Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | F. David Rodriguez | -- | 4364 | 2022-11-15 10:57:21 | | | |
| 2 | Vivi Li | -25 word(s) | 4339 | 2022-11-16 02:24:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Coveñas, R.; Rodríguez, F.D.; Muñoz, M. The Neurokinin-1 Receptor. Encyclopedia. Available online: https://encyclopedia.pub/entry/34711 (accessed on 08 February 2026).
Coveñas R, Rodríguez FD, Muñoz M. The Neurokinin-1 Receptor. Encyclopedia. Available at: https://encyclopedia.pub/entry/34711. Accessed February 08, 2026.
Coveñas, Rafael, Francisco D. Rodríguez, Miguel Muñoz. "The Neurokinin-1 Receptor" Encyclopedia, https://encyclopedia.pub/entry/34711 (accessed February 08, 2026).
Coveñas, R., Rodríguez, F.D., & Muñoz, M. (2022, November 15). The Neurokinin-1 Receptor. In Encyclopedia. https://encyclopedia.pub/entry/34711
Coveñas, Rafael, et al. "The Neurokinin-1 Receptor." Encyclopedia. Web. 15 November, 2022.
Copy Citation
Substance P (SP), through the neurokinin-1 receptor (NK-1R), behaves as a universal mitogen in cancer cells. The NK-1R is overexpressed in tumor cells and, in addition, affects the viability of cancer cells. NK-1R antagonists counteract all the previous actions mediated by SP through NK-1R. In a concentration-dependent manner, these antagonists promote tumor cell death by apoptosis. Therefore, NK-1R is a potential and promising therapeutic target for cancer treatment by using NK-1R antagonists (e.g., aprepitant) alone or in combination therapy with chemotherapy or radiotherapy.
NK-1 receptor
substance P
cancer
aprepitant
NK-1 receptor antagonists
1. Introduction
The exponential increase in cancer research has, unfortunately, not been translated into better perspectives for cancer patients despite developing promising research fields focused on stem cells, new cytostatic compounds, and the human genome. Novel anticancer strategies for translational research must be explored and developed to fight this global chronic problem and to improve the diagnosis and treatment of tumors. Current cancer treatments are surgery, radiotherapy, and chemotherapy; however, the results are unsatisfactory in many cases. Unfortunately, cancer is still a major health problem worldwide (causing nearly 10 million deaths in 2020). Despite its severe side effects on essential organs, chemotherapy is currently the most used treatment against the disease. However, drug resistance appears in many cases, causing the failure of this antitumor strategy and leading to cancer cell invasion and metastasis [1]. Drug resistance often occurs after the fourth cycle of chemotherapy [2]. Hence, chemotherapy is not always an effective antitumor strategy, and the contribution of curative/adjuvant chemotherapy for 5-year survival in adult malignancies is approximately 2%. In addition, many patients with cancer suffer severe chemotherapy-associated side effects because the drugs used in chemotherapy do not specifically target tumor cells [3]. Consequently, chemotherapy affords a minor contribution to the survival of cancer patients, and although cytostatics are currently used in clinical practice, they are not future drugs. This means that new specific antitumor targets as well as strategies showing enhanced antitumor action and decreased toxicity must be urgently developed.
Tumor cells and the tissues they form suffer proliferation, survival, invasion, and metastasis (over 90% of cancer deaths are due to metastases and not to a primary tumor); these mechanisms are regulated by ligands (e.g., peptides) and their receptors, and, hence, one of the main goals in cancer research is to inhibit the cellular events mediated by these ligands [4]. Accordingly, it is a promising line of research to investigate in depth the roles played by peptides and their receptors in cancer progression. Many peptides, including neurotensin, galanin, angiotensin II, endothelin-1, neuropeptide Y, cholecystokinin, gastrin, somatostatin, and calcitonin gene-related peptide, regulate cancer development [5][6][7][8]. It is widely known that the undecapeptide substance P (SP), through the neurokinin-1 receptor (NK-1R), is involved in chemotherapy-induced side effects (e.g., hepatotoxicity, neurotoxicity, nephrotoxicity, cardiotoxicity) and cancer development [1][9]. Moreover, the SP/NK-1R system has been related to poor prognosis, and this and other findings have opened up new research lines and antitumor therapeutic strategies [10][11][12][13][14]. Much published data has confirmed that NK-1R is a promising antitumor target (e.g., NK-1R is involved in the viability of tumor cells) [15][16]. Due to the vital role that the SP/NK-1R system plays in cancer, researchers update the involvement of NK-1R in cancer progression and the potential antitumor strategies targeting NK-1R.
2. Neurokinin-1 Receptor and Cancer
2.1. Neurokinin-1 Receptor: General Findings
NK-1R, also known as SP receptor or tachykinin 1 receptor, belongs to the rhodopsin-like G protein-coupled receptors family (seven-transmembrane domain receptors, serpentine receptors, or 7TM receptors). It is widely distributed by the whole body (e.g., nervous system, immune and endothelial cells, lung, gastrointestinal tract, skin), and it is highly conserved along the species [17][18]. The tachykinin receptor 1 (TACR1) gene with cytogenetic location 2p12 encodes the human NK-1R that expands through the plasma membrane, forming seven transmembrane domains and several intracellular and extracellular loops. The receptor’s function may be altered by anomalous expression, heterodimerization with other receptors, point mutations, or derangement of post-translational modifications. Specific amino acid positions may suffer post-translational changes, including glycosylation, formation of a disulfide bridge, and lipidation modification (Figure 1). Hundreds of single nucleotide variants (SNVs) have been described [19], but their pathological significance has not been analyzed thoroughly. They ask for further analysis related to specific pathologies, including cancer. Different classes of G protein-coupled receptors may assemble in operative homodimers, heterodimers, and oligomers [20]. These macromolecular structures amplify and diversify signaling responses and, in some cases, may serve as specific therapeutic targets for several pathological entities [20][21]. No information on the physiological formation of NK-1R dimers or oligomers has been provided. NK-1R functions through functional monomers residing in membrane microdomains where cholesterol is essential [22]. However, the capacity to heterodimerize appears possible. Artificially built heterodimers of NK-1R and β2-adrenergic receptors were functional and internalized as a complex [23]. Therefore, the formation of complexes with the intervention of NK-1R may have relevance for the homeostasis of NK-1R signaling in abnormal or adaptive circumstances and requires further exploration.
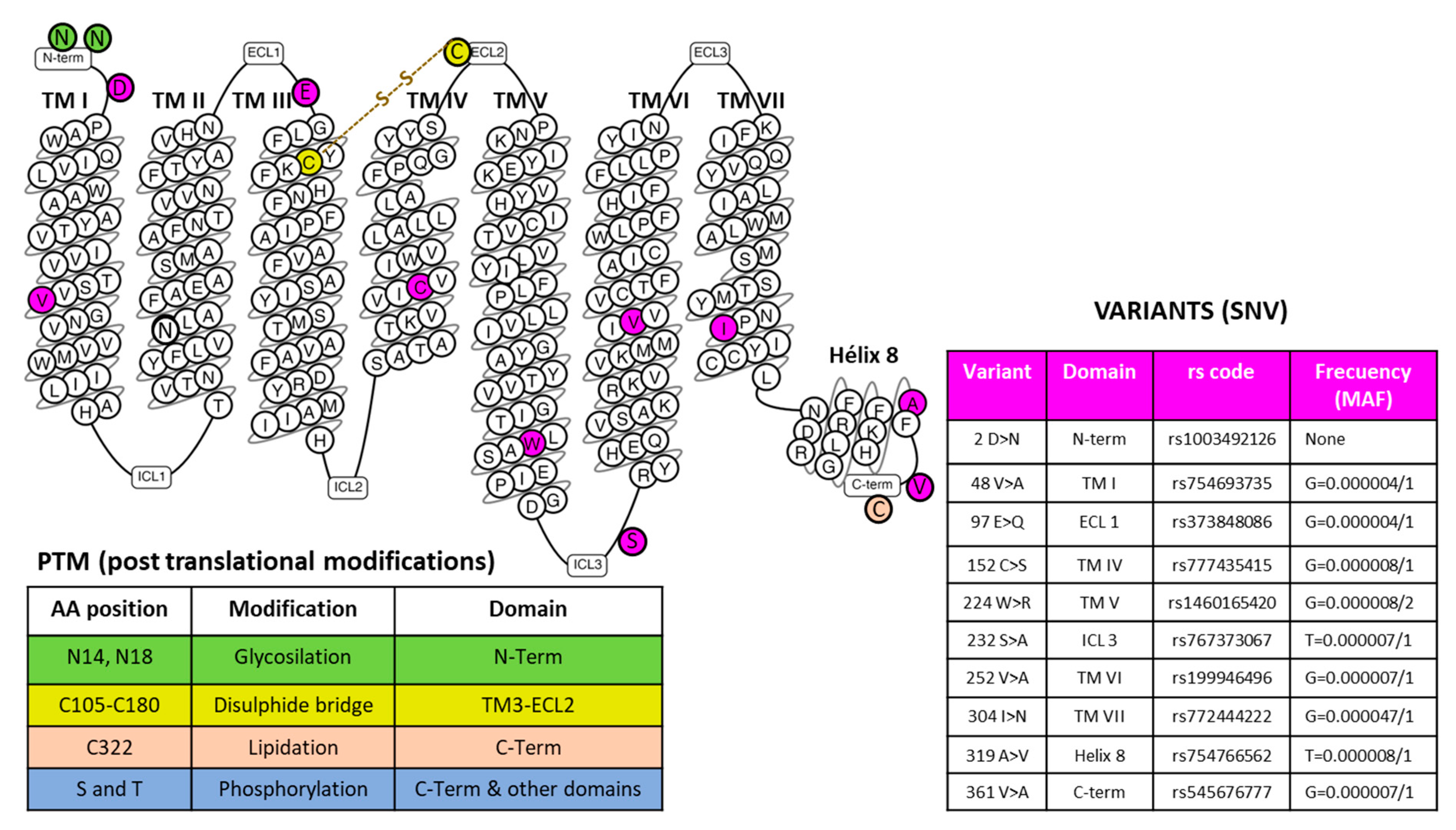
Figure 1. The snake plot of the human NK-1R (modified from [24]) highlights amino acid positions involved in post-translational modifications (PTM) and representative SNVs (Single Nucleotide Variations) in different receptor domains, as indicated. The table on the right-hand side of the figure showing the example variants depicts the amino acid position and change, the domain localization, the registered rs code, and the frequency acquired from the Single Nucleotide Polymorphism Database (dSNP) [19]. Abbreviations: ECL, extracellular loops; ICL, intracellular loops; MAF, minor allele frequency; TM, a transmembrane domain.
Seven-transmembrane helix receptors show a carboxy-terminal cytoplasmic domain (involved in the desensitization of the receptor), an amino-terminal extracellular domain (involved in the specificity of the receptor), and three intracellular and extracellular loops flanked by seven intermembrane domains [25]. The extracellular N-terminus and the intracellular C-terminus contain asparagine glycosylation and serine/threonine phosphorylation sites, respectively, which regulate NK-1R signaling [25]. NK-1R shows different active conformations, each of which has a different affinity for distinct agonists or antagonists: SP binds to the extracellular loops, and non-peptide NK-1R antagonists bind deep between the III and VI transmembrane segments of NK-1R [25]. Specific residues of this receptor (e.g., Gln165, His197, and 265) regulate the binding of non-peptide NK-1R antagonists [25]. NK-1R can be coupled to Gαq, Gαs, Gαi, Gαo, and Gα12/13 proteins, the activation of a determined G protein being regulated by the type of ligands and NK-1R conformation [25][26][27][28][29]. G proteins differ in the effectors/signaling pathways they activate, and via these pathways, the transcription of specific genes is controlled [2]. Gαi blocks adenylate cyclase activity, decreases the level of cyclic adenosine monophosphate, and increases the phosphorylation of extracellular signal-regulated kinases. Gαs activates adenylate cyclase; Gαq promotes the synthesis of inositol triphosphate, activates phosphatidylinositol-3 kinase (PI3K), and increases the intracellular concentrations of Ca++. Gαo activates the Wnt-β-catenin signaling pathway, and Gα12/13 activates the Rho/Rock signaling pathway and regulates cytoskeletal rearrangements [25][30][31][32][33]. Through these mechanisms, SP, after binding to NK-1R, regulates the anti-apoptotic cell proliferation and cell migration signaling pathways involved in cancer development [34]. Figure 2 shows the most representative NK-1R downstream pathways involved in cancer progression.
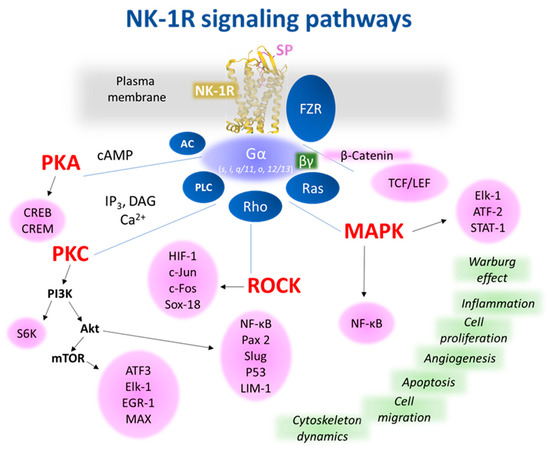
Figure 2. Representative NK-1R intracellular signaling pathways, which are possibly involved in cancer-associated processes. The receptor figure in yellow corresponding to PDB ID 7RMH [35] is from the Protein Data Bank [36] drawn with Mol* web-based open-source toolkit [37]. The transcription factors responsible for controlling cellular events (green) are in pink circles. Abbreviations: AC, adenylyl cyclase; Akt, Ak strain transforming, a protein kinase; ATF, activation transcription factor; cAMP, cyclic adenosine 5′monophosphate; CREB, cAMP response element-binding; CREM, cAMP responsive element modulator; c-Fos, transcription factor; c-Jun, transcription factor; DAG, diacylglycerol; EGR-1, early growth response; Elk-1, ETS-like protein; FZR, Frizzled receptor; HIF, hypoxia-Inducible Factor; IP3, inositol 1,4,5 trisphosphate; LIM-1, Lin11, Isl-1 y Mec-3; MAPK, mitogen-activated protein kinase; MAX, myc-associated factor X; mTOR, mammalian/mechanistic target of rapamycin, a protein kinase; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; p53, tumor suppressor protein; PAX-2, paired box gene 2; PI3K, phosphatidylinositol 3 kinase; PKA, protein kinase A; PKC, protein kinase C; PLC, phospholipase C; Ras, rat sarcoma virus, a small GTPase; Rho/ROCK, Ras homologous/Rho-associated protein kinase; Slug, SNAI2, a zinc-finger transcription factor; Sox18, SRY-related HMG-box; S6K, ribosomal protein S6 kinase; TCF/LEF, T cell factor/lymphoid enhancer factor family.
2.2. Substance P and Neurokinin-1 Receptor Antagonists
NK-1R shows a preferential affinity for SP and hemokinin-1 (a peripheral ligand of NK-1R), which belong to the tachykinin peptide family [17]. The affinity of the NK-1R for other members of the tachykinin peptide family, such as neurokinin A and B, is 100- and 500-fold lower than for SP. For this reason, NK-1R is also named the SP receptor [18]. SP and hemokinin-1 show a high and similar affinity for NK-1R. Both tachykinins share a homologous C-terminal sequence, favor angiogenesis, exert an anti-apoptotic effect, promote the proliferation and migration of tumor cells (e.g., hemokinin-1 increases the expression of matrix metalloproteinases, favors phosphorylation by protein kinase B and extracellular signal-regulated kinases (ERK), and enhances the action of the nuclear factor kappa-light-chain-enhancer of activated B cells) [18][38][39][40]. Accordingly, NK-1R antagonists inhibit the physiological actions mediated through the NK-1R by SP and hemokinin-1 [41]. SP is an undecapeptide derived from the pre-protachykinin A gene (the TAC1 gene locates on chromosome 7 in humans); it is hydrolyzed by p-endopeptidase (extracellular fluid) and angiotensin-converting enzyme (plasma), and it has a half-life from seconds/minutes (tissues) to hours (plasma) [25]. The undecapeptide is widely distributed by the whole body (e.g., immune and endothelial cells, smooth muscle, nervous system, fibroblasts, platelets, cerebrospinal fluid, blood) and, after binding to NK-1R, it is involved in many physiological and pathophysiological actions: micturition, chemotaxis of leukocytes, sensory perception, cardiovascular and respiratory mechanisms, salivation, movement control, immunological processes, sperm cell motility, platelet aggregation, cellular shape change, neuronal degeneration, memory, neurotransmitters release (e.g., acetylcholine, glutamate, histamine, dopamine), permeability of the blood–brain barrier, gastric motility, nausea and vomiting, obesity, pain, inflammation, anxiety, depression, bipolar disorder, pulpitis, neurocysticercosis, thrombosis, Hirschsprung’s and Crohn’s diseases, heart failure, myocarditis, cholestasis, emesis, migraine, mycosis, urinary incontinence, hepatitis, seizure, pruritus, dermatitis, acute pancreatitis, epilepsy, aggressive behavior, rheumatoid arthritis, asthma, chronic bronchitis, ulcerative colitis, viral and bacterial infection, alcohol addiction, and cancer [25][42][43]. The SP/NK-1R system is upregulated in many pathologies (e.g., inflammation, asthma, virus infection, acute pancreatitis, chronic stress, major depressive disorder, ulcerative colitis, Crohn’s disease, and cancer) [25]. Patents using NK-1R antagonists against corneal neovascularization, ocular pain, melanogenesis, cough, bacterial infection, respiratory tract diseases, cardiomyopathy, pruritus, emesis, and cancer have been reported [43]. Despite the potential therapeutic actions of NK-1R antagonists to treat many human pathologies, its potential is currently minimized. It is important to remark that many of the beneficial actions of NK-1R antagonists seen in preclinical studies have been ineffective in clinical trials [42][44]. This observation could be due to a lack of knowledge regarding the molecular interactions between NK-1R and NK-1R antagonists, the non-appropriate selection of patients/endpoints in the clinical trials, the non-appropriate use of experimental animal models, and the lower dose of NK-1R antagonists administered. Thus, studies focused on new NK-1R antagonists showing improved pharmacokinetic characteristics are crucial, and must urgently be performed. In addition, for each pathology, the correct dose of the NK-1R antagonist must be administered because, for example, in clinical trials, aprepitant either exerted (300 mg/day for 45 days) or did not exert (160 mg/day for 5–6 days) an antidepressant action, depending on the dose administered [45][46]. Thus, the lower dose did not reach the threshold required to promote an antidepressant activity, which could be due to the number of NK-1Rs that must be blocked with aprepitant; it seems that the appropriate dose links to the number of NK-1Rs expressed in cancer cells.
2.3. Neurokinin-1 Receptor Isoforms and Cancer
The expression levels of NK-1R are elevated in cancerous samples obtained from the gallbladder [47], pancreas [48], metastatic breast cells [49], acute myeloid leukemia blasts [50], esophageal squamous cell carcinoma [51], or lung cancer cells [52][53]. In contrast, neighboring non-affected tissues showed regular expression. Two isoforms of NK-1R have been reported: full-length (407 amino acids) and truncated (311 amino acids, the last 96 residues at the C-terminus are lost) forms [54]. In some breast cancer cells, the complete structure of NK-1R diminished, and the truncated variant was more abundant [55]. The two variants may be involved in segregated expressions, responses to ligand concentrations, and triggering different intracellular signaling mechanisms [56]. The implication of NK-1R increased activity through both receptor forms in cancer cells is supported by the fact that specific antagonists reverse the system’s participation in cell proliferation, migration, and metastasis [9]. Although precise mechanisms expect determination, there seems to be a dysregulated transcription, where different transcription factors may intervene. Indirect experimental evidence indicates that a putative regulator is a nuclear factor kappa B (NF-κB). This transcription factor governs the expression of NK-1R in macrophage cells by a process dependent on cytokine 1L-1β [57]. In addition, a pro-inflammatory cytokine cocktail (interferon-γ, tumor necrosis factor-α, and 1L-1β) provoked overexpression of NK-1R in colonic epithelial cells and colonic epithelial cell lines [58]. However, the order in which molecular events occur inside the cells requires extensive analysis [56]. Still, the data support that this transcription factor plays a significant role in the hyperactivation of the NK-1R system related to cancer [59]. The short variant of NK-1R may control the immune environment that could suffer alterations at high concentrations of the ligand SP [60]. The oncogenic isoform of NK-1R is the truncated form that mediates tumor growth and malignancy. In contrast, the full-length form interacts with β-arrestin, which is involved in the desensitization, internalization, and endocytosis of NK-1R [18]. The expression of NK-1R mRNA is lower in benign tissues than in malignant ones. Compared with fibroblasts, the expression of the TACR1 gene was augmented 7.5–30 times in human hepatoblastoma cell lines [48][61][62]. The truncated form is dominantly expressed in cervical and prostate cancer cell lines [11][63]. It seems that the truncated form prolongs the response of ligands, because its internalization and desensitization are affected (the absence of amino acid sequences at the C-terminus of NK-1R could block the internalization of the receptor, a clathrin-dependent mechanism, and the recycling processes, leading to SP longer response and cancer progression). In addition, human hepatoblastoma cell lines overexpress the truncated form. Still, negligible levels of this form were found in nonmalignant HEK-293 cells and human fibroblasts [61][64][65][66]. An increase in SP/NK-1R staining has been reported in metastatic tumors. In cultured normal epithelial cells, the level of SP was lower than that found in cultured cancer cells [62][67]. SP, via NK-1R, favors metastasis in human colorectal cancer cells [68]. The expression of NK-1R isoforms is important since activating the truncated form increases metastasis, whereas the activation of the full-length decreases it [55]. Moreover, the activation of the full-length isoform inhibited the proliferation of tumor cells, whereas the activation of the truncated form promoted its proliferation [55]. The tumor growth factor β regulates the expression of the truncated form, an action counteracted by the NK-1R antagonist aprepitant [69]. Pancreatic ductal adenocarcinoma cells mainly expressed the truncated form. Aprepitant exerted its highest antitumor action against them when tumor cells expressed higher levels of this isoform; in this entry, the authors highlighted that NK-1R could be an important target in future personalized medicine [70]. Moreover, the latter study also reported that the expression of NK-1R was lower in pancreatic ductal adenocarcinoma tissues than in normal tissues. A better overall survival rate was observed in patients showing a high level of NK-1R [70]. These are unexpected and contradictory findings compared with most of the previously published results; these findings may occur exclusively in pancreatic ductal adenocarcinomas [9][70]. This must be elucidated in future studies. A review focused on the structural dynamics and signaling cascades (e.g., mitogen-activated protein kinases (MAPK), hairy and enhancer of split 1 (Hes1)) of NK-1R has recently been published [71]. For example, Hes 1, a transcriptional blocker of the Notch signaling pathway, reduced the growth suppression of tumor cells when NK-1R was downregulated [72].
2.4. The Substance P/Neurokinin-1 Receptor System and Cancer: Key Points
The SP/NK-1R system is involved in the molecular bases of many human pathologies, including cancer. This fact means that in-depth knowledge of this system is a crucial step toward better understanding and management of cancer. In the last few years, this knowledge has dramatically increased. Currently, the known key points regarding the involvement of the SP/NK-1R system in cancer are the following: (1) cancer cells express/overexpress NK-1R (e.g., 40,000–60,000 NK-1Rs per glioma cell); (2) NK-1R is involved in the viability of tumor cells (e.g., acute lymphoblastic leukemia, melanoma, lung cancer); (3) NK-1R is not involved in the viability of normal cells; (4) tumor cells express a higher level of truncated NK-1R than normal cells; (5) tumor cells express a lower level of full-length NK-1R than normal cells; (6) SP is not relevant for the viability of cancer cells; (7) SP, after binding to NK-1R (activates MAPK cascade), promotes the mitogenesis/migration of cancer cells (solid and non-solid tumors); (8) cancer cells synthesize and release SP; (9) the undecapeptide acts through endocrine (from tumor mass), paracrine and autocrine mechanisms; (10) SP comes from multiple sources: cancer cells, immune cells placed in the tumor microenvironment, nerve terminals, and bloodstream; (11) SP increases the expression of NK-1R but not that of other tachykinin receptors (NK-2R and NK-3R); (12) SP exerts an anti-apoptotic effect (activating the basal kinase activity of the anti-apoptotic molecule protein kinase B (Akt)) and increases the glycolytic rate (Warburg effect) of tumor cells (which augment their metabolism due to the glucose obtained); (13) SP promotes the growth of blood vessels (angiogenesis, favoring the development of tumors by increasing the supply of blood; endothelial cells express NK-1R and SP), and (14) a higher serum SP level and a higher number of NK-1Rs have been observed in patients with cancer than in healthy individuals [1][9][15][25][73][74][75][76][77][78][79][80][81][82][83][84][85][86]. Moreover, the TAC and TACR1 genes are expressed in primary stem cells derived from human placental cord blood and human stem cell lines. SP promoted the proliferation and migration of stem cells; in the latter cells, SP activated the MAPK cascade via NK-1R, and NK-1R antagonists exerted an antitumor activity against cancer stem cells [87][88][89][90].
2.5. The Substance P/Neurokinin-1 Receptor System as a Cancer Predictive Factor
The overexpression of NK-1R by cancer cells can be used as a prognostic biomarker. It could also facilitate a specific antitumor treatment using NK-1R antagonists (e.g., aprepitant, CP-96,345, L-733,060, SR-140,333, L-732,138). In addition, an increased serum SP level can be used as a predictive factor, indicating a high risk of developing cancer [9][15][91]. The higher level of NK-1R in tumor cells has been related to cancer stage, tumor-node metastasis, poor prognosis, larger tumor size, and higher metastatic and invasion potential [1][72][73][75][92][93]. The expression of NK-1R has been associated with poor prognosis and advanced clinical stages of lung cancer [14]. A poor prognosis is associated with a high expression of truncated NK-1 in breast cancer, and the expression of SP/NK-1R has been suggested as a predictor for colorectal cancer [69][94]. NK-1R has been suggested as a tumor biomarker in hepatoblastoma, independent of the tumor biology and clinical stage. In non-tumor controls, the expression of the truncated NK-1R was lower than that reported in children with hepatoblastoma [2][95]. Tumor size and lymph-node metastasis relate to the number of fibers containing SP [96][97][98]. In breast cancer, the overexpression of SP is associated with a negative prognostic value, the expression of NK-1R with a high Ki-67 index (the nuclear Ki-67 protein is related to proliferative mechanisms), and higher tumor grade [99][100]. The data suggest that NK-1R and SP may serve as predictive cancer factors.
2.6. The Neurokinin-1 Receptor Is Crucial for the Viability of Cancer Cells
Another significant point is that the expression of the TACR1 gene is essential for the survival of tumor cells but not for the viability of normal cells; this means that NK-1R is a promising and specific therapeutic target for cancer treatment [15]. Because the stimulus mediated by SP is beneficial for the survival of cancer cells (e.g., proliferation, migration, anti-apoptotic effect, NK-1R synthesis increase), these cells overexpress NK-1R, ensuring SP binding. Blocking the stimulus with NK-1R antagonists or silencing the expression of the receptor, tumor cells suffer apoptotic mechanisms [78][101][102][103]. Antibodies against SP promoted apoptosis and decreased epidermal growth factor receptor (EGFR) phosphorylation, as well as the survival of tumor cells. An increase in EGFR expression is associated with an increased expression of SP and a worse prognosis [104][105]. When tumor cells do not receive the stimulus mediated by SP, several mechanisms occur: the synthesis of cell cycle proteins halts, the number of apoptotic cells and endothelial growth factor receptors increases, and the steady state of human epidermal growth factor receptor 2 (HER-2) and the MAPK signaling pathway decrease [104][105]. NK-1R overexpression could render cancer cells extremely dependent on the SP stimulus, which could counteract the death-signal pathways of tumor cells. This overexpression could neutralize such pathways because death signals can be overridden by the strong SP mitotic stimulus. When blocking NK-1R using NK-1R antagonists, the balance can favor apoptotic signals, leading to cellular death. It has recently been reported that the absence of NK-1R in glioma cells promoted the death of these cells by both apoptotic and necrotic mechanisms. An irreversible lesion, derived from a non-physiological situation, favors the breakage of glioma cell membranes, promoting their death by necrotic mechanisms [15]. This observation is another vital point worth studying in depth. Altogether, these findings demonstrate the crucial importance of the activation of NK-1R by SP for the survival of cancer cells.
2.7. Neurokinin-1 Receptor and EGFR, Akt, and HER-2
The activation of NK-1R by SP promotes EGFR transactivation, facilitates the formation of EGFR complex, activates the extracellular signal-regulated kinase (ERK) 2 and MAPK pathway, and induces DNA synthesis (Figure 2) [106]. ERK can be translocated into the nucleus, promoting proliferation and exerting an anti-apoptotic action. By activating ERK1/2, SP induced the proliferation and migration of cancer cells, and β-arrestin increased the sensitivity of cancer cells to NK-1R antagonists [107][108]. EGFR inhibitors blocked DNA synthesis and ERK2 activation mediated by SP, and EGFR transactivation mediated the mitogenic action promoted by SP [106]. EGFR and c-Src interact, and an augmented activity of c-Src has been associated with cancer progression, as this interaction enhances mitogenic signaling pathways [109]. Src kinase inhibitors block SP-dependent ERK phosphorylation and suppress the growth of tumor cells [110]. NK-1R, via EGFR transactivation, promoted non-small cell lung cancer progression (cell proliferation and migration) and aprepitant increased the sensitivity of lung cancer cells to gefitinib or osimertinib [111]. SP, via PI3K, augments the activity of protein kinase B (Akt), suppressing apoptosis. The inhibition of PI3K increased apoptosis and decreased cellular proliferation in cancer cells [29][79][112][113][114]. Akt activation has been related to poor prognosis and cellular processes that avoid the death of cells, leading to drug resistance and decreasing the antitumor effect of aprepitant [2][115][116]. Moreover, SP activates HER-2, which promotes drug resistance and malignant progression [104][117].
2.8. Neurokinin-1 Receptor and the Warburg Effect
SP, through NK-1R, mediates the Warburg effect (in tumor cells, compared with normal cells, the glycolytic rate is 200 times higher). This effect is blocked with NK-1R antagonists; then, tumor cells die by starvation (anti-Warburg effect) because NK-1R is needed to obtain glucose (Figure 2) [78][118]. SP activates glycogen synthase kinase-3 (GSK-3β), a finding associated with cancer progression and poor prognosis; its inhibition blocked tumorigenesis, counteracted the Warburg effect, increased apoptosis, and restrained cell motility [119][120]. NK-1R antagonists block GSK-3β activity and increase glycogen synthesis, counteracting the Warburg effect [117].
2.9. Neurokinin-1 Receptor Isoforms Balance
Cancer cells are highly responsive to NK-1R antagonists when they express a high quantity of truncated NK-1R. The overexpression of this form has been associated with an enhanced malignant potential, and the truncated form promoted the malignant transformation of non-tumorigenic cells. Cancer cells express more truncated than full-length forms, and the expression of the full-length form is inversely related to the proliferation of tumor cells, invasiveness, and metastasis [53][55][72][80][121]. The truncated form facilitated the synthesis of cytokines favoring growth-promoting actions, and the overexpression of miR-206 by tumor cells favored the malignant phenotype of these cells by maintaining a low level of the full-length isoform [92][122]. Thus, the antitumor effect mediated by NK-1R antagonists is associated with the differential expression of NK-1R isoforms; this is a significant point [2][34]. Accordingly, knowing the total number of full-length and truncated NK-1R isoforms expressed in tumors is necessary for the in-depth understanding of the mechanisms by which the SP/NK-1R system is involved in cancer, as well as the antitumor actions mediated by NK-1R antagonists.
2.10. Neurokinin-1 Receptor and Inflammation
SP enhanced the inflammatory-mediated tumor signaling pathways and promoted the expression of genes that facilitate tumor growth, invasion, and metastasis in head and neck cancers; these mechanisms were blocked with the NK-1R antagonist L-703,606 [123]. The SP/NK-1R system has been involved in the transition from chronic inflammation of the neck and head mucosa to preneoplastic/neoplastic transformation and development [111]. SP activates pro-inflammatory transcriptions factors (e.g., NF-κB) that control the expression of cytokines; NF-κB promotes the synthesis and release of pro-inflammatory cytokines (e.g., tumor necrosis factor; interleukins 1, 6, and 12), and an NF-κB binding site has been located in the promoter of the TACR1 gene [25][124]. SP increases the release of cytokines by monocytes and macrophages, and cancer cells synthesize interleukin 6, being that its level is related to an increased progression of tumors; in addition, this interleukin has been located in the tumor cyst and cerebrospinal fluids of patients with glioma [124][125][126][127]. Previous data highlight the crucial roles played by SP on inflammatory mechanisms and tumor microenvironment: SP acts as a pro-inflammatory agent, and crosstalk between immune and tumor cells occurs. Chronic inflammation is associated with an increased risk of cancer development and truncated NK-1R/protein levels were higher in colonic epithelial cells with high-grade dysplasia and carcinoma than in quiescent colitis [128][129].
References
- Robinson, P.; Coveñas, R.; Muñoz, M. Combination therapy of chemotherapy or radiotherapy and the neurokinin-1 receptor antagonist aprepitant: A new antitumor strategy? Curr. Med. Chem. 2022.
- Garnier, A.; Ilmer, M.; Kappler, R.; Berger, M. Therapeutic innovations for targeting hepatoblastoma. Anticancer Res. 2016, 36, 5577–5592.
- Morgan, G.; Ward, R.; Barton, M. The contribution of cytotoxic chemotherapy to 5-year survival in adult malignancies. Clin. Oncol. 2004, 16, 549–560.
- Sporn, M.B. The war on cancer. Lancet 1996, 347, 1377–1381.
- Sánchez, M.L.; Coveñas, R. The galaninergic system: A target for cancer treatment. Cancers 2022, 14, 3755.
- Sánchez, M.L.; Coveñas, R. The neurotensinergic system: A target for cancer treatment. Curr. Med. Chem. 2022, 29, 3221–3260.
- Kastin, A.J. Handbook of Biologically Active Peptides, 2nd ed.; Academic Press: Amsterdam, The Netherlands, 2013.
- Colucci, R.; Blandizzi, C.; Ghisu, N.; Florio, T.; Del Tacca, M. Somatostatin inhibits colon cancer cell growth through cyclooxygenase-2 downregulation. Br. J. Pharmacol. 2008, 155, 198–209.
- Coveñas, R.; Muñoz, M. Involvement of the substance P/neurokinin-1 receptor system in cancer. Cancers 2022, 14, 3539.
- Muñoz, M.; Coveñas, R. The neurokinin-1 receptor antagonist aprepitant, a new drug for the treatment of hematological malignancies: Focus on acute myeloid leukemia. J. Clin. Med. 2020, 9, 1659.
- Ebrahimi, S.; Mirzavi, F.; Aghaee-Bakhtiari, S.H.; Hashemy, S.I. SP/NK1R system regulates carcinogenesis in prostate cancer: Shedding light on the antitumoral function of aprepitant. Biochim. Biophys. Acta Mol. Cell. Res. 2022, 1869, 119221.
- Muñoz, M.; Coveñas, R. Neurokinin receptor antagonism: A patent review (2014-present). Expert Opin. Ther. Pat. 2020, 30, 527–539.
- Kolorz, J.; Demir, S.; Gottschlich, A.; Beirith, I.; Ilmer, M.; Lüthy, D.; Walz, C.; Dorostkar, M.M.; Magg, T.; Hauck, F.; et al. The neurokinin-1 receptor is a target in pediatric rhabdoid tumors. Curr. Oncol. 2021, 29, 94–110.
- Zhang, X.-W.; Li, L.; Hu, W.-Q.; Hu, M.-N.; Tao, Y.; Hu, H.; Miao, X.-K.; Yang, W.-L.; Zhu, Q.; Mou, L.-Y. Neurokinin-1 receptor promotes non-small cell lung cancer progression through transactivation of EGFR. Cell Death Dis. 2022, 13, 41.
- Muñoz, M.F.; Argüelles, S.; Rosso, M.; Medina, R.; Coveñas, R.; Ayala, A.; Muñoz, M. The neurokinin-1 receptor is essential for the viability of human glioma cells: A possible target for treating glioblastoma. Biomed. Res. Int. 2022, 2022, 6291504.
- González-Moles, M.A.; Ramos-García, P.; Esteban, F. Significance of the overexpression of substance P and its receptor NK-1R in head and neck carcinogenesis: A systematic review and meta-analysis. Cancers 2021, 13, 1349.
- Muñoz, M.; Rosso, M.; Coveñas, R. Neurokinin-1 receptor. In Encyclopedia of Signaling Molecules, 2nd ed.; Choi, S., Ed.; Springer: Cham, Switzerland, 2018; pp. 3437–3445.
- Muñoz, M.; Coveñas, R.; Substance, P. Encyclopedia of Endocrine Diseases, 2nd ed.; Huhtaniemi, I., Martini, L., Eds.; Academic Press: Oxford, UK, 2019; Volume 1, pp. 571–578.
- dSNP (Single Nucleotide Polymorphism Database). Single Nucleotide Polymorphisms. 2020. Available online: https://www-ncbi-nlm-nih-gov.ezproxy.usal.es/snp/ (accessed on 20 September 2022).
- Farran, B. An update on the physiological and therapeutic relevance of GPCR oligomers. Pharmacol. Res. 2017, 117, 303–327.
- Xue, L.; Sun, Q.; Zhao, H.; Rovira, X.; Gai, S.; He, Q.; Pin, J.-P.; Liu, J.; Rondard, P. Rearrangement of the transmembrane domain interfaces associated with the activation of a GPCR hetero-oligomer. Nat. Commun. 2019, 10, 2765.
- Meyer, B.H.; Segura, J.M.; Martinez, K.L.; Hovius, R.; George, N.; Johnsson, K.; Vogel, H. FRET imaging reveals that functional neurokinin-1 receptors are monomeric and reside in membrane microdomains of live cells. Proc. Natl. Acad. Sci. USA 2006, 103, 2138–2143.
- Terpager, M.; Scholl, D.J.; Kubale, V.; Martini, L.; Elling, C.E.; Schwartz, T.W. Construction of covalently coupled, concatameric dimers of 7TM receptors. J. Recept. Signal Transduct. Res. 2009, 29, 235–245.
- GPCR Database. 2022. Available online: https://gpcrdb.org/protein/nk1r_human (accessed on 20 September 2022).
- Muñoz, M.; Coveñas, R. Neurokinin/Tachykin receptors. In Encyclopedia of Molecular Pharmacology, 3rd ed.; Offermanns, S., Rosenthal, W., Eds.; Springer: Cham, Switzerland, 2021; pp. 1093–1103.
- Roush, E.D.; Kwatra, M.M. Human substance P receptor expressed in Chinese hamster ovary cells directly activates g (alpha q/11), g (alpha s), g (alpha o). FEBS Lett. 1998, 428, 291–294.
- Mitsuhashi, M.; Ohashi, Y.; Shichijo, S.; Christian, C.; Sudduth-Klinger, J.; Harrowe, G.; Payan, D.G. Multiple intracellular signaling pathways of the neuropeptide substance P receptor. J. Neurosci. Res. 1992, 32, 437–443.
- Sagan, S.; Chassaing, G.; Pradier, L.; Lavielle, S. Tachykinin peptides affect differently the second messenger pathways after binding to CHO-expressed human NK-1 receptors. J. Pharmacol. Exp. Ther. 1996, 276, 1039–1048.
- Takeda, Y.; Blount, P.; Sachais, B.S.; Hershey, A.D.; Raddatz, R.; Krause, J.E. Ligand binding kinetics of substance P and neurokinin A receptors stably expressed in Chinese hamster ovary cells and evidence for differential stimulation of inositol 1, 4, 5 trisphosphate and cyclic amp second messenger responses. J. Neurochem. 1992, 59, 740–745.
- Ramkissoon, S.H.; Patel, H.J.; Taborga, M.; Rameshwar, P. G protein-coupled receptors in haematopoietic disruption. Expert Opin. Biol. Ther. 2006, 6, 109–120.
- Nakajima, Y.; Tsuchida, K.; Negishi, M.; Ito, S.; Nakanishi, S. Direct linkage of three tachykinin receptors to stimulation of both phosphatidylinositol hydrolysis and cyclic AMP cascades in transfected Chinese hamster ovary cells. J. Biol. Chem. 1992, 267, 2437–2442.
- Holst, B.; Hastrup, H.; Raffetseder, U.; Martini, L.; Schwartz, T.W. Two active molecular phenotypes of the tachykinin NK1 receptor revealed by G-protein fusions and mutagenesis. J. Biol. Chem. 2001, 276, 19793–19799.
- Khawaja, A.M.; Rogers, D.F. Tachykinins: Receptor to effector. Int. J. Biochem. Cell Biol. 1996, 28, 721–738.
- Muñoz, M.; Rosso, M.; Coveñas, R. Neurokinin-1 receptor antagonists against hepatoblastoma. Cancers 2019, 11, 1258.
- Harris, G.C.; Aston-Jones, G. Involvement of D2 dopamine receptors in the nucleus accumbens in the opiate withdrawal syndrome. Nature 1994, 371, 155–157.
- PDB. Protein Data Bank (PDB). Available online: https://pdb101.rcsb.org (accessed on 20 September 2022).
- Sehnal, D.; Bittrich, S.; Deshpande, M.; Svobodová, R.; Berka, K.; Bazgier, V.; Velankar, S.; Burley, S.K.; Koča, J.; Rose, A.S. Mol* Viewer: A modern web app for 3D visualization and analysis of large biomolecular structures. Nucleic Acids Res. 2021, 49, W431–W437.
- Li, J.; Zeng, Q.; Zhang, Y.; Li, X.; Hu, H.; Miao, X.; Yang, W.; Zhang, W.; Song, X.; Mou, L.; et al. Neurokinin-1 receptor mediated breast cancer cell migration by increased expression of MMP-2 and MMP-14. Eur. J. Cell Biol. 2016, 95, 368–377.
- Zhang, Y.; Li, X.; Li, J.; Hu, H.; Miao, X.; Song, X.; Yang, W.; Zeng, Q.; Mou, L.; Wang, R. Human hemokinin-1 promotes migration of melanoma cells and increases MMP-2 and MT1-MMP expression by activating tumor cell NK1 receptors. Peptides 2016, 83, 8–15.
- Mou, L.; Kang, Y.; Zhou, Y.; Zeng, Q.; Song, H.; Wang, R. Neurokinin-1 receptor directly mediates glioma cell migration by up-regulation of matrix metalloproteinase-2 (MMP-2) and membrane type 1-matrix metalloproteinase (MT1-MMP). J. Biol. Chem. 2013, 288, 306–318.
- Berger, A.; Paige, C.J. Hemokinin-1 has substance P-like function in U-251 MG astrocytoma cells. A pharmacological and functional study. J. Neuroimmunol. 2005, 164, 48–56.
- Muñoz, M.; Coveñas, R. Involvement of substance P and the NK-1 receptor in human pathology. Amino Acids 2014, 46, 1727–1750.
- Muñoz, M.; Martínez-Armesto, J.; Coveñas, R. NK-1 receptor antagonists as antitumor drugs: A survey of the literature from 2000 to 2011. Expert Opin. Ther. Pat. 2012, 22, 735–746.
- Rost, K.; Fleischer, F.; Nieber, K. Neurokinin-1 receptor antagonists: Between hope and disappointment. Med. Monatsschr. Pharm. 2006, 29, 200–205.
- Kramer, M.S.; Cutler, N.; Feighner, J.; Shrivastava, R.; Carman, J.; Sramek, J.J.; Reines, S.A.; Liu, G.; Snavely, D.; Wyatt-Knowles, E.; et al. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science 1998, 281, 1640–1645.
- Keller, M.; Montgomery, S.; Ball, W.; Morrison, M.; Snavely, D.; Liu, G.; Hargreaves, R.; Hietala, J.; Lines, C.; Beebe, K.; et al. Lack of efficacy of the substance P (neurokinin 1 receptor) antagonist aprepitant in the treatment of major depressive disorder. Biol. Psychiatry 2006, 59, 216–223.
- Deng, X.T.; Tang, S.M.; Wu, P.Y.; Li, Q.P.; Ge, X.X.; Xu, B.M.; Wang, H.-S.; Miao, L. SP/NK-1R promotes gallbladder cancer cell proliferation and migration. J. Cell. Mol. Med. 2019, 23, 7961–7973.
- Friess, H.; Zhu, Z.; Liard, V.; Shi, X.; Shrikhande, S.V.; Wang, L.; Lieb, K.; Korc, M.; Palma, C.; Zimmermann, A.; et al. Neurokinin-1 receptor expression and its potential effects on tumor growth in human pancreatic cancer. Lab. Investig. 2003, 83, 731–742.
- Nizam, E.; Erin, N. Differential consequences of neurokinin receptor 1 and 2 antagonists in metastatic breast carcinoma cells; effects independent of substance P. Biomed. Pharmacother. 2018, 108, 263–270.
- Ge, Y.M. The role of human neutrophil elastase in the development of human emphysema. Zhonghua Jie He He Hu Xi Za Zhi 1989, 12, 347–349.
- Mohammadi, F.; Javid, H.; Afshari, A.R.; Mashkani, B.; Hashemy, S.I. Substance P accelerates the progression of human esophageal squamous cell carcinoma via MMP-2, MMP-9, VEGF-A, and VEGFR1 overexpression. Mol. Biol. Rep. 2020, 47, 4263–4272.
- Ebrahimi, S.; Javid, H.; Alaei, A.; Hashemy, S.I. New insight into the role of substance P/neurokinin-1 receptor system in breast cancer progression and its crosstalk with microRNAs. Clin. Genet. 2020, 98, 322–330.
- Zhang, L.; Wang, L.; Dong, D.; Wang, Z.; Ji, W.; Yu, M.; Zhang, F.; Niu, R.; Zhou, Y. MiR-34b/c-5p and the neurokinin-1 receptor regulate breast cancer cell proliferation and apoptosis. Cell Prolif. 2019, 52, e12527.
- Fong, T.M.; Anderson, S.A.; Yu, H.; Huang, R.R.; Strader, C.D. Differential activation of intracellular effector by two isoforms of human neurokinin-1 receptor. Mol. Pharmacol. 1992, 41, 24–30.
- Zhou, Y.; Zhao, L.; Xiong, T.; Chen, X.; Zhang, Y.; Yu, M.; Yang, J.; Yao, Z. Roles of full-length and truncated neurokinin-1 receptors on tumor progression and distant metastasis in human breast cancer. Breast Cancer Res. Treat. 2013, 140, 49–61.
- Lai, J.P.; Lai, S.; Tuluc, F.; Tansky, M.F.; Kilpatrick, L.E.; Leeman, S.E.; Douglas, S.D. Differences in the length of the carboxyl terminus mediate functional properties of neurokinin-1 receptor. Proc. Natl. Acad. Sci. USA 2008, 105, 12605–12610.
- Simeonidis, S.; Castagliuolo, I.; Pan, A.; Liu, J.; Wang, C.C.; Mykoniatis, A.; Pasha, A.; Valenick, L.; Sougioultzis, S.; Zhao, D.; et al. Regulation of the NK-1 receptor gene expression in human macrophage cells via an NF-kappa B site on its promoter. Proc. Natl. Acad. Sci. USA 2003, 100, 2957–2962.
- Goode, T.; O’Connor, T.; Hopkins, A.; Moriarty, D.; O’Sullivan, G.C.; Collins, J.K.; O’Donoghue, D.; Baird, A.W.; O’Connell, J.; Shanahan, F. Neurokinin-1 receptor (NK-1R) expression is induced in human colonic epithelial cells by pro-inflammatory cytokines and mediates proliferation in response to substance P. J. Cell. Physiol. 2003, 197, 30–41.
- Rosso, M.; Muñoz, M.; Berger, M. The role of the neurokinin-1 receptor in the microenvironment of inflammation and cancer. Sci. World J. 2012, 2012, 381434.
- Spitsin, S.; Pappa, V.; Douglas, S.D. Truncation of neurokinin-1 receptor—Negative regulation of substance P signaling. J. Leukoc. Biol. 2018, 103, 1043–1051.
- Berger, M.; Neth, O.; Ilmer, M.; Garnier, A.; Salinas-Martín, M.V.; de Agustin Asencio, J.C.; von Schweinitz, D.; Kappler, R.; Muñoz, M. Hepatoblastoma cells express truncated neurokinin-1 receptor and can be growth inhibited by aprepitant in vitro and in vivo. J. Hepatol. 2014, 60, 985–994.
- Singh, D.; Joshi, D.D.; Hameed, M.; Qian, J.; Gascón, P.; Maloof, P.B.; Mosenthal, A.; Rameshwar, P. Increased expression of preprotachykinin-I and neurokinin receptors in human breast cancer cells: Implications for bone marrow metastasis. Proc. Natl. Acad. Sci. USA 2000, 97, 1388–1393.
- Mozafari, M.; Ebrahimi, S.; Darban, R.A.; Hashemy, S.I. Potential in vitro therapeutic effects of targeting SP/NK1R system in cervical cancer. Mol. Biol. Rep. 2021, 49, 1067–1076.
- Douglas, S.D.; Leeman, S.E. Neurokinin-1 receptor: Functional significance in the immune system in reference to selected infections and inflammation. Ann. N. Y. Acad. Sci. 2011, 1217, 83–95.
- Henssen, A.G.; Odersky, A.; Szymansky, A.; Seiler, M.; Althoff, K.; Beckers, A.; Speleman, F.; Schäfers, S.; De Preter, K.; Astrahanseff, K.; et al. Targeting tachykinin receptors in neuroblastoma. Oncotarget 2017, 8, 430–443.
- Pohl, A.; Kappler, R.; Muhlig, J.; von Schweinitz, D.; Berger, M. Expression of truncated neurokinin-1 receptor in childhood neuroblastoma is independent of tumor biology and stage. Anticancer Res. 2017, 37, 6079–6085.
- Harford-Wright, E.; Lewis, K.M.; Vink, R.; Ghabriel, M.N. Evaluating the role of substance P in the growth of brain tumors. Neuroscience 2014, 261, 85–94.
- Golestaneh, M.; Firoozrai, M.; Javid, H.; Hashemy, S.I. The substance P/neurokinin-1 receptor signaling pathway mediates metastasis in human colorectal SW480 cancer cells. Mol. Biol. Rep. 2022, 49, 4893–4900.
- Zhou, Y.; Wang, L.; Wang, N.; Zhang, R.; Dong, D.; Liu, R.; Zhang, L.; Ji, W.; Yu, M.; Zhang, F.; et al. TGFβ regulates NK1R-Tr to affect the proliferation and apoptosis of breast cancer cells. Life Sci. 2020, 256, 117674.
- Beirith, I.; Renz, B.W.; Mudusetti, S.; Ring, N.S.; Kolorz, J.; Koch, D.; Bazhin, A.V.; Berger, M.; Wang, J.; Angele, M.K.; et al. Identification of the neurokinin-1 receptor as targetable stratification factor for drug repurposing in pancreatic cancer. Cancers 2021, 13, 2703.
- Rodríguez, F.D.; Coveñas, R. The neurokinin-1 receptor: Structure dynamics and signaling. Receptors 2022, 1, 54–71.
- Wang, F.; Liu, S.; Liu, J.; Feng, F.; Guo, Y.; Zhang, W.; Zheng, G.; Wang, Q.; Cai, L.; Guo, M.; et al. SP promotes cell proliferation in esophageal squamous cell carcinoma through the NK1R/Hes1 axis. Biochem. Biophys. Res. Commun. 2019, 514, 1210–1216.
- Davoodian, M.; Boroumand, N.; Mehrabi Bahar, M.; Jafarian, A.H.; Asadi, M.; Hashemy, S.I. Evaluation of serum level of substance P and tissue distribution of NK-1 receptor in breast cancer. Mol. Biol. Rep. 2019, 46, 1285–1293.
- Hennig, I.M.; Laissue, J.A.; Horisberger, U.; Reubi, J.C. Substance-P receptors in human primary neoplasms: Tumoral and vascular localization. Int. J. Cancer 1995, 61, 786–792.
- Gharaee, N.; Pourali, L.; Jafarian, A.H.; Hashemy, S.I. Evaluation of serum level of substance P and tissue distribution of NK-1 receptor in endometrial cancer. Mol. Biol. Rep. 2018, 45, 2257–2262.
- Ziche, M.; Morbidelli, L.; Pacini, M.; Geppetti, P.; Alessandri, G.; Maggi, C.A. Substance P stimulates neovascularization in vivo and proliferation of cultured endothelial cells. Microvasc. Res. 1990, 40, 264–278.
- Muñoz, M.; Coveñas, R. The neurokinin-1 receptor antagonist aprepitant: An intelligent bullet against cancer? Cancers 2020, 12, 2682.
- Muñoz, M.; Coveñas, R. Involvement of substance P and the NK-1 receptor in cancer progression. Peptides 2013, 48, 1–9.
- Koon, H.W.; Zhao, D.; Zhan, Y.; Moyer, M.P.; Pothoulakis, C. Substance P mediates antiapoptotic responses in human colonocytes by Akt activation. Proc. Natl. Acad. Sci. USA 2007, 104, 2013–2018.
- Fulenwider, H.D.; Smith, B.M.; Nichenko, A.S.; Carpenter, J.M.; Nennig, S.E.; Cheng, K.; Rice, K.C.; Schank, J.R. Cellular and behavioral effects of lipopolysaccharide treatment are dependent upon neurokinin-1 receptor activation. J. Neuroinflammation 2018, 15, 60.
- Walczak-Drzewiecka, A.; Ratajewski, M.; Wagner, W.; Dastych, J. HIF-1alpha is up-regulated in activated mast cells by a process that involves calcineurin and NFAT. J. Immunol. 2008, 181, 1665–1672.
- Muñoz, M.; Rosso, M. The NK-1 receptor antagonist aprepitant as a broad-spectrum antitumor drug. Investig. New Drugs 2010, 28, 187–193.
- Muñoz, M.; Rosso, M.; Robles-Frías, M.J.; Salinas-Martín, M.V.; Coveñas, R. Immunolocalization of the neurokinin-1 receptor: A new target in the treatment of human malignant melanoma. Lab. Investig. 2010, 90, 1259–1269.
- Muñoz, M.; Coveñas, R. Safety of neurokinin-1 receptor antagonists. Expert Opin. Drug Saf. 2013, 12, 673–685.
- Mehboob, R.; Kurdi, M.; Baeesa, S.; Fawzy Halawa, T.; Tanvir, I.; Maghrabi, Y.; Hakamy, S.; Saeedi, R.; Moshref, R.; Nasief, H.; et al. Immunolocalization of neurokinin 1 receptor in WHO grade 4 astrocytomas, oral squamous cell, and urothelial carcinoma. Folia Neuropathol. 2022, 60, 165–176.
- Muñoz, M.; Rosso, M.; Coveñas, R. A new frontier in the treatment of cancer: NK-1 receptor antagonists. Curr. Med. Chem. 2010, 17, 504–516.
- Li, Y.; Douglas, S.D.; Ho, W. Human stem cells express substance P gene and its receptor. J. Hematother. Stem Cell Res. 2000, 9, 445–452.
- Dubon, M.J.; Park, K.S. Substance P enhances the proliferation and migration potential of murine bone marrow-derived mesenchymal stem cell-like cell lines. Exp. Ther. Med. 2015, 9, 1185–1191.
- Kim, K.T.; Kim, H.J.; Cho, D.C.; Bae, J.S.; Park, S.W. Substance P stimulates proliferation of spinal neural stem cells in spinal cord injury via the mitogen-activated protein kinase signaling pathway. Spine J. 2015, 15, 2055–2065.
- Garnier, A.; Vykoukal, J.; Hubertus, J.; Alt, E.; von Schweinitz, D.; Kappler, R.; Berger, M.; Ilmer, M. Targeting the neurokinin-1 receptor inhibits growth of human colon cancer cells. Int. J. Oncol. 2015, 47, 151–160.
- Muñoz, M.; Coveñas, R. Neurokinin-1 receptor antagonists as anticancer drugs. Lett. Drug Des. Discov. 2019, 16, 1110–1129.
- Zhou, Y.; Wang, M.; Tong, Y.; Liu, X.; Zhang, L.; Dong, D.; Shao, J.; Zhou, Y. miR-206 promotes cancer progression by targeting full-length neurokinin-1 receptor in breast cancer. Technol. Cancer Res. Treat. 2019, 18, 1–14.
- Castro, T.A.; Cohen, M.C.; Rameshwar, P. The expression of neurokinin-1 and preprotachykinin-1 in breast cancer cells depends on the relative degree of invasive and metastatic potential. Clin. Exp. Metastasis 2005, 22, 621–628.
- Chen, X.Y.; Ru, G.Q.; Ma, Y.Y.; Xie, J.; Chen, W.Y.; Wang, H.J.; Wang, S.B.; Li, L.; Jin, K.T.; He, X.L.; et al. High expression of substance P and its receptor neurokinin-1 receptor in colorectal cancer is associated with tumor progression and prognosis. OncoTargets Ther. 2016, 9, 3595–3602.
- Garnier, A.; Ilmer, M.; Becker, K.; Häberle, B.; von Schweinitz, D.; Kappler, R.; Berger, M. Truncated neurokinin-1 receptor is an ubiquitous antitumor target in hepatoblastoma, and its expression is independent of tumor biology and stage. Oncol. Lett. 2016, 11, 870–878.
- Dong, J.; Feng, F.; Xu, G.; Zhang, H.; Hong, L.; Yang, J. Elevated SP/NK-1R in esophageal carcinoma promotes esophageal carcinoma cell proliferation and migration. Gene 2015, 560, 205–210.
- Feng, F.; Yang, J.; Tong, L.; Yuan, S.; Tian, Y.; Hong, L.; Wang, W.; Zhang, H. Substance P immunoreactive nerve fibres are related to gastric cancer differentiation status and could promote proliferation and migration of gastric cancer cells. Cell Biol. Int. 2011, 35, 623–629.
- González-Moles, M.A.; Brener, S.; Ruiz-Avila, I.; Gil-Montoya, J.A.; Tostes, D.; Bravo, M.; Esteban, F. Substance P and NK-1R expression in oral precancerous epithelium. Oncol. Rep. 2009, 22, 1325–1331.
- Al-Keilani, M.S.; Elstaty, R.I.; Alqudah, M.A.; Alkhateeb, A.M. Immunohistochemical expression of substance P in breast cancer and its association with prognostic parameters and Ki-67 index. PLoS ONE 2021, 16, e0252616.
- Al-Keilani, M.S.; Elstaty, R.I.; Alqudah, M.A. The prognostic potential of neurokinin 1 receptor in breast cancer and its relationship with Ki-67 index. Int. J. Breast Cancer 2022, 2022, 4987912.
- Muñoz, M.; Coveñas, R. Glioma, and neurokinin-1 receptor antagonists: A new therapeutic approach. Anticancer Agents Med. Chem. 2020, 19, 92–100.
- Muñoz, M.; Coveñas, R.; Esteban, F.; Redondo, M. The substance P/NK-1 receptor system: NK-1 receptor antagonists as anti-cancer drugs. J. Biosci. 2015, 40, 441–463.
- Gutierrez, S.; Boada, M.D. Neuropeptide-induced modulation of carcinogenesis in a metastatic breast cancer cell line (MDA-MB-231LUC+). Cancer Cell Int. 2018, 18, 216.
- García-Recio, S.; Fuster, G.; Fernández-Nogueira, P.; Pastor-Arroyo, E.M.; Park, S.Y.; Mayordomo, C.; Ametller, E.; Mancino, M.; González-Farré, X.; Russnes, H. Substance P autocrine signaling contributes to persistent HER2 activation that drives malignant progression and drug resistance in breast cancer. Cancer Res. 2013, 73, 6424–6434.
- Mayordomo, C.; García-Recio, S.; Ametller, E.; Fernández-Nogueira, P.; Pastor-Arroyo, E.M.; Vinyals, L.; Casas, I.; Gascón, P.; Almendro, V. Targeting of substance P induces cancer cell death and decreases the steady state of EGFR and Her2. J. Cell. Physiol. 2012, 227, 1358–1366.
- Castagliuolo, I.; Valenick, L.; Liu, J.; Pothoulakis, C. Epidermal growth factor receptor transactivation mediates substance P-induced mitogenic responses in U-373 MG cells. J. Biol. Chem. 2000, 275, 26545–26550.
- Kast, R.E. Glioblastoma: Synergy of growth promotion between CCL5 and NK-1R can be thwarted by blocking CCL5 with miraviroc, an FDA-approved anti-HIV drug, and blocking NK-1R with aprepitant, an FDA-approved anti-nausea drug. J. Clin. Pharm. Ther. 2010, 35, 657–663.
- Zhang, Y.X.; Li, X.F.; Yuan, G.Q.; Hu, H.; Song, X.Y.; Li, J.Y.; Miao, X.K.; Zhou, T.X.; Yang, W.L.; Zhang, X.W.; et al. β-Arrestin 1 has an essential role in neurokinin-1 receptor-mediated glioblastoma cell proliferation and G2/M phase transition. J. Biol. Chem. 2017, 292, 8933–8947.
- Almendro, V.; García-Recio, S.; Gascón, P. Tyrosine kinase receptor transactivation associated to G protein-coupled receptors. Curr. Drug Targets 2010, 11, 1169–1180.
- Yamaguchi, K.; Richardson, M.D.; Bigner, D.D.; Kwatra, M.M. Signal transduction through substance P receptor in human glioblastoma cells: Roles for Src and PKCδ. Cancer Chem. Pharmacol. 2005, 56, 585–593.
- Esteban, F.; Ramos-García, P.; Muñoz, M.; González-Moles, M.A. Substance P and neurokinin 1 receptor in chronic inflammation and cancer of the head and neck: A review of the literature. Int. J. Environ. Res. Public Health 2021, 19, 375.
- De Fea, K.A.; Vaughn, Z.D.; O’Bryan, E.M.; Nishijima, D.; Déry, O.; Bunnett, N.W. The proliferative and antiapoptotic effects of substance P are facilitated by formation of a beta-arrestin-dependent scaffolding complex. Proc. Natl. Acad. Sci. USA 2000, 97, 11086–11091.
- Bockmann, S.; Seep, J.; Jonas, L. Delay of neutrophil apoptosis by the neuropeptide substance P: Involvement of caspase cascade. Peptides 2001, 22, 661–670.
- Hartmann, W.; Küchler, J.; Koch, A.; Friedrichs, N.; Waha, A.; Endl, E.; Czerwitzki, J.; Metzger, D.; Steiner, S.; Wurst, P.; et al. Activation of phosphatidylinositol-30-kinase/AKT signaling is essential in hepatoblastoma survival. Clin. Cancer Res. 2009, 15, 4538–4545.
- Breuleux, M.; Klopfenstein, M.; Stephan, C.; Doughty, C.A.; Barys, L.; Maira, S.M.; Kwiatkowski, D.; Lane, H.A. Increased AKT s473 phosphorylation after mTORC1 inhibition is rictor dependent and does not predict tumor cell response to PI3K/mTOR inhibition. Mol. Cancer Ther. 2009, 8, 742–753.
- Chen, K.F.; Chen, H.L.; Tai, W.T.; Feng, W.C.; Hsu, C.H.; Chen, P.J.; Cheng, A.L. Activation of phosphatidylinositol 3-kinase/AKT signaling pathway mediates acquired resistance to sorafenib in hepatocellular carcinoma cells. J. Pharmacol. Exp. Ther. 2011, 337, 155–161.
- Akazawa, T.; Kwatra, S.G.; Goldsmith, L.E.; Richardson, M.D.; Cox, E.A.; Sampson, J.H.; Kwatra, M.M. A constitutively active form of neurokinin 1 receptor and neurokinin 1 receptor-mediated apoptosis in glioblastomas. J. Neurochem. 2009, 109, 1079–1086.
- Medrano, S.; Gruenstein, E.; Dimlich, R.V.W. Substance P receptors on human astrocytoma cells are linked to glycogen breakdown. Neurosci. Lett. 1994, 167, 14–18.
- Ougolkov, A.V.; Fernández-Zapico, M.E.; Savoy, D.N.; Urrutia, R.A.; Billadeau, D.D. Glycogen synthase kinase-3beta participates in nuclear factor kappaB-mediated gene transcription and cell survival in pancreatic cancer cells. Cancer Res. 2005, 65, 2076–2081.
- Zeng, J.; Liu, D.; Qiu, Z.; Huang, Y.; Chen, B.; Wang, L.; Xu, H.; Huang, N.; Liu, L.; Li, W. GSK3β overexpression indicates poor prognosis, and its inhibition reduces cell proliferation and survival of non-small cell lung cancer cells. PLoS ONE 2014, 9, e91231.
- Wierstra, I. The transcription factor FOXM1c is activated by protein kinase CK2, protein kinase A (PKA), c-Src, and Raf-1. Biochem. Biophys. Res. Commun. 2011, 413, 230–235.
- Ogo, H.; Kuroyanagi, N.; Inoue, A.; Nishio, H.; Hirai, Y.; Akiyama, M.; DiMaggio, D.A.; Krause, J.E.; Nakata, Y. Human astrocytoma cells (U-87 MG) exhibit a specific substance P binding site with the characteristics of an NK-1 receptor. J. Neurochem. 1996, 67, 1813–1820.
- Singh, S.; Kumaravel, S.; Dhole, S.; Roy, S.; Pavan, V.; Chakraborty, S. Neuropeptide substance P enhances inflammation-mediated tumor signaling pathways and migration and proliferation of head and neck cancers. Indian J. Surg. Oncol. 2021, 12, 93–102.
- Ho, W.Z.; Kaufman, D.; Uvaydova, M.; Douglas, S.D. Substance P augments interleukin-10 and tumor necrosis factor-alpha release by human cord blood monocytes and macrophages. J. Neuroimmunol. 1996, 71, 73–80.
- Weller, M.; Stevens, A.; Sommer, N.; Melms, A.; Dichgans, J.; Wiethölter, H. Comparative analysis of cytokine patterns in immunological, infectious, and oncological neurological disorders. J. Neurol. Sci. 1991, 104, 215–221.
- Frei, K.; Piani, D.; Malipiero, U.V.; Van Meir, E.; de Tribolet, N.; Fontana, A. Granulocyte-macrophage colony-stimulating factor GM-CSF production by glioblastoma cells. Despite the presence of inducing signals, GM-CSF is not expressed in vivo. J. Immunol. 1992, 148, 3140–3146.
- Lotz, M.; Vaughan, J.H.; Carson, D.A. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science 1988, 241, 1218–1221.
- Muñoz, M.; Coveñas, R. Targeting NK-1 receptors to prevent and treat pancreatic cancer: A new therapeutic approach. Cancers 2015, 7, 1215–1232.
- Gillespie, E.; Leeman, S.E.; Watts, L.A.; Coukos, J.A.; O’Brien, M.J.; Cerda, S.R.; Farraye, F.A.; Stucchi, A.F.; Becker, J.M. Truncated neurokinin-1 receptor is increased in colonic epithelial cells from patients with colitis-associated cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 17420–17425.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
16 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




