You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Jin Zhang.
Flux is a granular material that primarily consists of oxides and CaF2. The manufacturing method plays an important role in shaping the flux features.
- physicochemical properties
- calphad
- material design
1. Introduction
The atmosphere is primarily composed of N2 and O2. When the weld pool is exposed to the atmosphere, it tends to combine with O and N elements. The formation of oxides in the weld metal (WM) varies under different circumstances. The oxide layer on the metal surface may prevent the joining of the two workpieces together [1]. Additionally, an excess of O in the WM may incur unexpected problems, such as enhanced porosity, reduced toughness, and depreciated hardenability [2]. When N is absorbed by the molten metal, its presence exerts adverse impacts on the mechanical properties unless it combines with a strong nitride-forming element [3].
Submerged arc welding (SAW) is an automatic welding process particularly suitable for the joining of thick workpieces [4]. Instead of permitting the air to exist between the base metal (BM) and the electrode, the arc cavity and weld pool are submerged under a layer of granular flux [1]. Figure 1 illustrates the diagrammatic sketch of SAW. The SAW process is unique due to the permission of high welding currents in the absence of a violent arc [5]. Therefore, high deposition rates and welding efficiencies are expected when SAW is applied [6]. Flux is defined by the American Welding Society (AWS) as a material used for preventing, dissolving, or facilitating the removal of oxides and other undesirable surface substances [7].
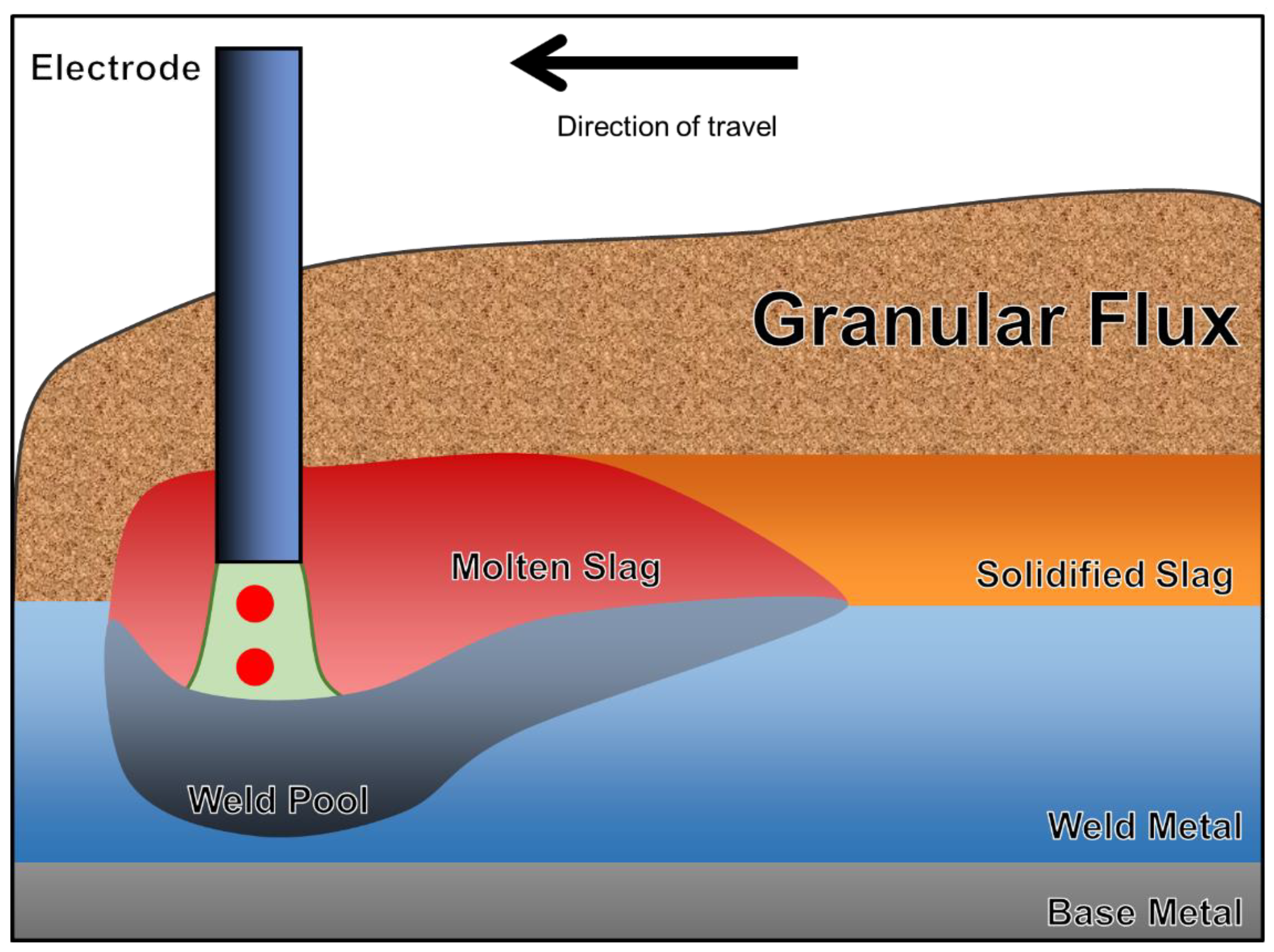
Figure 1.
Diagrammatic sketch of the SAW process.
2. Flux Classification
Flux is a granular material that primarily consists of oxides and CaF2 [4]. The manufacturing method plays an important role in shaping the flux features. In this section, the flux classification methods are documented to detail the manufacturing methods and to introduce an important definition widely applied in the field of flux design, namely, flux basicity.
2.1. Manufacturing Methods
2.1.1. Fused Flux
The fused flux is manufactured by melting the raw materials in a furnace at 1500 °C or higher. The melt is stirred for homogenization and then solidified by water or a metal plate. At last, the solidified fragments are screened to a proper size range [2].
2.1.2. Bonded Flux
Raw materials for producing the bonded flux are dry mixed and then combined with an addition of potassium silicate or sodium silicate at approximately 300 °C or higher. The resulting mixture is then dried at a relatively low temperature and screened [3].
2.1.3. Agglomerated Flux
The agglomerated flux is similar to the bonded flux except that it is bonded at a temperature higher than 760 °C. Table 1 summarizes the advantages and disadvantages of fused flux, bonded flux, and agglomerated flux [8].
Table 1.
| Flux | Advantages | Disadvantages |
|---|---|---|
| Fused flux | Excellent homogeneity. Nonhygroscopic Recyclable |
Inability to add deoxidizers and ferroalloys |
| Bonded flux | Permit the extensive use of deoxidizers and ferroalloys | Tendency to pick up moisture Require better protection during storage |
2.2. Basicity Assessment of SAW Flux
The most widely accepted BI was proposed by Tuliani et al. [16][9], as shown in Equation (1) (wt pct). The oxygen-free compound is CaF2. The basic oxides include CaO, CaF2, MgO, Na2O, K2O, MnO, and FeO, while the acid oxides include SiO2, Al2O3, Cr2O3, TiO2, and ZrO2. Fluxes can be classified into three categories: acidic (BI < 1.0), neutral (1 ≤ BI < 1.2), and basic (BI ≥ 1.2) [2]. Tuliania et al. [16][9] have regressed the tendency of O level as a function of BI, as shown by Equation (1). Eagar [28][10] then removed CaF2 from Equation (1) since he assumed that CaF2 should be considered as a neural component. Generally, the predicted O content decreases with increasing BI and then reaches a constant [2]. From the empirical relationship between BI and WM O content, the flux’s O potential can be estimated, as shown in Figure 2 [16,28,29][9][10][11].
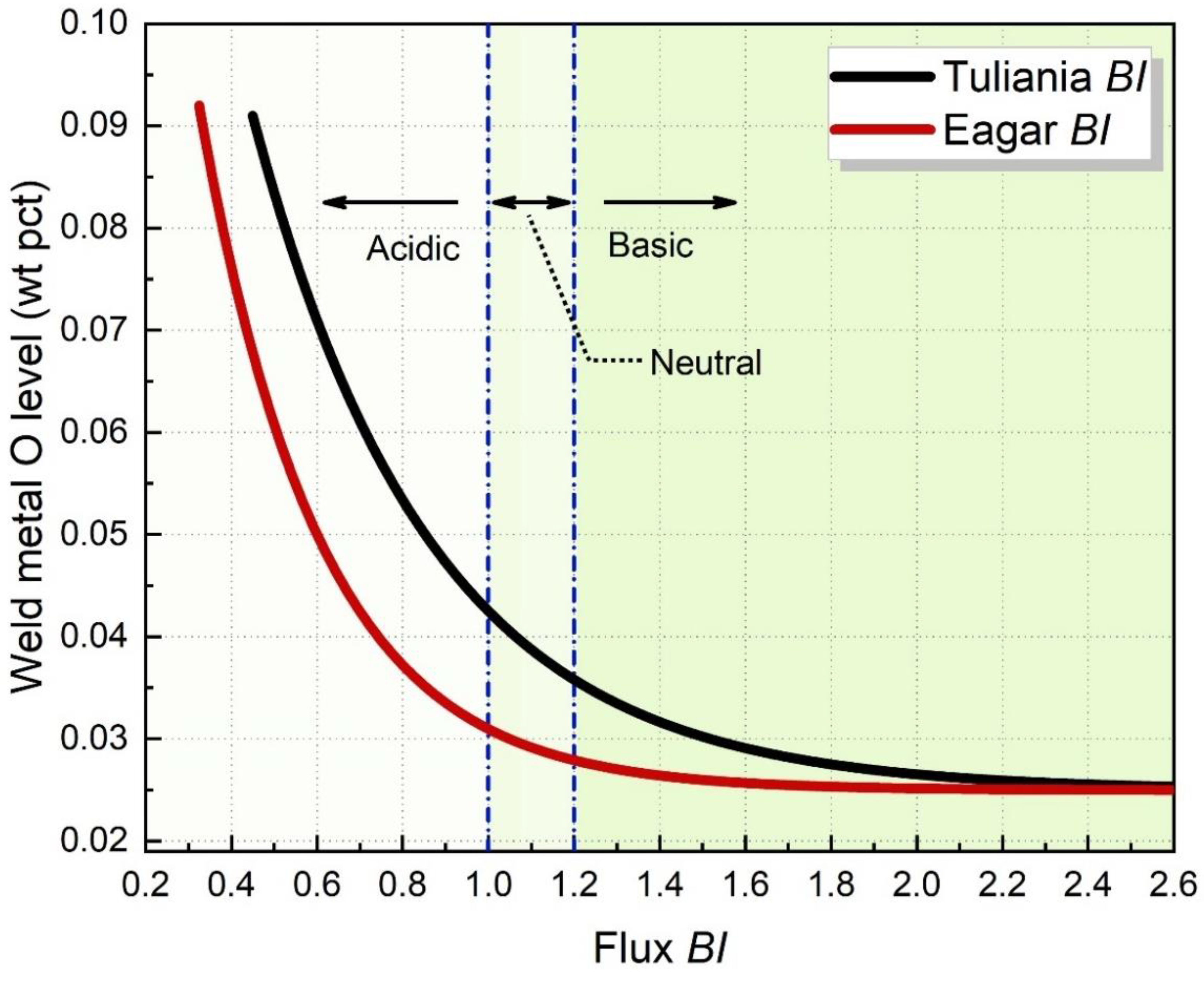
Figure 2. Predicted WM O level as a function of flux BI [16,28,29].
BI definition is a controversial subject. Much research has been performed in this area, but most of it has been empirical in nature [3]. Numbers of controversy centers around the BI definition subject to SAW and how different components in a flux contribute to the flux behavior [10][12]. Palm et al. [30][13] also pointed out that there is a lack of fundamental basis regarding the correlation between BI and flux O potential.
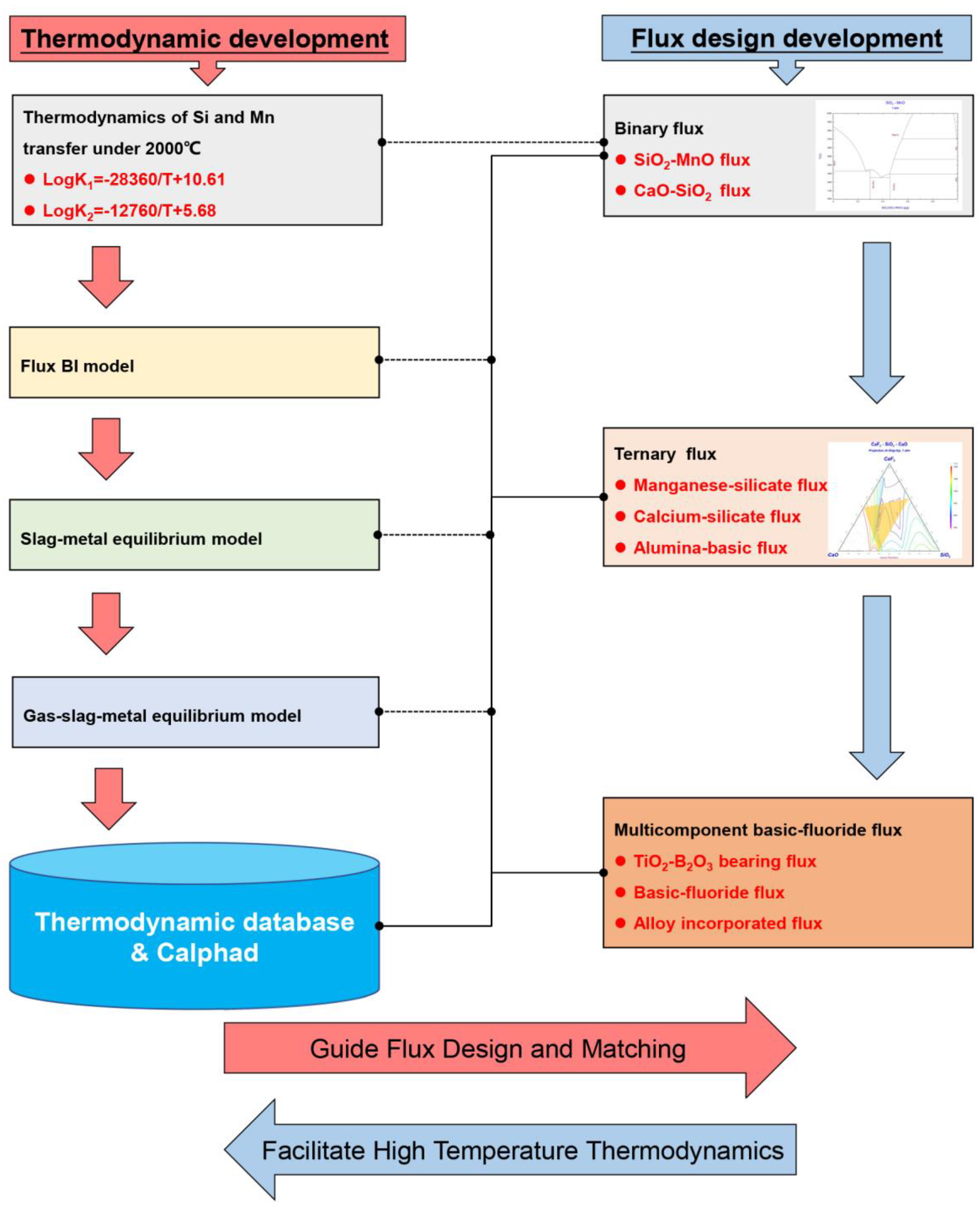
2.3. Workflow Methodology
Submerged arc welding is a metallurgical process with a temperature as high as 2000 °C, which puts forward techniques and scientific challenges for flux design. An understanding of the physicochemical properties of flux and slag, especially under high temperatures, is essential to optimize the flux formula. The overall flow diagram with respect to workflow methodology is plotted in Figure 3 for better readership. As shown in Figure 3, the development of the flux in submerged arc welding experiences three stages: the binary system, ternary system, and multicomponent system. Within this framework, the flux design stages have been documented and reviewed in detail. The design principles for fluxes are evaluated, and the limitations of each flux are elucidated. Furthermore, wresearchers explain and analyze the scientific significance of the designed fluxes upon the development of the welding metallurgy, especially in terms of the thermodynamic models developed to aid in the flux design. As last, the application of Calphad technology in welding metallurgy has been summarized.
Figure 3.
Flow diagram with respect to workflow methodology.
References
- Sengupta, V.; Havrylov, D.; Mendez, P. Physical Phenomena in the Weld Zone of Submerged Arc Welding—A Review. Weld. J. 2019, 98, 283–313.
- Olson, D.; Liu, S.; Frost, R.; Edwards, G.; Fleming, D. Nature and Behavior of Fluxes Used for Welding; ASM Handbook; ASM International: Almere, The Netherlands, 1993; Volume 6, pp. 55–63.
- Natalie, C.A.; Olson, D.L.; Blander, M. Physical and Chemical Behavior of Welding Fluxes. Annu. Rev. Mater. Sci. 1986, 16, 389–413.
- Cong, W.; Zhang, J. Fine-tuning Weld Metal Compositions via Flux Optimization in Submerged Arc Welding: An Overview. Acta Metall. Sin. 2022, 57, 1126–1140.
- Zhang, J.; Coetsee, T.; Dong, H.; Wang, C. Element Transfer Behaviors of Fused CaF2-SiO2-MnO Fluxes under High Heat Input Submerged Arc Welding. Metall. Mater. Trans. B 2020, 51, 885–890.
- Zhang, J.; Wang, C.; Coetsee, T. Thermodynamic Evaluation of Element Transfer Behaviors for Fused CaO-SiO2-MnO Fluxes Subjected to High Heat Input Submerged Arc Welding. Metall. Mater. Trans. B 2021, 52, 1937–1944.
- A 3.0 M/A3.0; Standard Welding Terms and Definitions. AWS: Bellevue, WA, USA, 2010.
- Jackson, C. Flux and Slag in Welding. Weld Res. Bull 1973, 190.
- Tuliani, S.; Boniszewski, T.; Eaton, N. Notch Toughness of Commercial Submerged Arc Weld Metal. Weld. Met. Fabr. 1969, 37, 327–339.
- Eagar, T. Sources of Weld Metal Oxygen Contamination during Submerged Arc Welding. Weld. J. 1978, 57, 76–80.
- Shao, G.; Liu, Z.; Fan, J.; Guo, Y.; Xu, Q.; Zhang, J. Evaluation of Flux Basicity Concept Geared toward Estimation for Oxygen Content in Submerged Arc Welded Metal. Metals 2022, 12, 1530.
- Kou, S. Welding Metallurgy, 3rd ed.; JohnWiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 22–122.
- Palm, J. How fluxes determine the metallurgical properties of submerged arc welds. Weld. J. 1972, 51, 358.
More
