Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Silvia Urbani | -- | 2499 | 2022-11-15 12:43:14 | | | |
| 2 | Vivi Li | Meta information modification | 2499 | 2022-11-16 02:38:13 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zamboni, M.; Mazzali, G.; Brunelli, A.; Saatchi, T.; Urbani, S.; Giani, A.; Rossi, A.P.; Zoico, E.; Fantin, F. Adipose Cells and Myocytes in Sarcopenic Obesity. Encyclopedia. Available online: https://encyclopedia.pub/entry/34722 (accessed on 07 February 2026).
Zamboni M, Mazzali G, Brunelli A, Saatchi T, Urbani S, Giani A, et al. Adipose Cells and Myocytes in Sarcopenic Obesity. Encyclopedia. Available at: https://encyclopedia.pub/entry/34722. Accessed February 07, 2026.
Zamboni, Mauro, Gloria Mazzali, Anna Brunelli, Tanaz Saatchi, Silvia Urbani, Anna Giani, Andrea P. Rossi, Elena Zoico, Francesco Fantin. "Adipose Cells and Myocytes in Sarcopenic Obesity" Encyclopedia, https://encyclopedia.pub/entry/34722 (accessed February 07, 2026).
Zamboni, M., Mazzali, G., Brunelli, A., Saatchi, T., Urbani, S., Giani, A., Rossi, A.P., Zoico, E., & Fantin, F. (2022, November 15). Adipose Cells and Myocytes in Sarcopenic Obesity. In Encyclopedia. https://encyclopedia.pub/entry/34722
Zamboni, Mauro, et al. "Adipose Cells and Myocytes in Sarcopenic Obesity." Encyclopedia. Web. 15 November, 2022.
Copy Citation
As a result of aging, body composition changes, with a decline in muscle mass and an increase in adipose tissue (AT), which reallocates from subcutaneous to visceral depots and stores ectopically in the liver, heart and muscles. Furthermore, with aging, muscle and AT, both of which have recognized endocrine activity, become dysfunctional and contribute, in the case of positive energy balance, to the development of sarcopenic obesity (SO). SO is defined as the co-existence of excess adiposity and low muscle mass and function, and its prevalence increases with age. SO is strongly associated with greater morbidity and mortality. The pathogenesis of SO is complex and multifactorial.
sarcopenic obesity
adipose tissue
skeletal muscle
myokines
adipokines
adipomyokines
1. Introduction
Normally, bone, muscle and fat mass grow in harmony in the body. However, this linkage may be lost as a result of aging and in several chronic diseases, when a progressive decline of muscle mass in stable weight subjects occurs, configuring a condition called sarcopenia, or when a combination of decline of muscle mass together with an increase in fat mass in overweight or obese subjects occurs, a condition called sarcopenic obesity (SO). SO has been recently defined as the co-existence of excess adiposity and low muscle mass and function. Its prevalence increases with age and has been recognized to be strongly associated with greater morbidity and mortality [1].
Much evidence shows that the consequences of SO are clinically relevant [1][2][3]. SO has consistently been demonstrated to be a strong and independent risk factor for frailty, metabolic disorders, hospitalization and mortality in the older population (Figure 1).
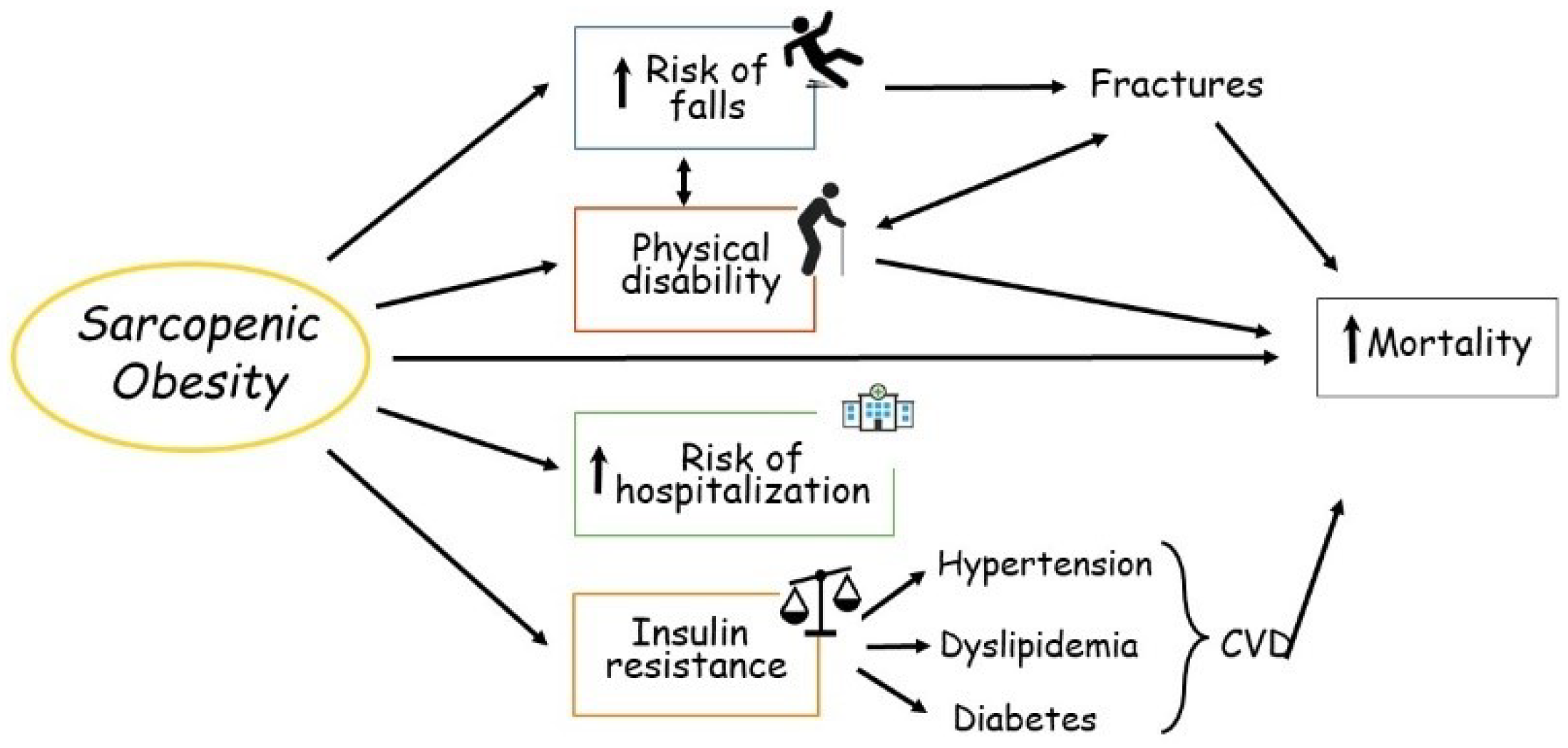
Figure 1. Main consequences of sarcopenic obesity in the elderly. CVD, cardiovascular disease.
Pathogenesis of SO is multi-factorial [3].
Gain in adipose tissue (WAT) and especially dysfunctional WAT may represent an independent determinant for the development of loss and dysfunction of muscle mass. In addition, a decline in muscle mass may facilitate WAT accumulation. SO is more frequently present in older adults, particularly because of the changes observed in body composition (i.e., changes in muscle mass and AT quantity and quality), which in general accompany the aging process.
Age-related changes in sex steroids should be also taken into account in the pathogenesis of SO in the elderly. In fact, in men as in women, age-related decline in sex steroids is strongly related to sarcopenia [4], in term of loss of muscle mass, quality and function, as well as to an increase of WAT and its redistribution (Figure 2) [5].
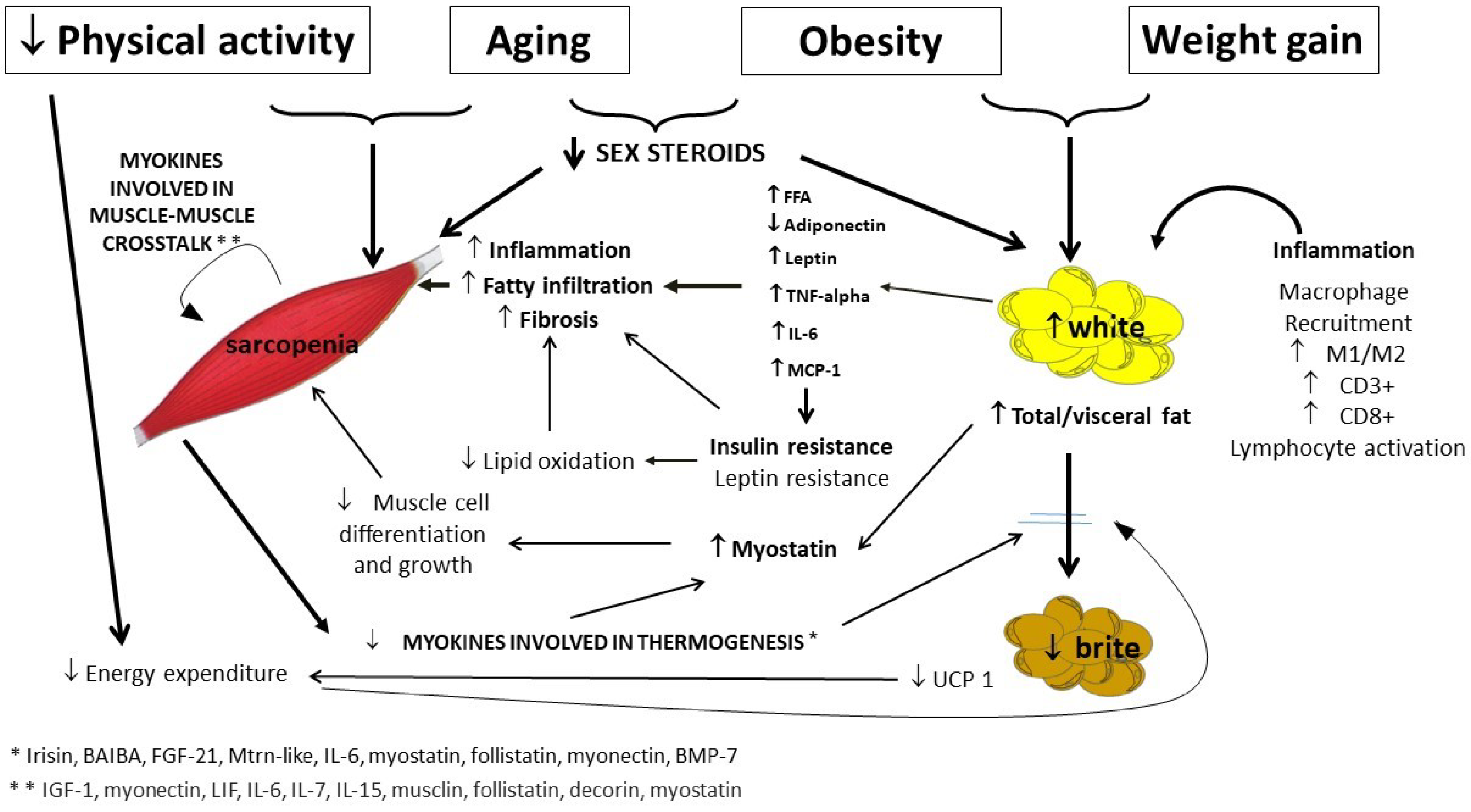
Figure 2. Pathogenesis of sarcopenic obesity in the elderly, with a focus on muscle and adipose tissue crosstalk. More relevant links in bold. FFA, free fatty acids; TNF-alpha, tumor necrosis factor-alpha; IL-6, Interleukin-6; MCP-1, monocyte chemoattractant protein-1; UCP-1, uncoupling protein 1.
Both WAT and muscle have been identified as endocrine organs, which influence each other through several mechanisms [6][7]. Defects in the crosstalk between adipose cells and myocytes may be a cause of SO; an understanding of the interplay between adipose cells and myocytes may be crucial for SO prevention and/or treatment.
2. Adipose Tissue as Endocrine Organ
The concept that WAT, apart from serving as an energy storage and mechanical protection, acts as an endocrine organ, representing a source of countless adipokines, proteins, metabolites, lipid molecules, non-coding RNAs and extracellular vesicles (Evs), as involved in tissue crosstalk, has been widely accepted in recent years [6]. Besides adipocytes, WAT contains stromovascular cells and immune cells acting as an integrated unit, all of which contribute to its endocrine activity [8]. Several adipocyte-derived proteins with endocrine function have been detected since the discovery of leptin many years ago [6].
WAT, distributed in different depots in the body, expresses specific features in relation to cellular composition and functions [6]. Furthermore, the anatomical location is relevant when considering that hormones produced by the visceral adipose tissue (VAT) are released into the portal system and go directly to the liver, while the systemic circulation receives the molecules produced by the subcutaneous adipose tissue (SAT) [8][9].
Moreover, WAT is a dynamic organ that modifies in response to changes in nutritional state, through modifications of its metabolism and cellularity, and subsequent shifts in adipokine secretion. In the lean state, SAT is characterized by smaller cell size and by an increased number of adipocytes, which have a different pattern of adipokine secretion compared with VAT. Weight gain causes adipocyte hypertrophy, with differences across sex, age and fat depot. Weight gain in women, at least until menopause, characterized by the enhanced accumulation of SAT, is associated with a low risk of type 2 diabetes and cardiovascular diseases [10]. In contrast, weight gain in men usually accumulates more VAT, resulting in higher metabolic risk [9].
In subjects with overweight and obesity, changes in WAT depots and cellularity, with a prevalent pro-inflammatory adipokine secretion profile, may directly contribute to the development of metabolic and cardiovascular consequences of obesity [6][9].
Adiponectin and leptin are the two most widely studied adipocyte-derived factors. Most leptin is secreted by SAT [11][12], flows into the bloodstream, passes through the blood brain barrier and arrives in areas of the brain involved in regulating hypothalamic energy balance. The link between neuroendocrine and sympathetic control of WAT endocrine function, and the existence of negative feedback between the brain and WAT, have been widely studied [13]. In the last few decades, several studies have shown the association between leptin and cardiovascular diseases [14].
Adiponectin is an adipokine with described anti-atherogenic, anti-inflammatory and insulin-sensitizing properties [15]. Obesity is associated with reduced adiponectin expression in VAT, and adiponectin levels have been shown to negatively correlate with the amount of VAT [16], suggesting that the obesity-associated decline in adiponectin could contribute to the detrimental effects of excessive VAT accumulation on whole body metabolism [17].
VAT expansion also triggers other proinflammatory cytokine expressions as well as the recruitment of immune cells [18]. Expansion of VAT is accompanied by increased interleukin 6 (IL-6) and tumor necrosis factor (TNF) secretion, which lead to a crown-like structure formation, and augmented hypoxia-inducible factor 1α expression to promote angiogenesis, which contributes to local and systemic inflammation [19][20][21].
Moreover, great attention has recently been focused on WAT contribution in inter-organ communication, not only by producing signaling mediators but also by converting or degrading signaling mediators from other organs (“signal metabolism” and “signal catabolism”) [6]. Increasing and specific interest has been given to Evs (carrying protein, lipids, small coding and noncoding RNAs), which are now considered an eminent way of communication between WAT and other organs, as well as between different cell populations within WAT itself [6].
3. Adipose Tissue Changes across Aging
With aging, quantity, distribution, and function of WAT changes. Fat mass increases and reaches its peak at about 65–75 years for men, and later for women [22]. The increase in fat mass is independent from changes in body weight, and this is due to the concurrent decline in muscle mass: so-called sarcopenia [23]. Fat storage is progressively redistributed from the body’s periphery (i.e., loss of SAT, in particular, gluteo-femoral SAT) to the abdomen (i.e., increase of VAT), and such abdominal fat accumulation is independent of weight gain [24][25]. It has been shown that involuntary SAT loss, in the absence of a negative energy balance, is associated with triglyceride (TG) spillover, which determines ectopic deposition of TG in muscle, liver, bone marrow and heart, contributing to the dysfunction of these organs [24][25].
With aging, WAT also becomes dysfunctional, showing an increased profile of pro-inflammatory adipokines produced by adipose cells, greater infiltration of inflammatory cells in WAT, and preadipocytes and adipocyte incompetence, together leading to inflammaging [26].
In general, serum levels of the majority of adipokines are higher in older than in younger individuals [24]. The relationship between aging and the endocrine function of WAT is complex to study in humans. As aging is associated with changes in fat mass and its distribution, as well as with high prevalence of metabolic syndrome, insulin resistance and obesity, the effect of aging itself is difficult to isolate. Indeed, age-related VAT increase, obesity and metabolic syndrome are all factors that can induce an increase in inflammatory and a decrease in anti-inflammatory adipokine production.
Older subjects show higher leptin levels [27], whose activity seems to be reduced, thereby determining a phenomenon called leptin resistance, a phenomenon that is not completely understood in humans [28].
It has been determined that the amount of serum adiponectin rises as humans age [29], and higher levels of adiponectin have been found in centenarians [30]. Although the beneficial metabolic and anti-inflammatory effects of adiponectin have been confirmed by some scientific studies, adiponectin’s role in the elderly is still controversial [30][31]. Indeed, a significant positive relationship was found between adiponectin and risk of incident disability and all-cause mortality among the subjects of the Health ABC Study [32], but this relationship was not significant after adjusting for weight loss and physical performance at baseline.
In an in vitro model of chronological aging of adipocytes, authors and other researchers observed that adipocyte secretion of proinflammatory cytokines, such as interleukin-6 and monocyte chemoattractant protein-1, was significantly higher in older than younger adipocytes [33][34][35]. Authors also found that in vitro aged adipocytes accumulate ROS, increase mRNA expression of key proteins related to the remodeling of the extracellular matrix and increase p53, p21 and p16 expression, compared to younger cells [34][35].
Moreover, dysfunction of WAT is also characterized by increased oxidative stress (OS), mitochondrial dysfunction, reduction in vascularization and hypoxia [36]. The age-related deregulation of WAT can initiate inflammatory cycles with monocyte recruitment and activation of macrophages. The ratio between pro-inflammatory M1 and anti-inflammatory M2 macrophages increases with aging [37][38], as well accumulation of CD3+ T and CD8+ T cells and activation of T and B lymphocytes [39][40]. Factors secreted by activated macrophages induce the release of fatty acids from adipocytes that inhibit differentiation of pre-adipocytes and cause de-differentiation of mesenchymal progenitors into mesenchymal adipocyte-like default cells [41]. These processes induce lipotoxicity both in WAT and in other organs, cause cellular stress responses, promote release of inflammatory cytokines, block adipogenesis and determine further release of lipotoxic fatty acid [42]. WAT in the elderly is also characterized by reduction of adipocyte size and increase of tissue fibrosis [43].
That adipocyte size is affected by aging was clearly shown by Donato et al., who observed significantly lower adipocyte areas from WAT in older than younger mice [44] and by the fact that a high fat diet was able to increase adipocyte diameter in young rats but not in old rats [45]. Furthermore, age-related decline of adipocyte capacity to stock TG has been shown to be related to fat infiltration inside the muscles, as well as to a deposition in muscles of toxic lipids such as ceramides [45]. Fibrosis, characterized by an increase in connective fiber content, may be due to an up-regulation of collagen protein [46]. The OS that occurs in WAT during aging causes oxidative damage to lipids, proteins and DNA. Finally, senescent cells are over-represented in aged WAT, providing a source of many pro-inflammatory cytokines and chemokines, impairing the production of extracellular matrix modifying proteases and further promoting the production of ROS [47][48].
4. Muscle as Endocrine Organ
Muscle has also been identified as having a secretory/endocrine function [7]. Cytokines and other peptides produced, expressed and released by muscle cells are called myokines. Proteomics analyses identified over 650 proteins and peptides produced by muscle cells, yet their precise biological role has been characterized only in a minority of cases [49]. Myokines act in an autocrine, paracrine or endocrine way. In fact, some myokines exert their effect on muscle itself, taking part in muscle hypertrophy and myogenesis, while other myokines are involved in the regulation of energy metabolism. There is now solid evidence that skeletal muscle, through the production of myokines, communicates with other key organs and regulates lipid mobilization from adipose tissue, liver endogenous glucose production, insulin secretion and thermogenesis [50].
Many myokines are produced in response to the contraction of muscle fibers and may indeed mediate protective effects of physical exercise and counteract the pathological consequences of a sedentary lifestyle.
Several myokines act precisely within skeletal muscle itself and are involved in muscle cell proliferation, differentiation, and regeneration [51][52]; others are involved in mediating energy supply during exercise (Table 1).
It is now believed that every stage of the myogenic process involves regulation by myokines, many of which contribute to myogenic regulation at different stages, from satellite cell proliferation to differentiation and cell survival [49][53].
The role of IGF-1 and IGF-2 as endocrine modulators of myogenesis has been extensively studied [54], as they seem indispensable for the initiation of differentiation [55]; IGF-1 leads to muscle hypertrophy by activating satellite cells and possibly inhibiting autophagy [56]. Impairment of IGF-1 signaling has been described in chronic disorders, and as such, represents a possible mechanism in muscle atrophy led by altered protein synthesis, autophagy, and impaired muscle regeneration [57].
Furthermore, IL-7 plays an essential role in myogenesis and may influence the differentiation of satellite cells into fully developed skeletal muscle cells [58].
On the other hand, TGF-beta has shown to be a strong inhibitor of myogenic differentiation in vitro [59]. Myostatin, the first discovered myokine, is a member of the TGF-beta superfamily and plays a key role in muscle growth and differentiation, by controlling the proliferation of myoblasts (as a major negative regulator of skeletal muscle growth) and suppressing satellite cell activation and myoblast proliferation [60][61]; it is known that the deletion of the myostatin gene causes massive muscle hypertrophy in animals [62][63].
More recently, follistatin and decorin have been identified as potent inducers of muscle hypertrophy with an anti-myostatin function [64]; in particular, decorin acts in an auto/paracrine manner as a direct antagonist of myostatin [65]. Although myokine IL-6 is mainly known for its role in the regulation of lipid and glucose metabolism, it has also been shown that it has an anabolic effect in the processes of myogenesis [66]. Leukemia inhibitory factor (LIF) also exerts an autocrine/paracrine action [67], and it has proven to be crucial for satellite cell proliferation and survival [67].
Apart from the regulatory effects on myogenesis, many myokines act on metabolic pathways in the modulation of energy metabolism. IL-6 [50] and brain-derived neurotrophic factor (BDNF) [68] are involved in activating fat oxidation in muscle cells. Indeed, IL-6 signaling within muscle cells appears to affect both glucose uptake and fat oxidation, and its role in GLUT4 translocation has been described [69]. In addition, several studies described an increase in intramyocellular and whole-body fatty acid oxidation in response to myokine IL-6 [69][70]. BDNF affects myogenesis through activation of satellite cells [71], especially in response to muscle injury; it has also been suggested as a regulator of neuromuscular function during the aging process, with possible implications in sarcopenia and SO [72]. Indeed, low levels of BDNF are described in subjects with obesity and T2D [73].
Table 1. Selected myokine functions.
| Function | Myokine | Aging | References |
|---|---|---|---|
| Myogenesis and muscle hypertrophy | myostatin LIF IL-6 IL-7 IL-15 musclin follistatin decorin myonectin IGF-1 musclin |
⇑ ? ⇑ ⇓ ⇓ ? ⇓ ⇓ ⇓ ⇓ ? |
[61][74] [67] [66][75] [58] [76] [77] [64][78] [79][80] [81] [56] [82] |
| Muscle-cell FFA oxidation | IL-6 BDNF irisin myonectin |
⇑ ⇓ ⇓ ⇓ |
[69][83] [68] [84] [81] |
| Insulin sensitivity | IL-6 IL-15 SPARC LIF BMP-7 mitsugumin 53 |
⇑ ⇓ ⇓ ? ⇓ ? |
[7][69] [85][86] [87] [88] [89] [90] |
| Osteogenesis | IGF-1 decorin IL-6 |
⇓ ⇓ ⇑ |
[91] [92] [93] |
| Browning of WAT | Irisin IL-6 meteorin-like FGF-21 BAIBA follistatin myonectin BMP-7 |
⇓ ⇑ ? ⇓ ⇓ ⇓ ? ⇓ |
[84] [94] [95] [96] [97][98] [99] [100] [101] |
| Lipolysis | IL-6 FGF-21 ANGPTL-4 |
⇑ ⇓ ? |
[7] [102] [103] |
| Muscle innervation | BDNF FGFBP-1 |
⇓ ? |
[104] [105] |
| Muscle angiogenesis | IL-8 VEGF-A |
⇓ ? |
[76] [106][107] |
LIF: leukemia inhibitory factor; IL-6: interleukin-6; IL-7: interleukin-7; IL-15: interleukin-15; BDNF: brain-derived neurotrophic factor; IGF-1: insulin-like growth factor-1; SPARC, secreted protein acidic and rich in cysteine; BMP-7, bone morphogenetic protein-7; FGF-21: fibroblast growth factor 21; BAIBA: β-aminoisobutyric acid; CNTFR-A: ciliary neurotrophic factor receptor-A; ANGPTL-4, angiopoietin-like protein 4; FGFBP-1, fibroblast growth factor binding protein 1; VEGF-A: vascular endothelial growth factor-A.
References
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes. Facts 2022, 15, 321–335.
- Zamboni, M.; Mazzali, G.; Fantin, F.; Rossi, A.; di Francesco, V. Sarcopenic Obesity: A New Category of Obesity in the Elderly. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 388–395.
- Batsis, J.A.; Villareal, D.T. Sarcopenic Obesity in Older Adults: Aetiology, Epidemiology and Treatment Strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537.
- Kim, Y.J.; Tamadon, A.; Park, H.T.; Kim, H.; Ku, S.-Y. The Role of Sex Steroid Hormones in the Pathophysiology and Treatment of Sarcopenia. Osteoporos. Sarcopenia 2016, 2, 140–155.
- Wawrzkiewicz-Jałowiecka, A.; Lalik, A.; Soveral, G. Recent Update on the Molecular Mechanisms of Gonadal Steroids Action in Adipose Tissue. Int. J. Mol. Sci. 2021, 22, 5226.
- Funcke, J.-B.; Scherer, P.E. Beyond Adiponectin and Leptin: Adipose Tissue-Derived Mediators of Inter-Organ Communication. J. Lipid. Res. 2019, 60, 1648–1684.
- Pedersen, B.K.; Febbraio, M.A. Muscle as an Endocrine Organ: Focus on Muscle-Derived Interleukin-6. Physiol. Rev. 2008, 88, 1379–1406.
- Kershaw, E.E.; Flier, J.S. Adipose Tissue as an Endocrine Organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556.
- Bosello, O.; Zamboni, M. Visceral Obesity and Metabolic Syndrome. Obes. Rev. 2000, 1, 47–56.
- Kwon, H.; Kim, D.; Kim, J.S. Body Fat Distribution and the Risk of Incident Metabolic Syndrome: A Longitudinal Cohort Study. Sci. Rep. 2017, 7, 10955.
- Hube, F.; Lietz, U.; Igel, M.; Jensen, P.; Tornqvist, H.; Joost, H.-G.; Hauner, H. Difference in Leptin MRNA Levels Between Omental and Subcutaneous Abdominal Adipose Tissue From Obese Humans. Horm. Metab. Res. 1996, 28, 690–693.
- Lefebvre, A.-M.; Laville, M.; Vega, N.; Riou, J.P.; van Gaal, L.; Auwerx, J.; Vidal, H. Depot-Specific Differences in Adipose Tissue Gene Expression in Lean and Obese Subjects. Diabetes 1998, 47, 98–103.
- Trayhurn, P.; Bing, C. Appetite and Energy Balance Signals from Adipocytes. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1237–1249.
- Zhao, S.; Kusminski, C.M.; Scherer, P.E. Adiponectin, Leptin and Cardiovascular Disorders. Circ. Res. 2021, 128, 136–149.
- Goldstein, B.J.; Scalia, R. Adiponectin: A Novel Adipokine Linking Adipocytes and Vascular Function. J. Clin. Endocrinol. Metab. 2004, 89, 2563–2568.
- Guenther, M.; James, R.; Marks, J.; Zhao, S.; Szabo, A.; Kidambi, S. Adiposity Distribution Influences Circulating Adiponectin Levels. Transl. Res. 2014, 164, 270–277.
- Ryo, M.; Nakamura, T.; Kihara, S.; Kumada, M.; Shibazaki, S.; Takahashi, M.; Nagai, M.; Matsuzawa, Y.; Funahashi, T. Adiponectin as a Biomarker of the Metabolic Syndrome. Circ. J. 2004, 68, 975–981.
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity Is Associated with Macrophage Accumulation in Adipose Tissue. J. Clin. Investig. 2003, 112, 1796–1808.
- Colleluori, G.; Villareal, D.T. Aging, Obesity, Sarcopenia and the Effect of Diet and Exercise Intervention. Exp. Gerontol. 2021, 155, 111561.
- Hotamisligil, G.S. Inflammation, Metaflammation and Immunometabolic Disorders. Nature 2017, 542, 177–185.
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte Death Defines Macrophage Localization and Function in Adipose Tissue of Obese Mice and Humans. J. Lipid. Res. 2005, 46, 2347–2355.
- Zamboni, M.; Mazzali, G.; Zoico, E.; Harris, T.B.; Meigs, J.B.; di Francesco, V.; Fantin, F.; Bissoli, L.; Bosello, O. Health Consequences of Obesity in the Elderly: A Review of Four Unresolved Questions. Int. J. Obes. 2005, 29, 1011–1029.
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31.
- Zamboni, M.; Rossi, A.P.; Fantin, F.; Budui, S.L.; Zoico, E.; Zamboni, G.A.; Mazzali, G. Predictors of Ectopic Fat in Humans. Curr. Obes. Rep. 2014, 3, 404–413.
- Zamboni, M.; Nori, N.; Brunelli, A.; Zoico, E. How Does Adipose Tissue Contribute to Inflammageing? Exp. Gerontol. 2021, 143, 111162.
- Santoro, A.; Bientinesi, E.; Monti, D. Immunosenescence and Inflammaging in the Aging Process: Age-Related Diseases or Longevity? Ageing Res. Rev. 2021, 71, 101422.
- Johnson, K.O.; Shannon, O.M.; Matu, J.; Holliday, A.; Ispoglou, T.; Deighton, K. Differences in Circulating Appetite-Related Hormone Concentrations between Younger and Older Adults: A Systematic Review and Meta-Analysis. Aging Clin. Exp. Res. 2020, 32, 1233–1244.
- Picard, F.; Carter, S.; Caron, A.; Richard, D. Role of Leptin Resistance in the Development of Obesity in Older Patients. Clin. Interv. Aging 2013, 8, 829.
- Kizer, J.R.; Arnold, A.M.; Jenny, N.S.; Cushman, M.; Strotmeyer, E.S.; Ives, D.G.; Ding, J.; Kritchevsky, S.B.; Chaves, P.H.M.; Hirsch, C.H.; et al. Longitudinal Changes in Adiponectin and Inflammatory Markers and Relation to Survival in the Oldest Old: The Cardiovascular Health Study All Stars Study. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 1100–1107.
- Arai, Y.; Kamide, K.; Hirose, N. Adipokines and Aging: Findings From Centenarians and the Very Old. Front. Endocrinol. 2019, 10, 142.
- Mancuso, P.; Bouchard, B. The Impact of Aging on Adipose Function and Adipokine Synthesis. Front. Endocrinol. 2019, 10, 137.
- Baker, J.F.; Newman, A.B.; Kanaya, A.; Leonard, M.B.; Zemel, B.; Miljkovic, I.; Long, J.; Weber, D.; Harris, T.B. The Adiponectin Paradox in the Elderly: Associations With Body Composition, Physical Functioning, and Mortality. J. Gerontol. Ser. A 2019, 74, 247–253.
- Yu, Y.-H.; Zhu, H. Chronological Changes in Metabolism and Functions of Cultured Adipocytes: A Hypothesis for Cell Aging in Mature Adipocytes. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E402–E410.
- Zoico, E.; Rizzatti, V.; Policastro, G.; Tebon, M.; Darra, E.; Rossi, A.P.; Mazzali, G.; Fantin, F.; Zamboni, M. In Vitro Model of Chronological Aging of Adipocytes: Interrelationships with Hypoxia and Oxidation. Exp. Gerontol. 2019, 121, 81–90.
- Zoico, E.; di Francesco, V.; Olioso, D.; Fratta Pasini, A.M.; Sepe, A.; Bosello, O.; Cinti, S.; Cominacini, L.; Zamboni, M. In Vitro Aging of 3T3-L1 Mouse Adipocytes Leads to Altered Metabolism and Response to Inflammation. Biogerontology 2010, 11, 111–122.
- Palmer, A.K.; Kirkland, J.L. Aging and Adipose Tissue: Potential Interventions for Diabetes and Regenerative Medicine. Exp. Gerontol. 2016, 86, 97–105.
- Lumeng, C.N.; Saltiel, A.R. Inflammatory Links between Obesity and Metabolic Disease. J. Clin. Investig. 2011, 121, 2111–2117.
- Garg, S.K.; Delaney, C.; Shi, H.; Yung, R. Changes in Adipose Tissue Macrophages and T Cells during Aging. Crit. Rev. Immunol. 2014, 34, 1–14.
- Nakagami, H. Cellular Senescence and Senescence-Associated T Cells as a Potential Therapeutic Target. Geriatr. Gerontol. Int. 2020, 20, 97–100.
- Kalathookunnel Antony, A.; Lian, Z.; Wu, H. T Cells in Adipose Tissue in Aging. Front. Immunol. 2018, 9, 2945.
- Kirkland, J.L.; Tchkonia, T.; Pirtskhalava, T.; Han, J.; Karagiannides, I. Adipogenesis and Aging: Does Aging Make Fat Go MAD? Exp. Gerontol. 2002, 37, 757–767.
- Carobbio, S.; Pellegrinelli, V.; Vidal-Puig, A. Adipose Tissue Function and Expandability as Determinants of Lipotoxicity and the Metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 960, 161–196.
- Sepe, A.; Tchkonia, T.; Thomou, T.; Zamboni, M.; Kirkland, J.L. Aging and Regional Differences in Fat Cell Progenitors—A Mini-Review. Gerontology 2011, 57, 66–75.
- Donato, A.J.; Henson, G.D.; Hart, C.R.; Layec, G.; Trinity, J.D.; Bramwell, R.C.; Enz, R.A.; Morgan, R.G.; Reihl, K.D.; Hazra, S.; et al. The Impact of Ageing on Adipose Structure, Function and Vasculature in the B6D2F1 Mouse: Evidence of Significant Multisystem Dysfunction. J. Physiol. 2014, 592, 4083–4096.
- Tardif, N.; Salles, J.; Guillet, C.; Tordjman, J.; Reggio, S.; Landrier, J.-F.; Giraudet, C.; Patrac, V.; Bertrand-Michel, J.; Migne, C.; et al. Muscle Ectopic Fat Deposition Contributes to Anabolic Resistance in Obese Sarcopenic Old Rats through EIF2α Activation. Aging Cell 2014, 13, 1001–1011.
- Khan, T.; Muise, E.S.; Iyengar, P.; Wang, Z.V.; Chandalia, M.; Abate, N.; Zhang, B.B.; Bonaldo, P.; Chua, S.; Scherer, P.E. Metabolic Dysregulation and Adipose Tissue Fibrosis: Role of Collagen VI. Mol. Cell. Biol. 2009, 29, 1575–1591.
- Tchkonia, T.; Lenburg, M.; Thomou, T.; Giorgadze, N.; Frampton, G.; Pirtskhalava, T.; Cartwright, A.; Cartwright, M.; Flanagan, J.; Karagiannides, I.; et al. Identification of Depot-Specific Human Fat Cell Progenitors through Distinct Expression Profiles and Developmental Gene Patterns. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E298–E307.
- Tchkonia, T.; Kirkland, J.L. Aging, Cell Senescence, and Chronic Disease: Emerging Therapeutic Strategies. JAMA 2018, 320, 1319–1320.
- Severinsen, M.C.K.; Pedersen, B.K. Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609.
- Pedersen, B.K.; Febbraio, M.A. Muscles, Exercise and Obesity: Skeletal Muscle as a Secretory Organ. Nat. Rev. Endocrinol. 2012, 8, 457–465.
- Henningsen, J.; Rigbolt, K.T.G.; Blagoev, B.; Pedersen, B.K.; Kratchmarova, I. Dynamics of the Skeletal Muscle Secretome during Myoblast Differentiation. Mol. Cell. Proteom. 2010, 9, 2482–2496.
- Henningsen, J.; Pedersen, B.K.; Kratchmarova, I. Quantitative Analysis of the Secretion of the MCP Family of Chemokines by Muscle Cells. Mol. Biosyst. 2011, 7, 311–321.
- Waldemer-Streyer, R.J.; Kim, D.; Chen, J. Muscle Cell-Derived Cytokines in Skeletal Muscle Regeneration. FEBS J. 2022, 24, 16372.
- Florini, J.R.; Ewton, D.Z.; Coolican, S.A. Growth Hormone and the Insulin-Like Growth Factor System in Myogenesis*. Endocr. Rev. 1996, 17, 481–517.
- Florini, J.R.; Magri, K.A.; Ewton, D.Z.; James, P.L.; Grindstaff, K.; Rotwein, P.S. “Spontaneous” Differentiation of Skeletal Myoblasts Is Dependent upon Autocrine Secretion of Insulin-like Growth Factor-II. J. Biol. Chem. 1991, 266, 15917–15923.
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970.
- Ahmad, S.S.; Ahmad, K.; Lee, E.J.; Lee, Y.-H.; Choi, I. Implications of Insulin-Like Growth Factor-1 in Skeletal Muscle and Various Diseases. Cells 2020, 9, 1773.
- Haugen, F.; Norheim, F.; Lian, H.; Wensaas, A.J.; Dueland, S.; Berg, O.; Funderud, A.; Skålhegg, B.S.; Raastad, T.; Drevon, C.A. IL-7 Is Expressed and Secreted by Human Skeletal Muscle Cells. Am. J. Physiol. Cell Physiol. 2010, 298, C807–C816.
- Olson, E.N.; Sternberg, E.; Hu, J.S.; Spizz, G.; Wilcox, C. Regulation of Myogenic Differentiation by Type Beta Transforming Growth Factor. J. Cell. Biol. 1986, 103, 1799–1805.
- Joulia, D.; Bernardi, H.; Garandel, V.; Rabenoelina, F.; Vernus, B.; Cabello, G. Mechanisms Involved in the Inhibition of Myoblast Proliferation and Differentiation by Myostatin. Exp. Cell. Res. 2003, 286, 263–275.
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of Skeletal Muscle Mass in Mice by a New TGF-Beta Superfamily Member. Nature 1997, 387, 83–90.
- Grobet, L.; Martin, L.J.; Poncelet, D.; Pirottin, D.; Brouwers, B.; Riquet, J.; Schoeberlein, A.; Dunner, S.; Ménissier, F.; Massabanda, J.; et al. A Deletion in the Bovine Myostatin Gene Causes the Double-Muscled Phenotype in Cattle. Nat. Genet. 1997, 17, 71–74.
- Mosher, D.S.; Quignon, P.; Bustamante, C.D.; Sutter, N.B.; Mellersh, C.S.; Parker, H.G.; Ostrander, E.A. A Mutation in the Myostatin Gene Increases Muscle Mass and Enhances Racing Performance in Heterozygote Dogs. PLoS Genet. 2007, 3, e79.
- Gilson, H.; Schakman, O.; Kalista, S.; Lause, P.; Tsuchida, K.; Thissen, J.-P. Follistatin Induces Muscle Hypertrophy through Satellite Cell Proliferation and Inhibition of Both Myostatin and Activin. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E157–E164.
- el Shafey, N.; Guesnon, M.; Simon, F.; Deprez, E.; Cosette, J.; Stockholm, D.; Scherman, D.; Bigey, P.; Kichler, A. Inhibition of the Myostatin/Smad Signaling Pathway by Short Decorin-Derived Peptides. Exp. Cell. Res. 2016, 341, 187–195.
- Serrano, A.L.; Baeza-Raja, B.; Perdiguero, E.; Jardí, M.; Muñoz-Cánoves, P. Interleukin-6 Is an Essential Regulator of Satellite Cell-Mediated Skeletal Muscle Hypertrophy. Cell Metab. 2008, 7, 33–44.
- Broholm, C.; Pedersen, B.K. Leukaemia Inhibitory Factor--an Exercise-Induced Myokine. Exerc. Immunol. Rev. 2010, 16, 77–85.
- Matthews, V.B.; Aström, M.-B.; Chan, M.H.S.; Bruce, C.R.; Krabbe, K.S.; Prelovsek, O.; Akerström, T.; Yfanti, C.; Broholm, C.; Mortensen, O.H.; et al. Brain-Derived Neurotrophic Factor Is Produced by Skeletal Muscle Cells in Response to Contraction and Enhances Fat Oxidation via Activation of AMP-Activated Protein Kinase. Diabetologia 2009, 52, 1409–1418.
- Carey, A.L.; Steinberg, G.R.; Macaulay, S.L.; Thomas, W.G.; Holmes, A.G.; Ramm, G.; Prelovsek, O.; Hohnen-Behrens, C.; Watt, M.J.; James, D.E.; et al. Interleukin-6 Increases Insulin-Stimulated Glucose Disposal in Humans and Glucose Uptake and Fatty Acid Oxidation In Vitro via AMP-Activated Protein Kinase. Diabetes 2006, 55, 2688–2697.
- Petersen, E.W.; Carey, A.L.; Sacchetti, M.; Steinberg, G.R.; Macaulay, S.L.; Febbraio, M.A.; Pedersen, B.K. Acute IL-6 Treatment Increases Fatty Acid Turnover in Elderly Humans in Vivo and in Tissue Culture in Vitro. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E155–E162.
- Colombo, E.; Bedogni, F.; Lorenzetti, I.; Landsberger, N.; Previtali, S.C.; Farina, C. Autocrine and Immune Cell-Derived BDNF in Human Skeletal Muscle: Implications for Myogenesis and Tissue Regeneration. J. Pathol. 2013, 231, 190–198.
- Guo, A.; Li, K.; Xiao, Q. Sarcopenic Obesity: Myokines as Potential Diagnostic Biomarkers and Therapeutic Targets? Exp. Gerontol. 2020, 139, 111022.
- Li, B.; Lang, N.; Cheng, Z.-F. Serum Levels of Brain-Derived Neurotrophic Factor Are Associated with Diabetes Risk, Complications, and Obesity: A Cohort Study from Chinese Patients with Type 2 Diabetes. Mol. Neurobiol. 2016, 53, 5492–5499.
- Allen, D.L.; Hittel, D.S.; McPherron, A.C. Expression and Function of Myostatin in Obesity, Diabetes, and Exercise Adaptation. Med. Sci. Sports Exerc. 2011, 43, 1828–1835.
- Gao, S.; Durstine, J.L.; Koh, H.-J.; Carver, W.E.; Frizzell, N.; Carson, J.A. Acute Myotube Protein Synthesis Regulation by IL-6-Related Cytokines. Am. J. Physiol. Cell Physiol. 2017, 313, C487–C500.
- Nielsen, A.R.; Pedersen, B.K. The Biological Roles of Exercise-Induced Cytokines: IL-6, IL-8, and IL-15. Appl. Physiol. Nutr. Metab. 2007, 32, 833–839.
- Re Cecconi, A.D.; Forti, M.; Chiappa, M.; Zhu, Z.; Zingman, L.V.; Cervo, L.; Beltrame, L.; Marchini, S.; Piccirillo, R. Musclin, A Myokine Induced by Aerobic Exercise, Retards Muscle Atrophy During Cancer Cachexia in Mice. Cancers 2019, 11, 1541.
- Kota, J.; Handy, C.R.; Haidet, A.M.; Montgomery, C.L.; Eagle, A.; Rodino-Klapac, L.R.; Tucker, D.; Shilling, C.J.; Therlfall, W.R.; Walker, C.M.; et al. Follistatin Gene Delivery Enhances Muscle Growth and Strength in Nonhuman Primates. Sci. Transl. Med. 2009, 1, 6ra15.
- Miura, T.; Kishioka, Y.; Wakamatsu, J.; Hattori, A.; Hennebry, A.; Berry, C.J.; Sharma, M.; Kambadur, R.; Nishimura, T. Decorin Binds Myostatin and Modulates Its Activity to Muscle Cells. Biochem. Biophys. Res. Commun. 2006, 340, 675–680.
- Kanzleiter, T.; Rath, M.; Görgens, S.W.; Jensen, J.; Tangen, D.S.; Kolnes, A.J.; Kolnes, K.J.; Lee, S.; Eckel, J.; Schürmann, A.; et al. The Myokine Decorin Is Regulated by Contraction and Involved in Muscle Hypertrophy. Biochem. Biophys. Res. Commun. 2014, 450, 1089–1094.
- Seldin, M.M.; Peterson, J.M.; Byerly, M.S.; Wei, Z.; Wong, G.W. Myonectin (CTRP15), a Novel Myokine That Links Skeletal Muscle to Systemic Lipid Homeostasis. J. Biol. Chem. 2012, 287, 11968–11980.
- Subbotina, E.; Sierra, A.; Zhu, Z.; Gao, Z.; Koganti, S.R.K.; Reyes, S.; Stepniak, E.; Walsh, S.A.; Acevedo, M.R.; Perez-Terzic, C.M.; et al. Musclin Is an Activity-Stimulated Myokine That Enhances Physical Endurance. Proc. Natl. Acad. Sci. USA 2015, 112, 16042–16047.
- Bruce, C.R.; Dyck, D.J. Cytokine Regulation of Skeletal Muscle Fatty Acid Metabolism: Effect of Interleukin-6 and Tumor Necrosis Factor-α. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E616–E621.
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468.
- Quinn, L.; Straitbodey, L.; Anderson, B.; Argiles, J.; Havel, P. Interleukin-15 Stimulates Adiponectin Secretion by 3T3-L1 Adipocytes: Evidence for a Skeletal Muscle-to-Fat Signaling Pathway. Cell. Biol. Int. 2005, 29, 449–457.
- Krolopp, J.E.; Thornton, S.M.; Abbott, M.J. IL-15 Activates the Jak3/STAT3 Signaling Pathway to Mediate Glucose Uptake in Skeletal Muscle Cells. Front. Physiol. 2016, 7, 626.
- Aoi, W.; Hirano, N.; Lassiter, D.G.; Björnholm, M.; Chibalin, A.V.; Sakuma, K.; Tanimura, Y.; Mizushima, K.; Takagi, T.; Naito, Y.; et al. Secreted Protein Acidic and Rich in Cysteine (SPARC) Improves Glucose Tolerance via AMP-activated Protein Kinase Activation. FASEB J. 2019, 33, 10551–10562.
- Brandt, N.; O’Neill, H.M.; Kleinert, M.; Schjerling, P.; Vernet, E.; Steinberg, G.R.; Richter, E.A.; Jørgensen, S.B. Leukemia Inhibitory Factor Increases Glucose Uptake in Mouse Skeletal Muscle. Am. J. Physiol. -Endocrinol. Metab. 2015, 309, E142–E153.
- Chattopadhyay, T.; Singh, R.R.; Gupta, S.; Surolia, A. Bone Morphogenetic Protein-7 (BMP-7) Augments Insulin Sensitivity in Mice with Type II Diabetes Mellitus by Potentiating PI3K/AKT Pathway. BioFactors 2017, 43, 195–209.
- Wu, H.-K.; Zhang, Y.; Cao, C.-M.; Hu, X.; Fang, M.; Yao, Y.; Jin, L.; Chen, G.; Jiang, P.; Zhang, S.; et al. Glucose-Sensitive Myokine/Cardiokine MG53 Regulates Systemic Insulin Response and Metabolic Homeostasis. Circulation 2019, 139, 901–914.
- Perrini, S.; Laviola, L.; Carreira, M.C.; Cignarelli, A.; Natalicchio, A.; Giorgino, F. The GH/IGF1 Axis and Signaling Pathways in the Muscle and Bone: Mechanisms Underlying Age-Related Skeletal Muscle Wasting and Osteoporosis. J. Endocrinol. 2010, 205, 201–210.
- Kaji, H. Effects of Myokines on Bone. Bonekey Rep. 2016, 5, 826.
- Axmann, R.; Böhm, C.; Krönke, G.; Zwerina, J.; Smolen, J.; Schett, G. Inhibition of Interleukin-6 Receptor Directly Blocks Osteoclast Formation In Vitro and In Vivo. Arthritis Rheum. 2009, 60, 2747–2756.
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a Novel Metabolic Regulator. J. Clin. Investig. 2005, 115, 1627–1635.
- Rao, R.R.; Long, J.Z.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.P.; Ruas, J.L.; Wrann, C.D.; Lo, J.C.; et al. Meteorin-like Is a Hormone That Regulates Immune-Adipose Interactions to Increase Beige Fat Thermogenesis. Cell 2014, 157, 1279–1291.
- Véniant, M.M.; Sivits, G.; Helmering, J.; Komorowski, R.; Lee, J.; Fan, W.; Moyer, C.; Lloyd, D.J. Pharmacologic Effects of FGF21 Are Independent of the “Browning” of White Adipose Tissue. Cell Metab. 2015, 21, 731–738.
- Roberts, L.D.; Boström, P.; O’Sullivan, J.F.; Schinzel, R.T.; Lewis, G.D.; Dejam, A.; Lee, Y.-K.; Palma, M.J.; Calhoun, S.; Georgiadi, A.; et al. β-Aminoisobutyric Acid Induces Browning of White Fat and Hepatic β-Oxidation and Is Inversely Correlated with Cardiometabolic Risk Factors. Cell Metab. 2014, 19, 96–108.
- Tanianskii, D.A.; Jarzebska, N.; Birkenfeld, A.L.; O’Sullivan, J.F.; Rodionov, R.N. Beta-Aminoisobutyric Acid as a Novel Regulator of Carbohydrate and Lipid Metabolism. Nutrients 2019, 11, 524.
- Singh, R.; Braga, M.; Pervin, S. Regulation of Brown Adipocyte Metabolism by Myostatin/Follistatin Signaling. Front. Cell Dev. Biol. 2014, 2, 60.
- Peterson, J.M.; Mart, R.; Bond, C.E. Effect of Obesity and Exercise on the Expression of the Novel Myokines, Myonectin and Fibronectin Type III Domain Containing 5. PeerJ 2014, 2, e605.
- Tseng, Y.-H.; Kokkotou, E.; Schulz, T.J.; Huang, T.L.; Winnay, J.N.; Taniguchi, C.M.; Tran, T.T.; Suzuki, R.; Espinoza, D.O.; Yamamoto, Y.; et al. New Role of Bone Morphogenetic Protein 7 in Brown Adipogenesis and Energy Expenditure. Nature 2008, 454, 1000–1004.
- Cuevas-Ramos, D.; Aguilar-Salinas, C.A. Modulation of Energy Balance by Fibroblast Growth Factor 21. Horm. Mol. Biol. Clin. Investig 2016, 30.
- Staiger, H.; Haas, C.; Machann, J.; Werner, R.; Weisser, M.; Schick, F.; Machicao, F.; Stefan, N.; Fritsche, A.; Häring, H.-U. Muscle-Derived Angiopoietin-Like Protein 4 Is Induced by Fatty Acids via Peroxisome Proliferator–Activated Receptor (PPAR)-δ and Is of Metabolic Relevance in Humans. Diabetes 2009, 58, 579–589.
- Sakuma, K.; Yamaguchi, A. The Recent Understanding of the Neurotrophin’s Role in Skeletal Muscle Adaptation. J. Biomed. Biotechnol. 2011, 2011, 201696.
- Taetzsch, T.; Tenga, M.J.; Valdez, G. Muscle Fibers Secrete FGFBP1 to Slow Degeneration of Neuromuscular Synapses during Aging and Progression of ALS. J. Neurosci. 2017, 37, 70–82.
- Hoier, B.; Hellsten, Y. Exercise-Induced Capillary Growth in Human Skeletal Muscle and the Dynamics of VEGF. Microcirculation 2014, 21, 301–314.
- Jensen, L.; Schjerling, P.; Hellsten, Y. Regulation of VEGF and BFGF MRNA Expression and Other Proliferative Compounds in Skeletal Muscle Cells. Angiogenesis 2004, 7, 255–267.
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
16 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




