Ni mixture[39]. 1–Octyl–4–methylimidazole (12) was used in the final treatment of galvanic wastewater containing Zn(II) ions[46].
2.3. The 1-Alkyl-2,4-Dimethylimidazole as a Carrier
The addition of two methyl groups into positions 2 and 4 of an imidazole ring highly increases the basicity of the molecule (Table 1). Among all homologous series of alkylimidazoles, 1-alkyl-2,4-dimethylimidazoles are the strongest bases. 1-Octyl-2,4-dimethylimidazole (14, Figure 5) was used to separate Zn(II) ions from a Zn–Cd–Ni mixture[39]. On the other hand, 1-decyl-2,4-dimethylimidazole (15, Figure 6) was used in the separation of Zn(II) from a Zn-Cd mixture[47].
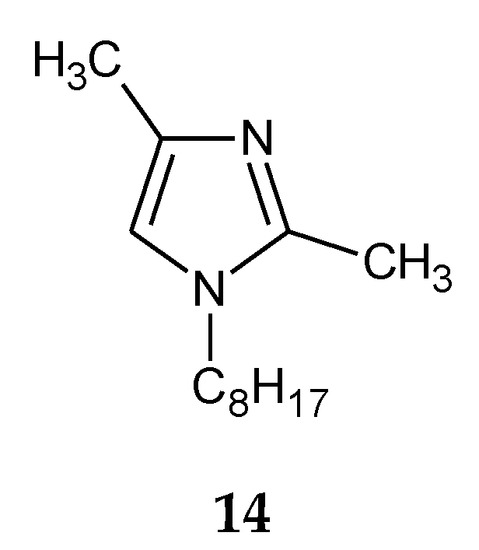
Figure 5. The 1-octyl-2,4-dimetylimidazole molecule.
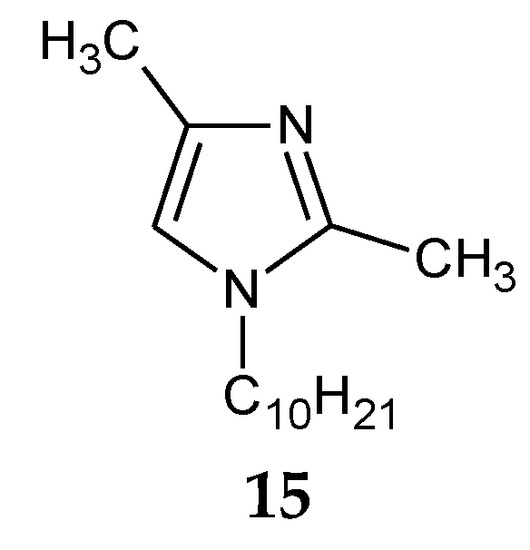
Figure 6. The 1-decyl-2,4-dimetylimidazole molecule.
3. Complexes of Alkylimidazoles
According to Bjerrum[48], in an aqueous solution, metal ions (Mz+) having complex-forming properties gradually bind base particles (L) (Equation 1):
|
[M(H2O)N−n+1Ln-1]z+ + L ↔ [M(H2O)N−nLn]z+ + H2O |
(1) |
in which N stands for the maximum coordination number of a metal ion.
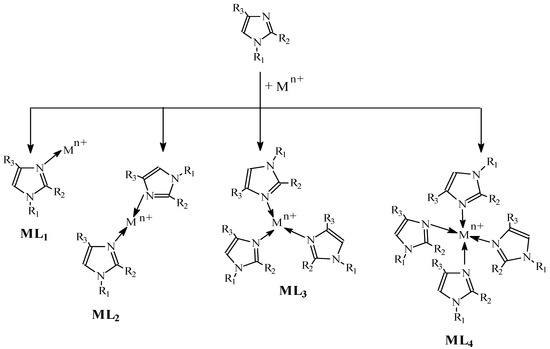
Figure 7. Complex formation.
This results in the establishment of equilibria for the gradual formation of single-core complexes (ML, ML2….MLN). The formation of complexes with alkylimidazoles in a membrane proceeds according to the pattern presented in Figure 7.
Another factor deciding about the usefulness of alkylimidazoles for the separation of heavy metal ions involves the stability of their complexes with the ions of these metals. Table 2 lists the values of stability constants (log βn) of Co(II), Ni(II), Cu(II) and Zn(II) complexes with 1-alkylimidazoles.
Table 2. The stability constants (log βn) of Co(II), Ni(II), Cu(II), and Zn(II) complexes with 1-alkylimidazoles in aqueous solutions at 25 °C, ionic strength 0.5 mol/dm3 (KNO3).
|
log βn |
Co(II)[13] |
Ni(II)[9] |
Cu(II)[17] |
Zn(II)[16] |
|
log β1 |
y = 0.302x + 1.653 |
y = 0.161x + 2.631 |
4.15 |
y = 0.229x + 1.986 |
|
log β2 |
y = 0.342x + 2.592 |
y = 0.164x + 4.790 |
7.57 |
y = 0.229x + 4.500 |
|
log β3 |
y = 0.377x + 3.881 |
y = 0.164x + 6.233 |
- |
y = 0.229x + 5.700 |
|
log β4 |
y = 0.434x + 4.780 |
y = 0.166x + 5.653 |
- |
- |
x—the number of carbon atoms in the 1-alkyl substituent.
Table 2 indicates that the stability of the complexes of Co(II), Ni(II), Cu(II) and Zn(II) ions increases in the following sequence: Co(II) < Zn(II) < Ni(II) < Cu(II). The values of stability constants of Cu(II) complexes with 1-alkylimidazoles do not depend on the length of the alkyl group[17], while the stability constants of Co(II)[13], Ni(II)[9] and Zn(II)[16] complexes increase linearly along with an increase in the length of the alkyl group (Table 2). Table 3 lists the values of stability constants (log βn) of Co(II), Cu(II) and Zn(II) complexes with selected 1-alkyl-2-methylimidazoles (6, 8, 9).
Table 3. The stability constants (log βn) of Co(II)[19], Cu(II)[18], and Zn(II)[18] complexes with 1-alkyl-2-methylimidazoles in aqueous solutions at 25 °C, ionic strength 0.5 mol/dm3 (KNO3).
|
Carrier |
Metal ion |
log β1 |
log β2 |
log β3 |
log β4 |
|
1-hexyl-2-methylimidazole (6) |
Co(II) |
1.96 |
2.18 |
3.02 |
5.61 |
|
Cu(II) |
3.52 |
6.63 |
8.98 |
- |
|
|
Zn(II) |
3.48 |
5.80 |
8.30 |
10.10 |
|
|
1-octyl-2-methylimidazole (8) |
Co(II) |
1.94 |
2.14 |
3.06 |
5.70 |
|
Cu(II) |
3.53 |
6.65 |
9.65 |
- |
|
|
Zn(II) |
4.45 |
6.80 |
9.10 |
- |
|
|
1-decyl-2-methylimidazole (9) |
Co(II) |
1.95 |
2.21 |
3.16 |
5.76 |
|
Cu(II) |
3.54 |
6.68 |
9.44 |
- |
|
|
Zn(II) |
5.10 |
7.75 |
9.90 |
- |
The data in Table 3 indicates that the stability of complexes of 1-alkyl-2-methylimidazoles with metal cations is lower by one order of magnitude compared to analogous compounds with 1-alkylimidazoles (Table 2), although 1-alkyl-2-methylimidazoles are harder bases and they should be bound more strongly with metal cations. Reduction in the stability of these complexes results from the so-called steric effect (steric hindrance) related to the presence of a substituent (e.g., a methyl) in direct vicinity of the donor nitrogen atom (Figure 1, N-3). It hinders the process of generating complexes. Table 4 lists the values of stability constants (log βn) of Co(II), Cu(II) and Zn(II) complexes with selected 1-alkyl-4-methylimidazoles (10, 12, 13).
Similar to the case of 1-alkyl-2-methylimidazoles, the steric effect of a methyl substituent in position 4 results in a reduction of the stability of complexes of 1-alkyl-4-methylimidazoles compared to analogous compounds with 1-alkylimidazoles. The influence of steric hindrance also depends on the complex forming properties of metal ions. It hinders the formation of octahedral or pseudo-octahedral complexes. It does not hinder the generation of complexes with the shape of a tetrahedron.
Table 4. The stability constants (log βn) of Co(II), Cu(II), Zn(II), Cd(II), and Ni(II) complexes with 1-alkyl-4-methylimidazoles in aqueous solutions at 25 °C, ionic strength 0.5 mol/dm3 (KNO3).
|
Carrier |
Metal Ion |
log β1 |
log β2 |
log β3 |
log β4 |
|
1-hexyl-4-methylimidazole (10) |
Co(II)[21] |
1.25 |
2.04 |
3.02 |
5.61 |
|
Cu(II)[28] |
3.72 |
4.55 |
6.53 |
- |
|
|
Zn(II)[20] |
2.95 |
5.60 |
6.30 |
- |
|
|
Cd(II)[20] |
2.20 |
3.93 |
5.11 |
5.81 |
|
|
Ni(II)[49] |
1.04 |
1.51 |
2.32 |
3.05 |
|
|
1-octyl-4-methylimidazole (12) |
Co(II)[21] |
1.34 |
2.14 |
3.28 |
5.79 |
|
Cu(II)[28] |
3.85 |
4.49 |
6.57 |
- |
|
|
Zn(II)[20] [42] |
2.04 |
3.50 |
6.20 |
6.90 |
|
|
Cd(II)[15] |
1.26 |
2.20 |
2.93 |
3.91 |
|
|
Ni(II)[15] |
0.69 |
1.04 |
2.00 |
2.92 |
|
|
1-decyl-4-methylimidazole (13) |
Co(II)[21] |
1.40 |
2.95 |
3.60 |
5.83 |
|
Cu(II)[28] |
3.94 |
4.53 |
6.60 |
- |
|
|
Zn(II)[20] |
4.30 |
6.90 |
7.70 |
12.50 |
|
|
Cd(II)[43] |
1.45 |
2.25 |
2.48 |
3.14 |
|
|
Ni(II)[43] |
0.55 |
0.86 |
1.48 |
- |
Table 4 indicates that the stability of the complexes of Co(II), Ni(II), Cu(II), Zn(II) and Cd(II) ions increases in the following sequence: Ni(II) < Cd(II), Co(II) < Zn(II) < Cu(II). In aqueous solutions, cations of Co(II), Ni(II), Cu(II), Zn(II), and Cd(II) exist in the form of octahedral aqua complexes [M(H2O)6]2+ [50]. In the case of Co(II), Zn(II) and Cd(II) ions, their octahedral aqueous complexes tend to change the coordinate number (c.n.) from 6 to 4, and at the same time change the symmetry of their coordination sphere into a square flat or deformed tetrahedron, in depending on the structure of their d-electron layer, which can be described in Equations (2):
|
[M(H2O)6−n+1Ln-1]2+ + L ↔ [M(H2O)4-nLn]2+ + 3H2O [M(H2O)6−nLn]2+ ↔ [M(H2O)4-nLn]2+ + 2H2O |
(2) |
The steric effect of the substituent in position 2 or 4 decreases the stability constants of octahedral complexes of all the studied metals, although it does not hinder the formation of tetrahedral species[15][18][19][20][21][22][23][24][25][29][49]. The Ni(II) ions form mostly 6-coordination complexes, because they have a rigid octahedral structure which is hard to deform. The complexes of Ni(II) both with 1-alkyl-2-methylimidazoles (Table 3) and with 1-alkyl-4-methylimidazoles (Table 4) have the lowest stability constants compared to 1-alkylimidazoles. The steric effect (Table 3 and Table 4) has a much lower impact on the stability of Cu(II) complexes due to the plasticity of its coordination sphere[51].
Table 5. The stability constants (log βn) of Cd(II), Ni(II), and Zn(II) complexes with 1-alkyl-2,4-dimethylimidazoles in aqueous solutions at 25 °C, ionic strength 0.5 mol/dm3 (KNO3).
|
Carrier |
Metal ion |
log β1 |
log β2 |
log β3 |
log β4 |
|
1-octyl-2,4-dimethylimidazole (14)[39] |
Zn(II) |
1.65 |
2.17 |
4.48 |
6.39 |
|
Cd(II) |
1.17 |
2.53 |
4.21 |
5.68 |
|
|
Ni(II) |
0.09 |
0.22 |
1.11 |
2.05 |
|
|
1-decyl-2,4-dimethylimidazole (15)[47] |
Zn(II) |
2.17 |
4.48 |
6.39 |
9.87 |
|
Cd(II) |
2.53 |
4.21 |
5.68 |
7.56 |
Table 5 lists the values of stability constants (log βn) of Co(II), Cu(II) and Zn(II) complexes with selected 1-alkyl-2,4-dimethylimidazoles 14, 15. In the case of 1-octyl-2,4-dimethylimidazole (14), the stability constants of Zn(II), Cd(II) and Ni(II) complexes increase in the following order: Zn(II) > Cd(II) > Ni(II) (Table 5); for 1-decyl-2,4-dimethylimidazole (15), complexes with Zn(II) are more stable than those with Ni(II), except for the first formed complex (ML). The steric effect caused by the presence of methyl substituents in positions 2 and 4 causes the values of stability constants for the complexes of metals (Table 5) with 1-alkyl-2,4-dimethylimidazoles to be lower compared to 1,2- and 1,4-dialkylimidazoles.
References
- M. Inês G.S. Almeida; Robert W. Cattrall; Spas D. Kolev; Recent trends in extraction and transport of metal ions using polymer inclusion membranes (PIMs). Journal of Membrane Science 2012, 415, 9-23, 10.1016/j.memsci.2012.06.006.
- Kislik, V.S. (Ed.) Liquid Membranes: Principles and Applications in Chemical Separations and Wastewater Treatment; Elsevier: Burlington, VT, USA, 2010; ISBN 978-0-444-53218-3.
- Bambang Kuswandi; Fidelis Nitti; M. Inês G. S. Almeida; Spas D. Kolev; Water monitoring using polymer inclusion membranes: a review. Environmental Chemistry Letters 2019, 18, 129-150, 10.1007/s10311-019-00930-9.
- Ralph G. Pearson; Hard and soft acids and bases—the evolution of a chemical concept. Coordination Chemistry Reviews 1990, 100, 403-425, 10.1016/0010-8545(90)85016-l.
- D. Bougeard; 1,2,4‐Triazole: Vibrational spectra, normal coordinate calculations, and hydrogen bonding. The Journal of Chemical Physics 1976, 64, 5152-5164, 10.1063/1.432190.
- Bauman, J.E., Jr.; Wang, J.C. Imidazole Complexes of Nickel(II), Copper(II), Zinc(II), and Silver(I). Inorg. Chem. 1964, 3, 368–373.
- Sundberg, R.J.; Martin, R.B. Interactions of histidine and other imidazole derivatives with transition metal ions in chemical and biological systems. Chem. Rev. 1974, 74, 471–517.
- Rao, G.N.; Li, N.C. Preparation and characterization of some binary and ternary metal complexes of imidazole and amino acids. Can. J. Chem. 1966, 44, 1637–1641.
- Godlewska, S.; Jezierska, J.; Baranowska, K.; Augustin, E.; Dołęga, A. Copper(II) complexes with substituted imidazole and chlorido ligands: X-ray, UV–Vis, magnetic and EPR studies and chemotherapeutic potential. Polyhedron 2013, 65, 288–297.
- Soayed, A.A.; Refaat, H.A.; El-Din, D.A.N. Metal complexes of moxifloxacin–imidazole mixed ligands: Characterization and biological studies. Inorg. Chim. Acta 2013, 406, 230–240.
- Pekel, N.; Güven, O. Separation of heavy metal ions by complexation on poly (N-vinyl imidazole) hydrogels. Polym. Bull. 2004, 51, 307–314.
- Ajji, Z.; Ali, A.M. Separation of copper ions from iron ions using PVA-g-(acrylic acid/N-vinyl imidazole) membranes prepared by radiation-induced grafting. J. Hazard. Mat. 2010, 173, 71–74.
- Lenarcik, B.; Ojczenasz, P. Investigation of the Stability Constants of Co(II) Complexes with a Homologous Series of 1-Alkylimidazoles in Aqueous Solution by Using a Partition Method with Several Solvents. Sep. Sci. Technol. 2004, 39, 199–226.
- Lenarcik, B.; Rauckyte, T. The Influence of Alkyl Length on Extraction Equilibria of Ni(II) Complexes with 1-Alkylimidazoles in Aqueous Solution/Organic Solvent Systems. Sep. Sci. Technol. 2004, 39, 3353–3372.
- Radzyminska-Lenarcik, E. Search for the possibility of utilizing the differences in complex-forming capacities of alkylimidazoles for selective extraction of some metal ions from aqueous solutions. Pol. J. Chem. Technol. 2008, 10, 73–78.
- Lenarcik, B.; Kierzkowska, A. The Influence of Alkyl Length on Stability Constants of Zn(II) Complexes with 1-Alkylimidazoles in Aqueous Solutions and Their Partition Between Aqueous Phase and Organic Solvent. Solvent Ext. Ion Exch. 2004, 22, 449–471.
- Radzyminska-Lenarcik, E. The influence of the alkyl chain length on extraction equilibrium of Cu(II) complexes with 1-alkylimidazole in aqueous solution/organic solvent system. Solvent Ext. Ion Exch. 2006, 25, 53–64.
- Lenarcik, B.; Kierzkowska, A. The Influence of Alkyl Chain Length and Steric Effect on Extraction of Zinc(II) Complexes with 1-Alkyl-2-methylimidazoles. Solvent Ext. Ion Exch. 2006, 24, 433–445.
- Lenarcik, B.; Barszcz, B. Stability and structure of transition metal complexes with azoles in aqueous solutions, Part XXI. A comparison of complex-forming of 1,2-dimethylimidazole with that of other 1,3-diazoles. J. Chem. Soc. Dalton Trans. 1980, 24–28.
- Lenarcik, B.; Kierzkowska, A. The Influence of Alkyl Chain Length and Steric Effect on Stability Constants and Extractability of Zn(II) Complexes with 1-Alkyl-4(5)-methylimidazoles. Sep. Sci. Technol. 2004, 39, 3485–3508.
- Lenarcik, B.; Ojczenasz, P.; Kopkowski, A. The Influence of the Alkyl Chain Length and Steric Effect on Stability Constants and Extractability of Co(II) Complexes with 1-Alkyl-4(5)-methylimidazoles. Sep. Sci. Technol. 2006, 41, 1697–1724.
- Radzyminska-Lenarcik, E. Influence of the solvent donor number on the O/W partition ratio of Cu(II) complexes of 1,2-dialkylimidazoles. Chem. Pap. 2011, 65, 226–232.
- Lenarcik, B.; Kurdziel, K. Stability and Structure of Transition Metal Complexes of Azoles in Aqueous Solutions, Part XXV. The Effect of the Size and Position of an Alkyl Substituent on the Stability and Structure of Alkylimidazole Complexes. Pol. J. Chem. 1982, 56, 3–14.
- Radzyminska-Lenarcik, E. Effect of alkyl chain length on the extraction of Cu(II) complexes with 1-alkyl-2-methylimidazole. Sep. Sci. Technol. 2007, 42, 2661–2675.
- Radzyminska-Lenarcik, E.; Witt, K. The influence of alkyl chain length and steric effect on the stability constants and extractability of Co(II) complexes with 1-alkyl-2-methylimidazoles. Sep. Sci. Technol. 2015, 50, 676–682.
- Radzyminska-Lenarcik, E. Influence of the steric hindrance, ligand hydrophobicity and DN of solvents on structure and extraction of Cu(II) complexes with 1-alkyl-2-ethylimidazole. Sep. Sci. Technol. 2008, 43, 794–814.
- Radzyminska-Lenarcik, E. The influence of alkyl chain length in 1,2-dialkylimidazoles on the extraction capacity and structure of their copper(II) complexes. Sep. Sci. Technol. 2009, 44, 954–970.
- Radzyminska-Lenarcik, E. The influence of steric effect, alkyl chain length and donor number of solvents on the extraction of copper(II) complexes with 1-alkyl-4-methylimidazoles. Solvent Ext. Ion Exch. 2010, 28, 636–652.
- Lenarcik, B.; Kulig, J.; Laider, P. Stability and structure of transition metal complexes with azoles in aqueous solutions, Part II. 2-methylimidazole complexes of Co(II), Cu(II) and Zn(II). Rocz. Chem. 1974, 48, 1151–1158.
- Lenarcik, B.; Kurdziel, K.; Czopek, R. Stability and structure of transition metal complexes with azoles in aqueous solutions. Part XXVIII. The steric effect in the complex formation of 2-n-propylimidazole and 2-isopropylimidazole. Pol. J. Chem. 1991, 65, 815–820.
- Lenarcik, B.; Kurdziel, K.; Czopek, R. Stability and structure of transition metal complexes with azoles in aqueous solutions. Part XXIX. The influence of the size and structure of the alkyl group on the formation of 2-alkylimidazole complexes. Pol. J. Chem. 1991, 65, 1235–1241.
- Beniamin Lenarcik; Piotr Ojczenasz; The influence of the size and position of the alkyl groups in alkylimidazole molecules on their acid-base properties. Journal of Heterocyclic Chemistry 2002, 39, 287-290, 10.1002/jhet.5570390206.
- Cupery, M.E. N-Imidazole Compounds and Their Complex Metal Derivatives. U.S. Patent 3,843,667, 22 October 1974.
- Schakers, J.M.; du Preez, J.G.H. Solvent Extraction Mixture Comprising Substituted Imidazole or Benzimidazole for the Purification of Groups of Base Metals. U.S. Patent 20040208808, 21 October 2004.
- Radzyminska-Lenarcik, E.; Ulewicz, M. Selective transport of Cu(II) across a polymer inclusion membrane with 1-alkylimidazole from nitrate solutions. Sep. Sci. Technol. 2012, 47, 1113–1118.
- Radzyminska-Lenarcik, E.; Ulewicz, M. The use of the steric effect of the carrier molecule in the polymer inclusion membranes for the separation of cobalt(II), nickel(II), copper(II), and zinc(II) ions. Pol. J. Chem. Technol. 2015, 17, 51–56.
- Ulewicz, M.; Radzyminska-Lenarcik, E.; Transport of metal ions across polymer inclusion membrane with 1-alkylimidazole. Physicochem. Probl. Miner. Process. 2011, 46, 119–130.
- Radzyminska-Lenarcik, E.; Ulewicz, M.; The use of 1-alkylimidazoles for selective separation of zinc ions in the transport process across a polymer inclusion membrane. Physicochem. Probl. Miner. Process. 2014, 50, 131–142., 10.5277/ppmp140112.
- Elzbieta Radzyminska-Lenarcik; Malgorzata Ulewicz; Radzyminska- Lenarcik; Polymer Inclusion Membranes (PIMs) Doped with Alkylimidazole and their Application in the Separation of Non-Ferrous Metal Ions. Polymers 2019, 11, 1780, 10.3390/polym11111780.
- Elzbieta Radzyminska-Lenarcik; Malgorzata Ulewicz; The Application of Polymer Inclusion Membranes Based on CTA with 1-alkylimidazole for the Separation of Zinc(II) and Manganese(II) Ions from Aqueous Solutions. Polymers 2019, 11, 242, 10.3390/polym11020242.
- Radzyminska-Lenarcik, E.; Ulewicz, M. Application of polymer and supported membranes with 1-alkyl-2-methylimidazoles for separation of some transition metal ions. Desal. Water Treat. 2017, 64, 425–431.
- Ulewicz, M.; Radzyminska-Lenarcik, E. Supported liquid (SLM) and polymer inclusion (PIM) membranes pertraction of copper(II) from aqueous nitrate solutions by 1-hexyl-2-methylimidazole. Sep. Sci. Technol. 2012, 47, 1383–1389.
- Ulewicz, M.; Radzyminska-Lenarcik, E.; Application of Polymer and Supported Membranes with 1-Decyl-4-Methylimidazole for Pertraction of Transition Metal Ions. Physicochem. Probl. Miner. Process. 2012, 48, 91–102, 10..
- Ulewicz, M.; Radzyminska-Lenarcik, E. Application of Polymer and Supported Membranes with 1-Decyl-4-Methylimidazole for Pertraction of Transition Metal Ions. Sep. Sci. Technol. 2014, 49, 1713–1721.
- Ulewicz, M.; Radzyminska-Lenarcik, E. Application of polymer membranes doped with 1-hexyl-4-methylimidazole for pertraction of zinc(II) ions. Physicochem. Probl. Miner. Process. 2015, 51, 447–460.
- Elzbieta Radzyminska-Lenarcik; Robert Ulewicz; Malgorzata Ulewicz; Zinc recovery from model and waste solutions using polymer inclusion membranes (PIMs) with 1-octyl-4-methylimidazole. DESALINATION AND WATER TREATMENT 2008, 102, 211-219, 10.5004/dwt.2018.21826.
- Elzbieta Radzyminska-Lenarcik; Katarzyna Witt; The application of membrane extraction in the separation of zinc and cadmium ions. DESALINATION AND WATER TREATMENT 2018, 128, 140-147, 10.5004/dwt.2018.22628.
- Bjerrum, J. Metal Ammine Formation in Aqueous Solution: Theory of the Reversible Step Reactions, P.; Hasse: Copenhagen, Denmark, 1957.
- Lenarcik, B.; Kurdziel, K.; Czopek, R.; Search for optimum conditions of extraction of metal complexes with alkylimidazoles, III. Structure—Extractability relationships for 1,4-dimethylimidazole complexes of Co(II), Ni(II), Cu(II), Zn(II), and Cd(II). Solvent Ext. Ion Exch. 1986, 4, 165–182.
- Cieślak-Golonka, M.; Starosta, J.; Wasielewski, M. Wstęp Do Chemii Koordynacyjnej; PWN: Warszawa, Poland, 2010.
- J Gaazo; J. Gaǎzo; I.B. Bersuker; J. Garaj; M. Kabešová; J. Kohout; H. Langfelderová; M. Melník; M. Serator; F. Valach; et al. Plasticity of the coordination sphere of copper(II) complexes, its manifestation and causes. Coordination Chemistry Reviews 1976, 19, 253-297, 10.1016/s0010-8545(00)80317-3.





