1. Gut Microbiome and Its’ Influence on the Colon
As mentioned earlier, one of the key factors playing a role in colorectal carcinogenesis is the environment of the gut microbiome. The gut microbiome constitutes a rich and diverse community of microorganisms
[1]. This ecosystem is formed before birth and develops to become a fully functioning and stable microbiome within 2 to 3 years
[2][3]. The human intestine is estimated to contain more than 2000 microbial species
[4]. In addition, the most heavily microbial colonized section of the digestive system is the colon. It is estimated to contain around 70% of the human microbiome
[1].
These microbial species perform a variety of functions, some of which include metabolizing indigestible food, modulating immune response, and synthesizing nutrients
[4]. Moreover, it is now evident that the process of acquiring and maintaining gut microbes is fundamental for an individual’s health
[5]. These microbes are vital in the formation of mucosal immunity
[6]. For example, a class of microbicidal proteins in Paneth cells known as angiogenin-4 can be secreted against microbes into the gut lumen
[6]. The commensal bacteria residing within the intestine are capable of enhancing the intestines’ innate immunity by modulating toll-like receptors (TLRs) expression on immune cells’ surface via pathogen-associated molecular patterns leading to the expression of antimicrobial peptides (AMPs)
[7]. Microbes lead to the activation of T-cells by activating the nuclear factor-kappa B signaling pathway, which in turn leads to the stimulation of cytokine production and overexpression of costimulatory molecules on the antigen-presenting cells (APCs)
[7]. In turn, the TLRs activation leads to the induction of islet-derived protein 3 gamma (Reg3g) expression
[7]. The TLR activation induces the inhibition of inflammatory action contributing to intestinal homeostasis
[7].
Additionally, the microbes found within the gastrointestinal tract are capable of communicating with each other, as well as with the host
[5]. This communication feature may ultimately result in great effects on disease and health development
[5]. In addition to immune response, this communication is also essential for appropriate mucosal function
[2]. Furthermore, this crosstalk is mediated by metabolites, proteins, and small RNAs
[2]. These communicate together via epithelium
[2]. In this regard, since the gut microbiome interacts with the host, it contributes to the process of carcinogenesis
[1]. Furthermore, the colon is thought to be the most disposed to cancer development upon its comparison to other sections of the digestive tract
[1].
Alterations in the gut microbiome may contribute to various diseases. This may be because of their role in metabolism and immune function
[4]. These alterations are known as dysbiosis and are facilitated through changes in the Mus musculus miRNA
[2]. For example, changes occurring within the intestinal microbiome may result in the initiation and promotion of colorectal cancer
[4].
Fusobacterium nucleatum and
Escherichia coli, through the uptake of specific human sncRNA, regulate the expression of microbial genes, thus affecting their growth
[2].
Fusobacterium nucleatum is the most found gut bacterium in CRC patients; this bacterium is a gram-negative anaerobe
[8]. Furthermore, this bacterium acts as a prognostic biomarker; at higher levels it usually means a shorter overall survival
[8].
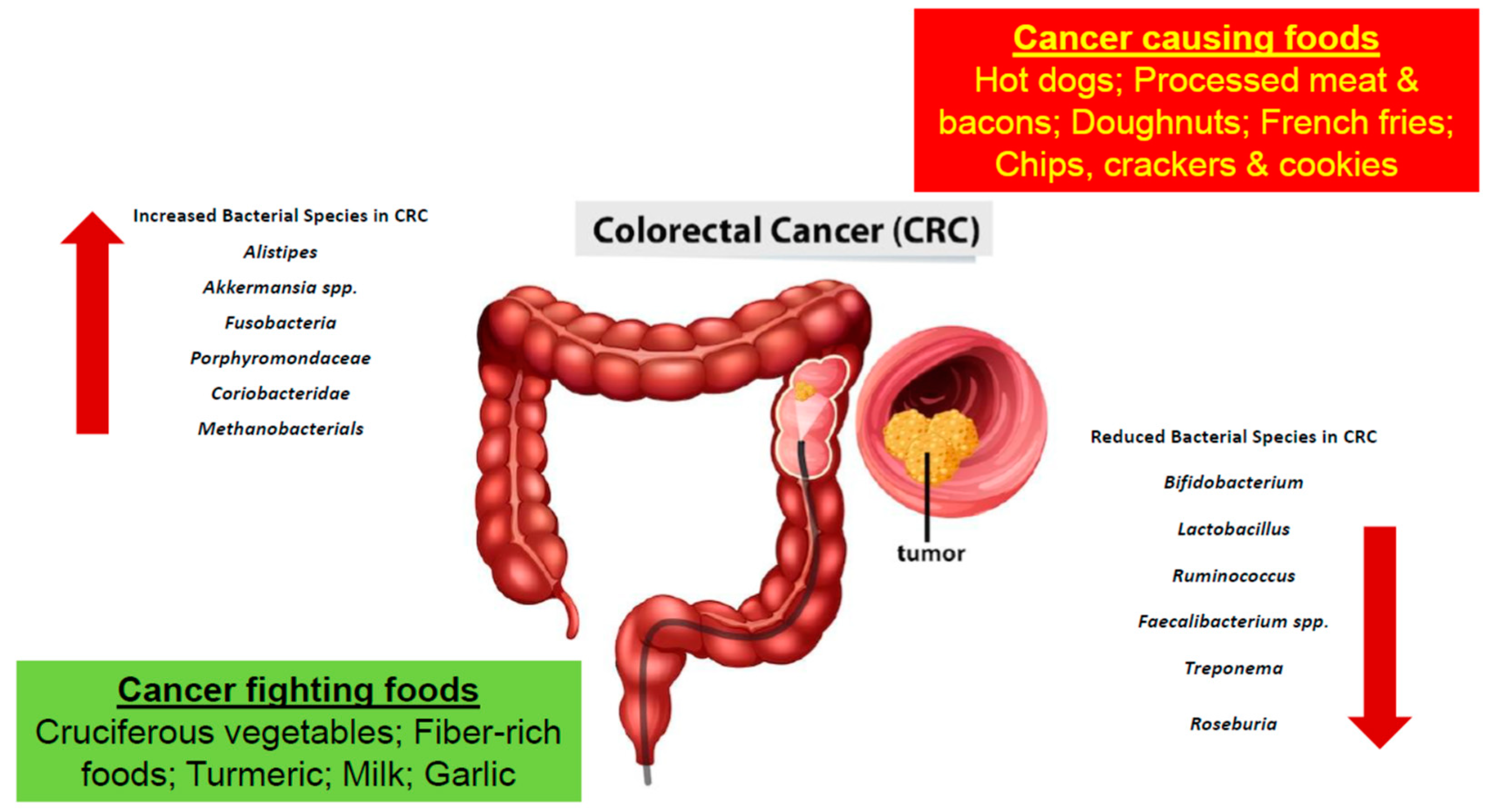
Furthermore, based on studies obtained, certain bacteria are found to be greater in number in CRC patients, while others are found to lessen
[8][9]. Bacteria such as
Alistipes,
Akkermansia spp.
Fusobacteria,
Porphyromonadaceae,
Coriobacteridae, and Methanobacteriales were found to be increasing in number in the colon microbiota of a CRC patient
[9][10]. More specifically,
Bacteroides fragilis,
Escherichia coli,
Fusobacterium nucleatum,
Enterococcus faecalis, and
Streptococcus gallolyticus were all linked to CRC
[11]. In a study conducted, an increase in polyketide synthase pks island-positive
Escherichia coli was found in the colon tissues isolated from CRC patients
[12]. Additionally, in another study conducted with CRC patients,
F. nucleatum, an oral bacterium, which will be elaborated on further in the upcoming sections, was also found within the colorectal tumors of the patients
[12]. Furthermore, in another study conducted on mice, tumor-bearing mice were found to have an increase in the number of Bacteroides within their fecal samples
[13]. Moreover, bacteria such as
Bifidobacterium,
Lactobacillus,
Ruminococcus,
Faecalibacterium spp.,
Treponema, and
Roseburia were decreasing in number (
Table 1 and
Figure 1)
[9][10]. Further studies were performed using germ-free mice. The fecal samples of patients with CRC and healthy individuals were transplanted into the mice in which cancer was promoted chemically. It was recorded that the rate upon which the tumor was generated was associated.
Figure 1. The development of colorectal cancer is a result of a multifactorial process involving the consumption of various foods and gut microbial dysbiosis.
Table 1. Various bacterial species are found to associate positively or negatively with CRC. In patients diagnosed with CRC the bacteria families found to have increased in number include
Alistipes,
Akkermansia spp.,
Fusobacteria,
Porphyromondaceae,
Coriobacteridae and
Methanobacterials. Whereas the bacteria species belonging to
Bifidobacterium,
Lactobacillus,
Ruminococcus,
Faecalibacterium spp.,
Treponema and
Roseburia are decreased in number
[12].
With microbial composition. Gram-positive bacteria such as Clostridium group XIVa were found to associate negatively with tumor generation. In contrast, Gram negative bacteria such as
Alistipes, Akkermansia,
Parabacteroides, and
Bacteroides are associated positively with tumor generation
[9][10][14].
Moreover, through evidence obtained, it is proven that alterations in the gut microbiome occur during the initial stages of colorectal carcinogenesis
[4]. It is hypothesized that alterations in colonic flora may create a more favorable microenvironment for tumor development
[1]. Bacterial micro vesicles may play a role in tumorigenesis, and in fact, their role is underestimated
[2]. There is a possibility that the extracellular vesicles from the host and microbiota in the intestinal ecosystem promote tumor survival and multi-drug resistance
[2]. Furthermore, with changes in the gut microbiota, it may be possible to identify the precursor lesion for CRC: colorectal adenoma, for individuals at risk
[4]. It may be possible to modify the intestinal microbiome to aid in the prevention of CRC
[4].
2. Oral Bacteria and Its Role in Colorectal Cancer
The discovery of oral bacteria dates back to the 1670s, when Antony Van Leeuwenhoek reported the presence of various microbes within the plaque on tooth surfaces, since then to examine multispecies microbial communities, researchers have studied the human oral microbiome
[15][16]. The oral microbiome is a complex ecosystem containing billions of bacteria, with approximately 700 predominant taxa
[17][18]. The bacterial taxa colonizing the oral cavity contribute to oral health and oral diseases
[18]. The various bacteria present create their own balanced ecosystem in which they are capable of surviving
[17].
Oral bacteria have been shown through molecular methods to be involved in colorectal cancer
[2]. In fact, through various studies, it has been observed that various oral bacteria may play an essential role in the development of colorectal cancer
[19]. Tissue samples from the intestinal mucosa have been collected from CRC patients, in which higher numbers of
Fusobacterium,
Peptostreptococcus,
Mogibacterium spp., and
Porphyromonas have been found (
Table 2)
[17]. Additionally, in another study, fecal samples were collected, and an increase in oral bacterial species was found. Those bacteria include
Actinomyces,
Corynebacterium,
Mogibacterium,
Haemophilus, and
Porphyromonas [17].
Table 2. The oral bacteria found within tissue samples of CRC patients include
Fusobacterium,
Peptostreptococcus,
Mogibacterium spp. and
Porphyromona [17].
Furthermore, oral bacterial species such as
Fusobacterium and Bacteroides fragilis are found in both primary and metastatic CRC in humans
[2]. A high abundance of the
Fusobacterium is believed to be associated with tumor location and regional lymph node metastases
[19]. Moreover, a type of
Fusobacterium known as
Fusobacterium nucleatum is of specific interest since it has only recently been linked to colorectal cancer
[20]. Associations between the gram-negative
F. nucleatum bacteria and colorectal cancer in humans have been found in patients during the different disease stages
[20].
As mentioned earlier, this bacterium’s role has only recently been discovered hence why its role as a cancer-causing microbiota is still emerging; with new revelations being found regarding its various contributions to the development, growth, and spreading of cancer
[20]. Moreover, the nucleic acids of
F. nucleatum present in CRC tissues have been studied; this bacterium was found to be present within the tissues using the various molecular approaches, including 16 s ribosomal RNA (rRNA) gene amplicon sequencing, RNA sequencing (RNA-seq), directed quantitative PCR (qPCR) and DNA sequencing (DNA-seq)
[20]. Further studies to elucidate the precise mechanism of the role of this bacterium in the development of CRC are thus warranted.
3. The Relationship between an Individual’s Diet and Colorectal Cancer
An individual’s lifestyle, as well as the food they consume, can increase or decrease their chances of being diagnosed with colorectal cancer. As dietary patterns are changing in developed countries, individuals are increasing in weight and heading towards the obese and overweight sections of the scale
[21]. Furthermore, overweight individuals whose body mass index (BMI) is between 25 kg/m
2 to 29.9 kg/m
2 are 19% more likely to be diagnosed with CRC compared to individuals whose BMI is between 20 kg/m
2 to 25 kg/m
2 [21]. Studies have shown that certain diets may tend to increase the risk of CRC, while others, such as the Mediterranean diet, tend to decrease it
[22].
As mentioned earlier, due to the changing dietary patterns in developed countries, and the increase in consumption of red meat, there is an increase in CRC cases. In fact, approximately 60% of CRC cases occurring in developed countries are due to the unhealthy diet and lifestyles being followed
[22]. Individuals that are maintaining a diet rich in processed or red meat and high-fat dairy products, as well as various fast foods and drinks, are more prone to being diagnosed with CRC
[21]. Furthermore, with an increase in fat intake, there is a higher secondary bile production as well as insulin resistance which leads to the facilitation of carcinogenesis
[21]. With this increase in fat intake and the maintenance of an animal-based diet, there is a greater number of bile-tolerant microorganisms such as
Alistipes,
Bacteroides, and
Bilophila (
Table 3)
[23].
Table 3. The diet an individual follows plays a major role in their chances of developing CRC. Moreover, the diet an individual follows impacts the diversity of microorganisms inhabiting the gut. Alterations in the gut microbiome leads to an increased risk of CRC development.
Moreover, maintaining a diet rich in fiber is important as well. In fact, individuals sustaining a diet rich in whole-grain consumption were found to have a reduction in their chances of developing CRC
[25]. The term “dietary fiber” is a broad term referring to carbohydrate polymers consisting of ten or more monomeric units which the small intestine can neither digest nor absorb
[24]. Although they are incapable of being digested by the small intestine, dietary fibers are very beneficial. They have been proven to affect the metabolic activities in an individual’s gastrointestinal tract
[25]. It was found that the relation between an individual’s fiber intake density and their risk of developing CRC was inversely proportional. In addition, a high intake of dietary fiber is also associated with higher survival rates
[25]. Furthermore, the fermentation of certain fibers to short-chain fatty acids plays a major role in CRC prevention. The importance of short-fatty acids will be further explained in the coming paragraphs.
As stated earlier, the diet an individual follows has a great influence on their gut microbiota. For example, one diet may have the ability to promote the growth of certain bacterial species, which in turn may alter various processes such as fermentative metabolism
[23]. This alteration will lead to changes in an individual’s intestinal pH, thus increasing the chances of the development of pathogenic flora
[23]. To further elaborate, individuals with high fat are not only more prone to CRC, but the diet these individuals follow may lead to the promotion of pro-inflammatory gut microbiota
[23]. As a result, the intestine becomes more permeable, and pathogenic bacteria can take over. To further understand what is meant when stating that any type of alteration to the gut microbiota can lead to various gut-microbiota diseases, the changes (diet-depended) in the microorganisms of the gut-microbiota is going to be further elaborated
[23].
Initially, of the complete microbiota, four major microbial phyla are believed to represent more than 90% of the bacteria present in the gut. These phyla are
Firmicutes,
Bacteroides,
Proteobacteria, and
Actinobacteria [23][27]. Furthermore, the gut microbiota has three enterotypes. The word enterotype refers to the stratification of the human gut microbiota, which serves to reduce a large number of global microbiome variations into just a couple of categories; this term first appeared in Nature back in 2011
[28]. Within the three enterotypes, there is a specific group of bacteria that is more abundant when compared to the others. For example, in enterotype 1, Bacteroides are more abundant, while in enterotypes 2 and 3,
Prevotella and
Ruminococcus, are more prevalent, respectively
[23]. When an individual pursues a high-fat and protein diet, the growth of bacteria within enterotypes 1 and 3 are enhanced, compared to an individual pursuing a diet rich in carbohydrates whose enterotype 2 would be increased
[23][29].
Moreover, studies were conducted to understand the changes in the gut microbiota when different diets are followed. The gut microbiota of African and European children was studied
[23]. The African children came from rural Africa, whereas the Italian children came from urban areas
[23]. Based on what mentioned before, it was found that the African children contained more Bacteroidetes, whereas the European children contained more
Enterobacteriaceae [23]. Furthermore, the high consumption of red meat and low-fiber food, a form of diet referred to as the “Western diet,” is believed to cause an increase in the number of bacteria in the
Bacteroides phyla and
Ruminococcus [23]. Basically, a high-fat diet means a prevalence of
Bacteroides and
Actinobacteria, while a high fiber intake means less of
Bacteroides and
Actinobacteria [23]. On the other hand, a high fiber intake means an increase in
Firmicutes and
Proteobacteria [23]. Bacteroides-prevalent enterotype is associated with animal fats and proteins, while Prevotella-led enterotypes are associated with the high consumption of sugars and carbohydrates
[23].
Additionally, individuals maintaining a diet rich in fruits and vegetables, as well as whole grain cereals, white meat, and fish, are less likely to be diagnosed with CRC
[21]. In fact, individuals living along the Mediterranean coast follow a Mediterranean diet, which has proven to show a decrease in cancer mortality rates
[22]. Olive oil, red grapes, and tomatoes are three constituents of the diet that have been proven to reduce the risks of CRC
[22]. Olive oil, the very center of the Mediterranean diet, is a polyphenol that is believed to reduce the risk for CRC
[22]. This polyphenol is believed to contain many chemopreventive effects since it interferes with the initiation, promotion, and progression of the cancerogenesis pathway
[22]. Furthermore, phenolic derivatives generally tend to contribute to the cell adhesion processes, as well as tumor angiogenesis and migration
[21]. Red grapes contain resveratrol on their external skin, which is a phenolic compound that is found mainly in red wine
[22]. This compound contains various pharmacologic properties, including affecting the number of molecular targets of different cancer types
[22]. Furthermore, this compound has the capability to deregulate the multiple pathways which affect cancer cell growth as well as oncogenic signaling
[22]. Finally, of the many benefits tomatoes possess, one of them may be the prevention of cancer
[22]. An individual who consumes tomatoes daily has a 20% decrease in their risk of obtaining CRC
[22]. The reason tomatoes may have such a tremendous effect on CRC is possibly due to their high level of carotenoids, especially Beta-carotene and lycopene
[22]. However, prospective studies are needed to comprehend the precise role of these carotenoids.