Video Upload Options
Flux is a granular material that primarily consists of oxides and CaF2. The manufacturing method plays an important role in shaping the flux features.
1. Introduction
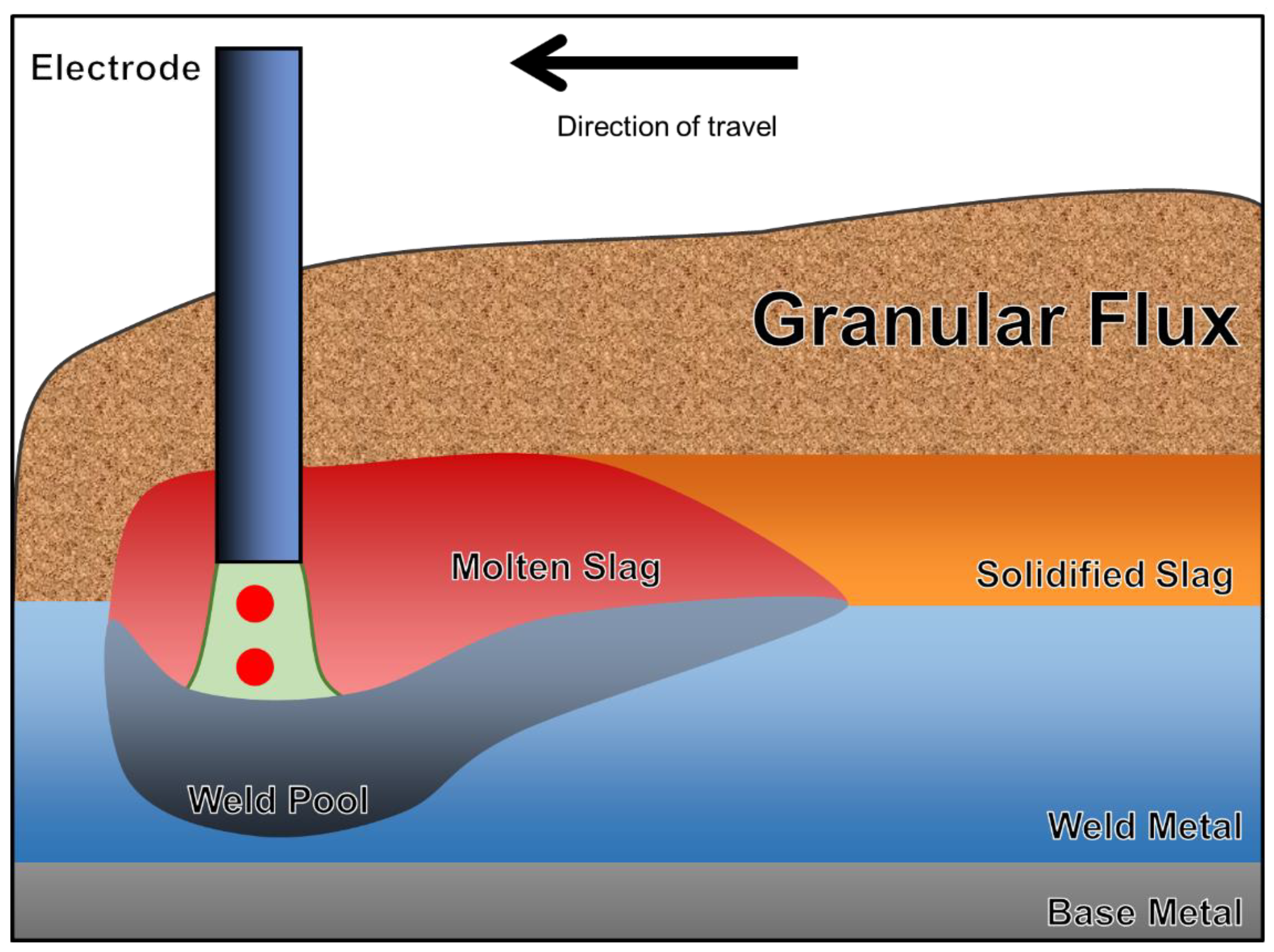
2. Flux Classification
2.1. Manufacturing Methods
2.1.1. Fused Flux
2.1.2. Bonded Flux
2.1.3. Agglomerated Flux
| Flux | Advantages | Disadvantages |
|---|---|---|
| Fused flux | Excellent homogeneity. Nonhygroscopic Recyclable |
Inability to add deoxidizers and ferroalloys |
| Bonded flux | Permit the extensive use of deoxidizers and ferroalloys | Tendency to pick up moisture Require better protection during storage |
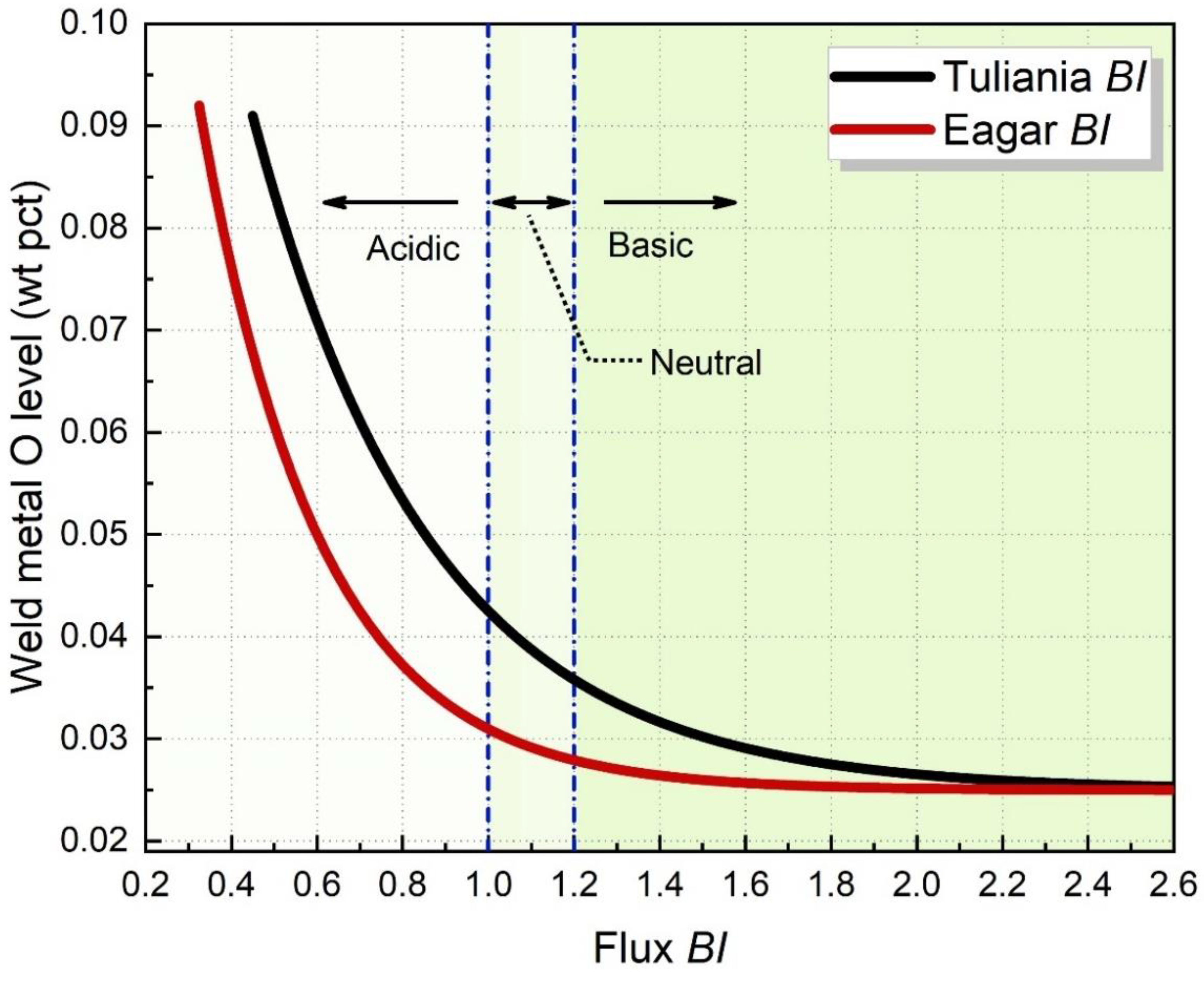
2.2. Basicity Assessment of SAW Flux
2.3. Workflow Methodology
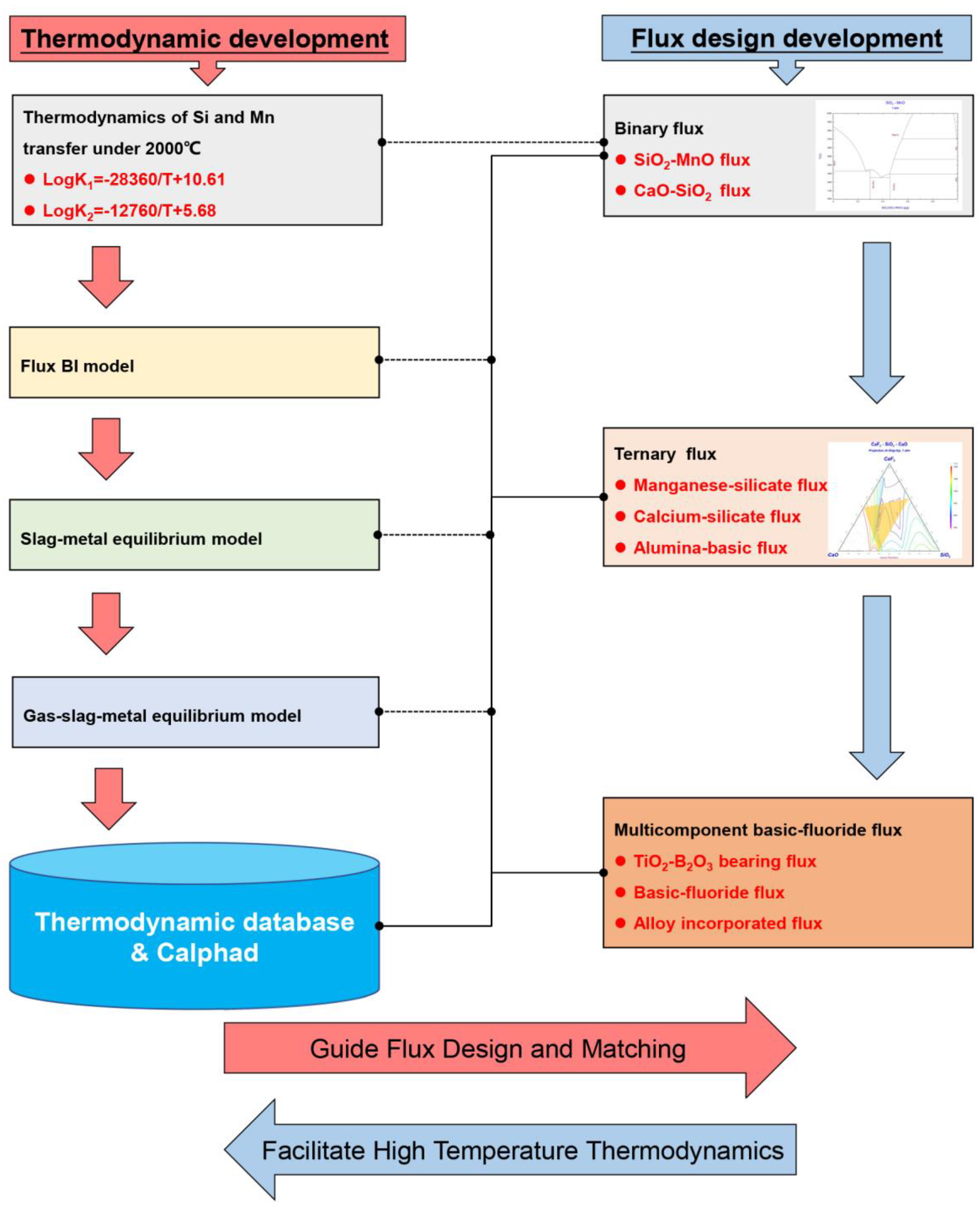
The review above has demonstrated that the thermodynamics is vital for the development of the flux design, while the ongoing flux design requirement further foster the research in thermodynamics subject to high temperature. From the perspective of technology, the measurement of thermodynamic data under the temperature of 2000℃ is impossible[4]. Therefore, to further improve the thermodynamic calculation accuracy, the development of the thermodynamic data under high temperature is anticipated.
Then, a significant feature of SAW concerns the chemical interaction between plasma and the alloy element. For intendance, it has been extensively established that the element loss of Mn is favored during SAW process, and the sole consideration of thermodynamic equilibrium is insufficient to explain such loss. Therefore, a deeper understand of the interconnection between plasma and welding consumables is required.
The development of new fluxes for submerged arc welding is ongoing. The present study provides a revealing insight into the flux design at various stages from the thermodynamic perspective: The flux design philosophy regarding binary, ternary, and multicomponent systems have been systematically reviewed. The contribution of each flux system to the development of welding thermodynamics and technology are documented. The Claphad technology is introduced to facilitate the flux design, flux selection, and the matching of welding consumables. Based on the critical outcome, the following conclusions can be drawn:
- Binary fluxes were mainly applied in the early trails of SAW primarily focused on the flux capability to perform the submerging function with high currents and facilitate high deposition rates. Despite of several limitations, the binary fluxes promote the development of welding thermodynamics, especially in terms of the transfer of Si and Mn mechanisms at slag-metal interface.
- Ternary fluxes were developed based on binary fluxes benefiting from deeper understandings of the thermodynamics. Within this stages, various types of fluxes, such as manganese-silicate fluxes, calcium-silicate fluxes, and alumina-basic fluxes have been developed to fulfill different SAW conditions. With the development of welding metallurgy and growing demand in the arctic regions for large welded structures, investigators began trying the development of multicomponent fluxes.
- The Calphad technology and progressive thermodynamic databases facilitate the flux design process and strengthen the understanding of SAW process. By using Calphad technology, gas-slag-metal equilibrium model has been developed, which processes stronger universality than the traditional BI slag-metal equilibrium models.
- Then Viscosity module, Phase Diagram module, and Equilib module is able to aid in the flux design so that some random experiments regarding flux design can be replace, thereby saving human and material resources.
References
- Sengupta, V.; Havrylov, D.; Mendez, P. Physical Phenomena in the Weld Zone of Submerged Arc Welding—A Review. Weld. J. 2019, 98, 283–313.
- Olson, D.; Liu, S.; Frost, R.; Edwards, G.; Fleming, D. Nature and Behavior of Fluxes Used for Welding; ASM Handbook; ASM International: Almere, The Netherlands, 1993; Volume 6, pp. 55–63.
- Natalie, C.A.; Olson, D.L.; Blander, M. Physical and Chemical Behavior of Welding Fluxes. Annu. Rev. Mater. Sci. 1986, 16, 389–413.
- Cong, W.; Zhang, J. Fine-tuning Weld Metal Compositions via Flux Optimization in Submerged Arc Welding: An Overview. Acta Metall. Sin. 2022, 57, 1126–1140.
- Zhang, J.; Coetsee, T.; Dong, H.; Wang, C. Element Transfer Behaviors of Fused CaF2-SiO2-MnO Fluxes under High Heat Input Submerged Arc Welding. Metall. Mater. Trans. B 2020, 51, 885–890.
- Zhang, J.; Wang, C.; Coetsee, T. Thermodynamic Evaluation of Element Transfer Behaviors for Fused CaO-SiO2-MnO Fluxes Subjected to High Heat Input Submerged Arc Welding. Metall. Mater. Trans. B 2021, 52, 1937–1944.
- A 3.0 M/A3.0; Standard Welding Terms and Definitions. AWS: Bellevue, WA, USA, 2010.
- Jackson, C. Flux and Slag in Welding. Weld Res. Bull 1973, 190.
- Tuliani, S.; Boniszewski, T.; Eaton, N. Notch Toughness of Commercial Submerged Arc Weld Metal. Weld. Met. Fabr. 1969, 37, 327–339.
- Eagar, T. Sources of Weld Metal Oxygen Contamination during Submerged Arc Welding. Weld. J. 1978, 57, 76–80.
- Shao, G.; Liu, Z.; Fan, J.; Guo, Y.; Xu, Q.; Zhang, J. Evaluation of Flux Basicity Concept Geared toward Estimation for Oxygen Content in Submerged Arc Welded Metal. Metals 2022, 12, 1530.
- Kou, S. Welding Metallurgy, 3rd ed.; JohnWiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 22–122.
- Palm, J. How fluxes determine the metallurgical properties of submerged arc welds. Weld. J. 1972, 51, 358.