Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Meghan Dukes | -- | 1297 | 2022-11-16 04:23:16 | | | |
| 2 | Vivi Li | -4 word(s) | 1293 | 2022-11-17 02:51:12 | | | | |
| 3 | Vivi Li | Meta information modification | 1293 | 2022-11-23 08:16:25 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Dukes, M.W.; Meade, T.J. Hedgehog Signaling for Basal Cell Carcinoma. Encyclopedia. Available online: https://encyclopedia.pub/entry/34775 (accessed on 07 February 2026).
Dukes MW, Meade TJ. Hedgehog Signaling for Basal Cell Carcinoma. Encyclopedia. Available at: https://encyclopedia.pub/entry/34775. Accessed February 07, 2026.
Dukes, Meghan W., Thomas J. Meade. "Hedgehog Signaling for Basal Cell Carcinoma" Encyclopedia, https://encyclopedia.pub/entry/34775 (accessed February 07, 2026).
Dukes, M.W., & Meade, T.J. (2022, November 16). Hedgehog Signaling for Basal Cell Carcinoma. In Encyclopedia. https://encyclopedia.pub/entry/34775
Dukes, Meghan W. and Thomas J. Meade. "Hedgehog Signaling for Basal Cell Carcinoma." Encyclopedia. Web. 16 November, 2022.
Copy Citation
Basal Cell Carcinoma (BCC) is the most commonly diagnosed cancer worldwide. While the survivability of BCC is high, many patients are excluded from clinically available treatments due to health risks or personal choice. Further, patients with advanced or metastatic disease have severely limited treatment options. The dysregulation of the Hedgehog (Hh) signaling cascade drives onset and progression of BCC. As such, the modulation of this pathway has driven advancements in BCC research.
basal cell carcinoma
HEDGEHOG signaling
smoothened inhibitors
Gli inhibitors
pre-clinical models
1. Introduction
Keratinocyte cancers, or nonmelanoma skin cancers (NMSCs), are the most commonly diagnosed cancers worldwide [1][2]. In the United States alone, one in every three to five Caucasian people are expected to develop an NMSC in their lifetime, with estimates as high as 4 million cases diagnosed each year [3][4][5]. Approximately 80% of all NMSCs are characterized as basal cell carcinomas (BCC), where uncontrolled growth of the basal cell population of the epidermis leads to tumorigenesis (Scheme 1) [6][7]. The overwhelming number of BCC diagnoses requires ample research and medical attention for the development of effective treatment and prevention strategies.
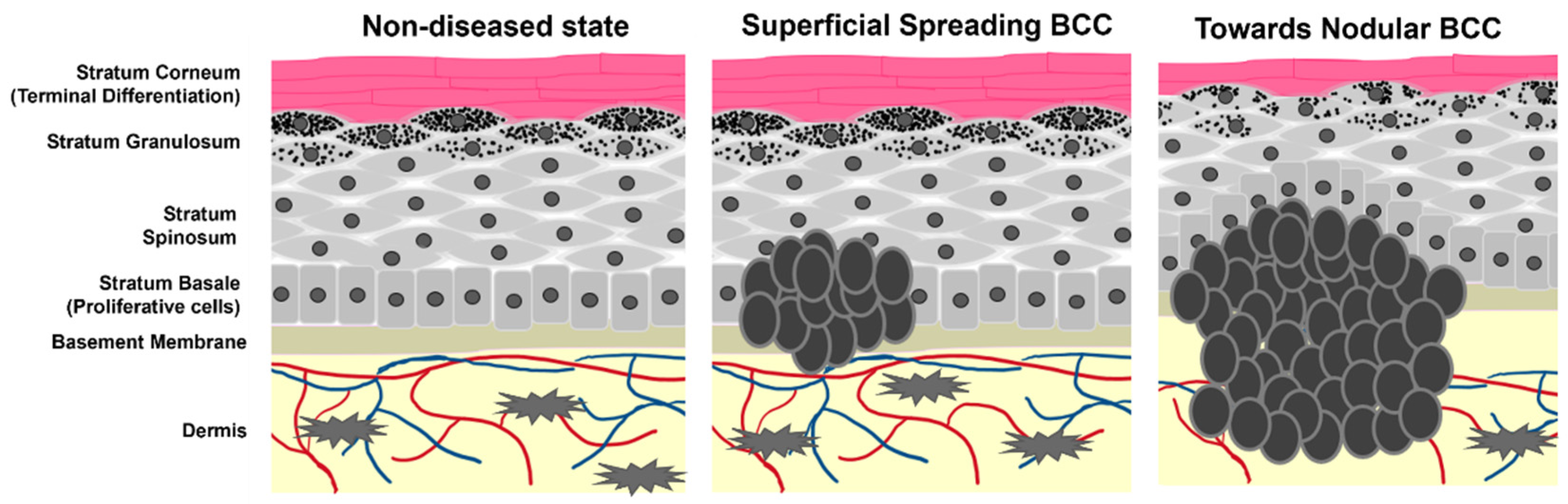
Scheme 1. Cartoon representation of epidermal layers and the growth of BCC. Tumor cells originate from the stratum basale where they maintain stemness and hyperproliferative capacity. As the tumors progress, they begin to spread and migrate into the dermis as well as alter the pathology of non-tumor keratinocyte differentiation.
Gorlin Syndrome (GS) is a rare autosomal dominant disease comprising a small percentage of the BCC community and approximately 0.05% of the population [8]. In total, 90% of patients with GS experience the uncontrolled growth of multiple BCCs alongside various developmental abnormalities including those associated with holoprosencephaly and malignant medulloblastomas [8][9]. Sporadic BCC accounts for the predominant population of BCC patients. The primary cause of sporadic BCC is prolonged exposure to ultraviolet (UV) radiation from the sun [10][11]. The risk of developing BCC increases with light-skin pigmentation, age, and sunburn frequency during youth [12]. Other risk factors include family history of melanoma, blonde/red hair phenotype, and men are more susceptible to BCC than women [12][13].
A significant burden to the BCC patient community is the high rate of recurrence. BCC is clinically designated as low or high risk depending on the likelihood of recurrence [14]. However, BCC more commonly recurs in an entirely different location on the body. For primary tumor locations, the recurrence rate depends heavily on the method of treatment, with a range from 1 to 70% after 5 years [15][16]. Larger tumors also experience an increased likelihood of relapse [16]. More strikingly, the three-year risk of developing a second BCC lesion is estimated between 41–44% [15][17][18][19], and the likelihood increases with each additional lesion. Once diagnosed, approximately 50% of patients will battle BCC again.
BCC is classified in three primary identities: superficial (10–30%), nodular (60–80%), and morpheaform/infiltrative (<10%) [7][20]. Each differ in physical and histopathological behavior and exhibit differential relapse rates [21]. Superficial and nodular BCCs are less likely to recur, whereas infiltrative BCCs are more challenging to treat permanently [22]. Additionally, the different subtypes have variable occurrence rates on different skin areas. Nodular BCC is most commonly found on the face, while superficial BCC affects the torso and hands more frequently [23]. Infiltrative BCC is the most aggressive form and can often lead to the destruction of nearby healthy tissue [24]. Due to these differences in risk classification and behavior, selective care must be taken when deciding which treatment option to pursue for an individual BCC patient.
BCC’s overall survivability is very high, with estimates for mortality being less than 0.5% [25][26]. However, the exceptionally high number of BCC diagnoses means that even a low mortality rate produces significant BCC-related cancer deaths [5]. The American Cancer Society estimates this population at around 2000 NMSC-related deaths annually, primarily attributed to metastatic BCC complications.
2. Predominant Treatment Options for BCC
Treatment strategies for BCC vary by subtype of the disease, size of the lesion, and patient age and preference. Table 1 outlines the advantages and disadvantages of each, including any imperative patient restrictions.
Table 1. Select list and commentary of treatment options for BCC.
| Treatment | Advantages | Disadvantages | Patient Restrictions |
|---|---|---|---|
| Wide Local Excision (WLE) | Low recurrence rates upon complete excision Short procedure times |
Excision likely to be incomplete and lead to higher recurrence rate Highly invasive |
Elderly patients where surgery is considered risky When loss of anatomical function is a risk |
| Mohs Micrographic Surgery (MMS) | Promotes excision of poorly defined tumor margins Suggested for more aggressive/high-risk tumors Minimizes harm to non-diseased tissue |
Long treatment times Requires highly trained physicians, and access may be limited to patients in underdeveloped areas |
Elderly patients where surgery is considered risky When loss of anatomical function is a risk |
| Radiation Therapy | Can treat tumors in locations where the loss of anatomical function is a risk Can be used in higher-risk BCC Boosts the efficacy of incomplete surgical resection when used in tandem |
Use of ionizing radiation Increases risk of melanoma Not as effective in larger Tumors Cannot determine the complete clearance of tumor tissue |
Not advised for patients with Gorlin Syndrome Not advised for younger patients due to the long-term impact of ionizing radiation |
| Ablative Laser Therapy |
Locally delivered, less destructive to non-diseased tissue Low recurrence rates Favorable cosmetic outcomes |
Not applicable to larger, deeper tissues May induce increased sensitivity to the sun |
Not recommended for patients with high sunlight sensitivity Not applicable for patients with nodular or infiltrative BCC |
| Photodynamic Therapy (PDT) |
Well characterized mechanisms of cytotoxicity Local administration of non-harmful laser light Safe for patients with Gorlin Syndrome |
High variability of treatment efficacy Multiple treatment sessions Increased sensitivity to Sunlight Severely limited depth penetration |
Not recommended for patients with high sunlight sensitivity Not applicable for patients with nodular or infiltrative BCC |
| Imiquimod Topical Therapy |
Well-characterized induction of immune response Topical, localized application reduces harm to healthy tissue |
Only approved for small superficial BCCs Many patients report skin Irritation Recurrence rates understudied |
Not applicable for patients with nodular or infiltrative BCC |
| 5-Fluorouracil Topical Therapy |
Well-characterized inhibition of DNA synthesis Topical, localized application reduces harm to healthy tissue High cure rate |
Not specific to tumor tissue and may cause harm to non-diseased skin Many patients report skin Irritation Recurrence rates understudied |
Not applicable for patients with nodular or infiltrative BCC |
3. The Hedgehog Signaling Cascade in BCC
In the 1990s, genetic evaluation of BCCs of patients with GS revealed the most crucial discovery in BCC research history: BCC lesions are often linked to mutations in the patched1 (PTCH1) gene loci [27][28][29][30]. Since then, it has become commonly accepted that the Hedgehog (Hh) signaling cascade, to which PTCH1 proteins belong, is BCC’s primary oncogenic driver [31][32][33]. The Hh pathway is canonically activated (Figure 1) by the binding of Hh ligands to the transmembrane protein PTCH1, which releases smoothened (SMO) inhibition. Suppressor of fused (SUFU) is signaled to release glioma-associated oncogene (Gli) transcription factors where they are activated in the cytosol. Translocation into the nucleus activates the expression of target genes for cellular processes such as proliferation and migration [34][35]. Dysregulation of this pathway is associated with many cancers but is causative of BCC [31][32][33][36][37]. As such, it is a promising chemotherapeutic target for BCC.

Figure 1. Key regulators of the Hedgehog signaling cascade. 1. Hh signaling is activated by the binding of Hh ligands (Sonic Hedgehog, Indian Hedgehog, and Desert Hedgehog) to the transmembrane protein PTCH1. 2. PTCH1 is a suppressor of SMO activity. Upon the binding of Hh proteins, inhibition of SMO is released, represented by the red X. 3. Upon activation, SMO signals for the SUFU complex to release the Gli family of transcription factors. 4. Gli transcription factors are activated in the cytosol prior to translocating into the nucleus. 5. Gli transcription factors transcribe pro-proliferative and migratory genes that lead to tumorigenesis in BCC. Crystal structures images were made from the following Protein Data Bank files: Ptch1/SHH complex, 6N7H. SMO, 6D35. SUFU/Gli complex: 4BLB. Gli1/DNA complex, 2Gli.
Approximately 90% of sporadic BCCs arise from mutations of one PTCH1 allele, and 10% harbor mutations to downstream protein SMO [38]. Mutations in tumor suppressor p53 (p53) are also observed in BCC [39]. These mutations are consistent with genetic modifications commonly caused by UV exposure that ultimately leads to increased proliferation, maintained stemness, and tumorigenesis [23][38][40][41][42]. Additionally, activation of Hh signaling is often associated with the overexpression of programmed cell death ligand (PD-L1), promoting immunogenic escape and tumor cell proliferation [43][44].
References
- Lai, V.; Cranwell, W.; Sinclair, R. Epidemiology skin cancer in the mature patient. Clin. Dermatol. 2018, 36, 167–176.
- Albert, M.R.; Weinstock, M.A. Keratinocyte Carcinoma. CA Cancer J. Clin. 2003, 53, 292–302.
- Stern, R.S. Prevalence of a history of skin cancer in 2007: Results of an incidence-based model. Arch. Dermatol. 2010, 146, 279–282.
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015, 151, 1081–1086.
- Wong, C.S.M.; Strange, R.C.; Lear, J.T. Basal cell carcinoma. BMJ 2003, 327, 794–798.
- Delishaj, D.; Rembielak, A.; Manfredi, B.; Ursino, S.; Pasqualetti, F.; Laliscia, C.; Orlandi, F.; Morganti, R.; Fabrini, M.G.; Paiar, F. Non-melanoma skin cancer treated with high-dose-rate brachytherapy: A review of literature. J. Contemp. Brachyther. 2016, 8, 533–540.
- Cojocaru, A.; Marinescu, E.-A.; Nica, O.; Ilinoiu, E.; Negrila, A.; Ciurea, M.-E. Basal Cell Carcinoma and its Impact on Different Anatomical Regions. Curr. Health Sci. J. 2021, 47, 75–83.
- Jones, E.A.; Sajid, M.I.; Shenton, A.; Evans, D.G. Basal Cell Carcinomas in Gorlin Syndrome: A Review of 202 Patients. J. Ski. Cancer 2011, 2011, 217378.
- Kiwilsza, M.; Sporniak-Tutak, K. Gorlin-Goltz syndrome—A medical condition requiring a multidisciplinary approach. Med. Sci. Monit. 2012, 18, RA145–RA153.
- Hoban, P.R.; Ramachandran, S.; Strange, R.C. Environment, phenotype and genetics: Risk factors associated with BCC of the skin. Expert Rev. Anticancer Ther. 2002, 2, 570–579.
- Situm, M.; Buljan, M.; Bulat, V.; Lugović Mihić, L.; Bolanca, Z.; Simić, D. The role of UV radiation in the development of basal cell carcinoma. Coll. Antropol. 2008, 32 (Suppl. S2), 167–170.
- Wu, S.; Han, J.; Li, W.-Q.; Li, T.; Qureshi, A.A. Basal-cell carcinoma incidence and associated risk factors in U.S. women and men. Am. J. Epidemiol. 2013, 178, 890–897.
- Berlin, N.L.; Cartmel, B.; Leffell, D.J.; Bale, A.E.; Mayne, S.T.; Ferrucci, L.M. Family history of skin cancer is associated with early-onset basal cell carcinoma independent of MC1R genotype. Cancer Epidemiol. 2015, 39, 1078–1083.
- Puig, S.; Berrocal, A. Management of high-risk and advanced basal cell carcinoma. Clin. Transl. Oncol. 2015, 17, 497–503.
- Chung, S. Basal cell carcinoma. Arch. Plast. Surg. 2012, 39, 166–170.
- Bøgelund, F.S.; Philipsen, P.A.; Gniadecki, R. Factors affecting the recurrence rate of basal cell carcinoma. Acta Derm. Venereol. 2007, 87, 330–334.
- Marcil, I.; Stern, R.S. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: A critical review of the literature and meta-analysis. Arch. Dermatol. 2000, 136, 1524–1530.
- Levi, F.; Randimbison, L.; Maspoli, M.; Te, V.C.; La Vecchia, C. High incidence of second basal cell skin cancers. Int. J. Cancer 2006, 119, 1505–1507.
- Bartos, V. Development of Multiple-Lesion Basal Cell Carcinoma of the Skin: A Comprehensive Review. Sisli Etfal Hast. Tip Bülteni 2019, 53, 323–328.
- Villani, R.; Murigneux, V.; Alexis, J.; Sim, S.-L.; Wagels, M.; Saunders, N.; Soyer, H.P.; Parmentier, L.; Nikolaev, S.; Fink, J.L.; et al. Subtype-Specific Analyses Reveal Infiltrative Basal Cell Carcinomas Are Highly Interactive with their Environment. J. Investig. Dermatol. 2021, 141, 2380–2390.
- Cameron, M.C.; Lee, E.; Hibler, B.P.; Barker, C.A.; Mori, S.; Cordova, M.; Nehal, K.S.; Rossi, A.M. Basal cell carcinoma: Epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J. Am. Acad. Dermatol. 2019, 80, 303–317.
- Armstrong, L.T.D.; Magnusson, M.R.; Guppy, M.P.B. Risk factors for recurrence of facial basal cell carcinoma after surgical excision: A follow-up analysis. J. Plast. Reconstr. Aesthetic Surg. 2017, 70, 1738–1745.
- Fania, L.; Didona, D.; Morese, R.; Campana, I.; Coco, V.; Di Pietro, F.R.; Ricci, F.; Pallotta, S.; Candi, E.; Abeni, D.; et al. Basal Cell Carcinoma: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2020, 8, 449.
- Hendrix, J.D., Jr.; Parlette, H.L. Duplicitous growth of infiltrative basal cell carcinoma: Analysis of clinically undetected tumor extent in a paired case-control study. Dermatol. Surg. 1996, 22, 535–539.
- Wehner, M.R.; Cidre Serrano, W.; Nosrati, A.; Schoen, P.M.; Chren, M.-M.; Boscardin, J.; Linos, E. All-cause mortality in patients with basal and squamous cell carcinoma: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2018, 78, 663–672.e663.
- Kim, D.P.; Kus, K.J.B.; Ruiz, E. Basal Cell Carcinoma Review. Hematol. Oncol. Clin. N. Am. 2019, 33, 13–24.
- Gailani, M.R.; Bale, S.J.; Leffell, D.J.; DiGiovanna, J.J.; Peck, G.L.; Poliak, S.; Drum, M.A.; Pastakia, B.; McBride, O.W.; Kase, R.; et al. Developmental defects in Gorlin syndrome related to a putative tumor suppressor gene on chromosome 9. Cell 1992, 69, 111–117.
- Hahn, H.; Wicking, C.; Zaphiropoulous, P.G.; Gailani, M.R.; Shanley, S.; Chidambaram, A.; Vorechovsky, I.; Holmberg, E.; Unden, A.B.; Gillies, S.; et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell 1996, 85, 841–851.
- Johnson, R.L.; Rothman, A.L.; Xie, J.; Goodrich, L.V.; Bare, J.W.; Bonifas, J.M.; Quinn, A.G.; Myers, R.M.; Cox, D.R.; Epstein, E.H., Jr.; et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science 1996, 272, 1668–1671.
- Klein, R.D.; Dykas, D.J.; Bale, A.E. Clinical testing for the nevoid basal cell carcinoma syndrome in a DNA diagnostic laboratory. Genet. Med. 2005, 7, 611–619.
- Gupta, S.; Takebe, N.; Lorusso, P. Targeting the Hedgehog pathway in cancer. Ther. Adv. Med. Oncol. 2010, 2, 237–250.
- Ming, J.E.; Nanni, L.; Muenke, M.; Meinecke, P.; Pierpont, M.E.M.; Robin, N.H.; Young, I.D.; Roessler, E.; Steinhaus, K.; Bocian, M.; et al. The Mutational Spectrum of the Sonic Hedgehog Gene in Holoprosencephaly: SHH Mutations Cause a Significant Proportion of Autosomal Dominant Holoprosencephaly. Hum. Mol. Genet. 1999, 8, 2479–2488.
- Di Magliano, M.P.; Hebrok, M. Hedgehog signalling in cancer formation and maintenance. Nat. Rev. Cancer 2003, 3, 903–911.
- Fujii, K.; Miyashita, T. Gorlin syndrome (nevoid basal cell carcinoma syndrome): Update and literature review. Pediatrics Int. 2014, 56, 667–674.
- Sahebjam, S.; Siu, L.L.; Razak, A.A. The Utility of Hedgehog Signaling Pathway Inhibition for Cancer. Oncologist 2012, 17, 1090–1099.
- Chahal, K.K.; Parle, M.; Abagyan, R. Hedgehog pathway and smoothened inhibitors in cancer therapies. Anti-Cancer Drugs 2018, 29, 387–401.
- Skoda, A.M.; Simovic, D.; Karin, V.; Kardum, V.; Vranic, S.; Serman, L. The role of the Hedgehog signaling pathway in cancer: A comprehensive review. Bosn. J. Basic Med. Sci. 2018, 18, 8–20.
- Epstein, E.H. Basal cell carcinomas: Attack of the hedgehog. Nat. Rev. Cancer 2008, 8, 743–754.
- Ouhtit, A.; Nakazawa, H.; Armstrong, B.K.; Kricker, A.; Tan, E.; Yamasaki, H.; English, D.R. UV-radiation-specific p53 mutation frequency in normal skin as a predictor of risk of basal cell carcinoma. J. Natl. Cancer Inst. 1998, 90, 523–531.
- Furth, N.; Aylon, Y.; Oren, M. p53 shades of Hippo. Cell Death Differ. 2018, 25, 81–92.
- Mancuso, M.; Pazzaglia, S.; Tanori, M.; Hahn, H.; Merola, P.; Rebessi, S.; Atkinson, M.J.; Di Majo, V.; Covelli, V.; Saran, A. Basal Cell Carcinoma and Its Development: Insights from Radiation-Induced Tumors in Ptch1-Deficient Mice. Cancer Res. 2004, 64, 934–941.
- Mercurio, L.; Albanesi, C.; Madonna, S. Recent Updates on the Involvement of PI3K/AKT/mTOR Molecular Cascade in the Pathogenesis of Hyperproliferative Skin Disorders. Front. Med. 2021, 8, 665647.
- Chakrabarti, J.; Holokai, L.; Syu, L.; Steele, N.G.; Chang, J.; Wang, J.; Ahmed, S.; Dlugosz, A.; Zavros, Y. Hedgehog signaling induces PD-L1 expression and tumor cell proliferation in gastric cancer. Oncotarget 2018, 9, 37439–37457.
- Lipson, E.J.; Lilo, M.T.; Ogurtsova, A.; Esandrio, J.; Xu, H.; Brothers, P.; Schollenberger, M.; Sharfman, W.H.; Taube, J.M. Basal cell carcinoma: PD-L1/PD-1 checkpoint expression and tumor regression after PD-1 blockade. J. ImmunoTherapy Cancer 2017, 5, 23.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
3 times
(View History)
Update Date:
23 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




