Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Meghan Dukes and Version 3 by Vivi Li.
Basal Cell Carcinoma (BCC) is the most commonly diagnosed cancer worldwide. While the survivability of BCC is high, many patients are excluded from clinically available treatments due to health risks or personal choice. Further, patients with advanced or metastatic disease have severely limited treatment options. The dysregulation of the Hedgehog (Hh) signaling cascade drives onset and progression of BCC. As such, the modulation of this pathway has driven advancements in BCC research.
- basal cell carcinoma
- HEDGEHOG signaling
- smoothened inhibitors
- Gli inhibitors
- pre-clinical models
1. Introduction
Keratinocyte cancers, or nonmelanoma skin cancers (NMSCs), are the most commonly diagnosed cancers worldwide [1][2][1,2]. In the United States alone, one in every three to five Caucasian people are expected to develop an NMSC in their lifetime, with estimates as high as 4 million cases diagnosed each year [3][4][5][3,4,5]. Approximately 80% of all NMSCs are characterized as basal cell carcinomas (BCC), where uncontrolled growth of the basal cell population of the epidermis leads to tumorigenesis (Scheme 1) [6][7][6,7]. The overwhelming number of BCC diagnoses requires ample research and medical attention for the development of effective treatment and prevention strategies.
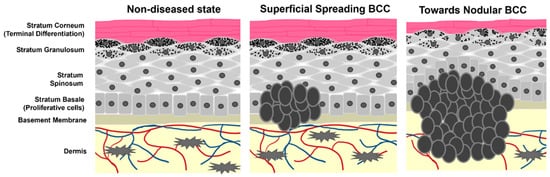
Scheme 1. Cartoon representation of epidermal layers and the growth of BCC. Tumor cells originate from the stratum basale where they maintain stemness and hyperproliferative capacity. As the tumors progress, they begin to spread and migrate into the dermis as well as alter the pathology of non-tumor keratinocyte differentiation.
Gorlin Syndrome (GS) is a rare autosomal dominant disease comprising a small percentage of the BCC community and approximately 0.05% of the population [8]. In total, 90% of patients with GS experience the uncontrolled growth of multiple BCCs alongside various developmental abnormalities including those associated with holoprosencephaly and malignant medulloblastomas [8][9][8,9]. Sporadic BCC accounts for the predominant population of BCC patients. The primary cause of sporadic BCC is prolonged exposure to ultraviolet (UV) radiation from the sun [10][11][10,11]. The risk of developing BCC increases with light-skin pigmentation, age, and sunburn frequency during youth [12]. Other risk factors include family history of melanoma, blonde/red hair phenotype, and men are more susceptible to BCC than women [12][13][12,13].
A significant burden to the BCC patient community is the high rate of recurrence. BCC is clinically designated as low or high risk depending on the likelihood of recurrence [14]. However, BCC more commonly recurs in an entirely different location on the body. For primary tumor locations, the recurrence rate depends heavily on the method of treatment (discussed in depth in Section 2), with a range from 1 to 70% after 5 years [15][16][15,16]. Larger tumors also experience an increased likelihood of relapse [16]. More strikingly, the three-year risk of developing a second BCC lesion is estimated between 41–44% [15][17][18][19][15,17,18,19], and the likelihood increases with each additional lesion. Once diagnosed, approximately 50% of patients will battle BCC again.
BCC is classified in three primary identities: superficial (10–30%), nodular (60–80%), and morpheaform/infiltrative (<10%) [7][20][7,20]. Each differ in physical and histopathological behavior and exhibit differential relapse rates [21]. Superficial and nodular BCCs are less likely to recur, whereas infiltrative BCCs are more challenging to treat permanently [22]. Additionally, the different subtypes have variable occurrence rates on different skin areas. Nodular BCC is most commonly found on the face, while superficial BCC affects the torso and hands more frequently [23]. Infiltrative BCC is the most aggressive form and can often lead to the destruction of nearby healthy tissue [24]. Due to these differences in risk classification and behavior, selective care must be taken when deciding which treatment option to pursue for an individual BCC patient.
BCC’s overall survivability is very high, with estimates for mortality being less than 0.5% [25][26][25,26]. However, the exceptionally high number of BCC diagnoses means that even a low mortality rate produces significant BCC-related cancer deaths [5]. The American Cancer Society estimates this population at around 2000 NMSC-related deaths annually, primarily attributed to metastatic BCC complications.
2. Predominant Treatment Options for BCC
Treatment strategies for BCC vary by subtype of the disease, size of the lesion, and patient age and preference. Table 1 outlines the advantages and disadvantages of each, including any imperative patient restrictions.Table 1.
Select list and commentary of treatment options for BCC.
| Treatment | Advantages | Disadvantages | Patient Restrictions |
|---|---|---|---|
| Wide Local Excision (WLE) | Low recurrence rates upon complete excision Short procedure times |
Excision likely to be incomplete and lead to higher recurrence rate Highly invasive |
Elderly patients where surgery is considered risky When loss of anatomical function is a risk |
| Mohs Micrographic Surgery (MMS) | Promotes excision of poorly defined tumor margins Suggested for more aggressive/high-risk tumors Minimizes harm to non-diseased tissue |
Long treatment times Requires highly trained physicians, and access may be limited to patients in underdeveloped areas |
Elderly patients where surgery is considered risky When loss of anatomical function is a risk |
| Radiation Therapy | Can treat tumors in locations where the loss of anatomical function is a risk Can be used in higher-risk BCC Boosts the efficacy of incomplete surgical resection when used in tandem |
Use of ionizing radiation Increases risk of melanoma Not as effective in larger Tumors Cannot determine the complete clearance of tumor tissue |
Not advised for patients with Gorlin Syndrome Not advised for younger patients due to the long-term impact of ionizing radiation |
| Ablative Laser Therapy |
Locally delivered, less destructive to non-diseased tissue Low recurrence rates Favorable cosmetic outcomes |
Not applicable to larger, deeper tissues May induce increased sensitivity to the sun |
Not recommended for patients with high sunlight sensitivity Not applicable for patients with nodular or infiltrative BCC |
| Photodynamic Therapy (PDT) |
Well characterized mechanisms of cytotoxicity Local administration of non-harmful laser light Safe for patients with Gorlin Syndrome |
High variability of treatment efficacy Multiple treatment sessions Increased sensitivity to Sunlight Severely limited depth penetration |
Not recommended for patients with high sunlight sensitivity Not applicable for patients with nodular or infiltrative BCC |
| Imiquimod Topical Therapy |
Well-characterized induction of immune response Topical, localized application reduces harm to healthy tissue |
Only approved for small superficial BCCs Many patients report skin Irritation Recurrence rates understudied |
Not applicable for patients with nodular or infiltrative BCC |
| 5-Fluorouracil Topical Therapy |
Well-characterized inhibition of DNA synthesis Topical, localized application reduces harm to healthy tissue High cure rate |
Not specific to tumor tissue and may cause harm to non-diseased skin Many patients report skin Irritation Recurrence rates understudied |
Not applicable for patients with nodular or infiltrative BCC |
3. The Hedgehog Signaling Cascade in BCC
In the 1990s, genetic evaluation of BCCs of patients with GS revealed the most crucial discovery in BCC research history: BCC lesions are often linked to mutations in the patched1 (PTCH1) gene loci [27][28][29][30][54,55,56,57]. Since then, it has become commonly accepted that the Hedgehog (Hh) signaling cascade, to which PTCH1 proteins belong, is BCC’s primary oncogenic driver [31][32][33][58,59,60]. The Hh pathway is canonically activated (Figure 12) by the binding of Hh ligands to the transmembrane protein PTCH1, which releases smoothened (SMO) inhibition. Suppressor of fused (SUFU) is signaled to release glioma-associated oncogene (Gli) transcription factors where they are activated in the cytosol. Translocation into the nucleus activates the expression of target genes for cellular processes such as proliferation and migration [34][35][61,62]. Dysregulation of this pathway is associated with many cancers but is causative of BCC [31][32][33][36][37][58,59,60,63,64]. As such, it is a promising chemotherapeutic target for BCC.
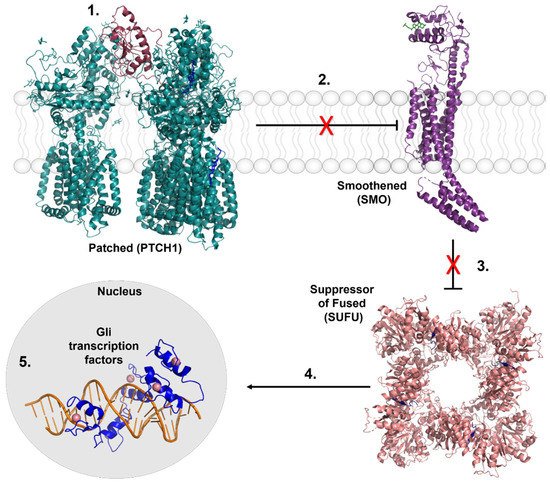

Figure 12. Key regulators of the Hedgehog signaling cascade. 1. Hh signaling is activated by the binding of Hh ligands (Sonic Hedgehog, Indian Hedgehog, and Desert Hedgehog) to the transmembrane protein PTCH1. 2. PTCH1 is a suppressor of SMO activity. Upon the binding of Hh proteins, inhibition of SMO is released, represented by the red X. 3. Upon activation, SMO signals for the SUFU complex to release the Gli family of transcription factors. 4. Gli transcription factors are activated in the cytosol prior to translocating into the nucleus. 5. Gli transcription factors transcribe pro-proliferative and migratory genes that lead to tumorigenesis in BCC. Crystal structures images were made from the following Protein Data Bank files: Ptch1/SHH complex, 6N7H. SMO, 6D35. SUFU/Gli complex: 4BLB. Gli1/DNA complex, 2Gli.
Approximately 90% of sporadic BCCs arise from mutations of one PTCH1 allele, and 10% harbor mutations to downstream protein SMO [38][65]. Mutations in tumor suppressor p53 (p53) are also observed in BCC [39][66]. These mutations are consistent with genetic modifications commonly caused by UV exposure that ultimately leads to increased proliferation, maintained stemness, and tumorigenesis [23][38][40][41][42][23,65,67,68,69]. Additionally, activation of Hh signaling is often associated with the overexpression of programmed cell death ligand (PD-L1), promoting immunogenic escape and tumor cell proliferation [43][44][70,71].
