Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kok Yong Chin | -- | 1279 | 2022-11-08 04:57:03 | | | |
| 2 | Conner Chen | Meta information modification | 1279 | 2022-11-15 09:00:18 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chin, K.; Ng, B.N.; Rostam, M.K.I.; Fadzil, N.F.D.M.; Raman, V.; Yunus, F.M.; Hashim, S.A.S.; Ekeuku, S.O. Pathophysiology of Osteoporosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/34482 (accessed on 26 January 2026).
Chin K, Ng BN, Rostam MKI, Fadzil NFDM, Raman V, Yunus FM, et al. Pathophysiology of Osteoporosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/34482. Accessed January 26, 2026.
Chin, Kok-Yong, Ben Nett Ng, Muhd Khairik Imran Rostam, Nur Farah Dhaniyah Muhammad Fadzil, Vaishnavi Raman, Farzana Mohamed Yunus, Syed Alhafiz Syed Hashim, Sophia Ogechi Ekeuku. "Pathophysiology of Osteoporosis" Encyclopedia, https://encyclopedia.pub/entry/34482 (accessed January 26, 2026).
Chin, K., Ng, B.N., Rostam, M.K.I., Fadzil, N.F.D.M., Raman, V., Yunus, F.M., Hashim, S.A.S., & Ekeuku, S.O. (2022, November 14). Pathophysiology of Osteoporosis. In Encyclopedia. https://encyclopedia.pub/entry/34482
Chin, Kok-Yong, et al. "Pathophysiology of Osteoporosis." Encyclopedia. Web. 14 November, 2022.
Copy Citation
Osteoporosis refers to excessive bone loss as reflected by the deterioration of bone mass and microarchitecture, which compromises bone strength. It is a complex multifactorial endocrine disease. Its pathogenesis relies on the presence of several endogenous and exogenous risk factors, which skew the physiological bone remodelling to a more catabolic process that results in net bone loss.
bone
menopause
osteoblast
osteoclast
1. Introduction
The world has been experiencing an increase in lifespan due to improved medical care and living environment, but this has not kept pace with the increase in healthy life expectancy [1]. Ageing causes multiple adverse physiological changes to the body due to the lifetime accumulation of molecular and cellular damage [2]. Among these geriatric diseases, the ageing of the skeleton is one aspect often overlooked by the community and medical professionals alike. Generally, humans achieve peak bone mass in the third decade of life, but the exact age varies with sex and skeletal sites [3]. After peaking, both sexes experience a decline in bone mass [4], which is accelerated during menopause in women [5].
Osteoporosis is a skeletal disease characterised by reduced bone strength due to deteriorating bone mass and bone microarchitecture, leading to increased susceptibility to fracture [6]. Owing to a lower peak bone mass and faster bone loss during menopause, women are at greater risk for osteoporosis than men [5]. While the development of osteoporosis is mostly asymptomatic, its ultimate consequences, i.e., fragility fractures, pose tremendous medical and economical challenges to the patients and society [7]. Despite the availability of effective therapy, a substantial number of patients with osteoporosis remain untreated [8].
2. Pathophysiology of Osteoporosis
The traditional pathophysiological models of osteoporosis are based on endocrine mechanisms. Two examples are estrogen deficiency in postmenopausal women and secondary hyperparathyroidism in the elderly due to menopause and vitamin D deficiency. In reality, osteoporosis is a multifactorial disease caused by a complex interplay of genetic, intrinsic, exogenous, and lifestyle factors [9].
A basic understanding of the bone remodelling cycle will facilitate the discussion on the pathophysiology of osteoporosis (Figure 1). Osteoclasts, osteoblasts, and osteocytes are the three main players in bone remodelling. When bone damage occurs, the macrophage polykaryon-derived osteoclasts migrate to the damage site and perform bone resorption [10]. At the end of bone resorption, osteoclasts undergo apoptosis and produce apoptotic bodies that may play a role in the subsequent osteogenesis [10]. After the reversal phase, the mesenchymal stem cell-derived osteoblasts will migrate to the cavity and perform bone formation [11]. Some osteoblasts will be embedded in the bone matrix they synthesise and differentiate into osteocytes. Osteocytes act as a mechanosensor and play regulatory roles in regulating the bone remodelling process through signalling proteins and via perilacunar remodelling directly [12].
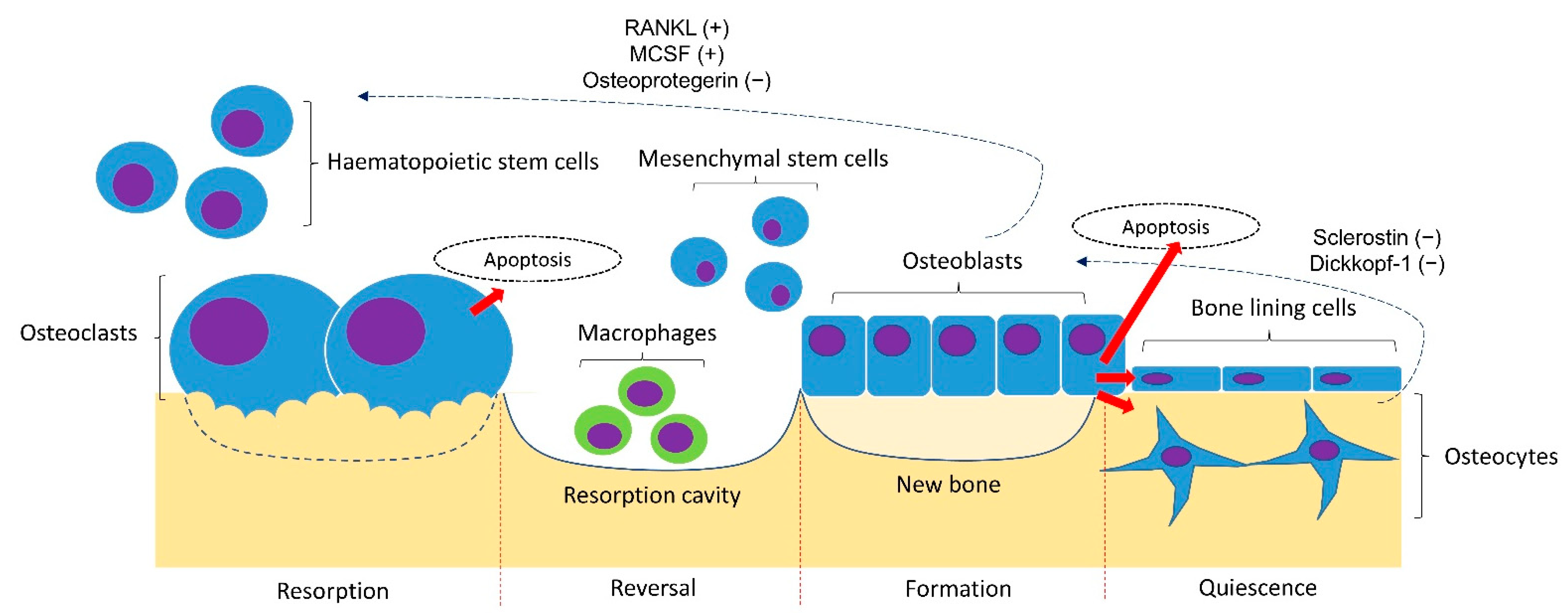
Figure 1. Bone remodelling cycle. The bone remodelling cycle is governed by osteoclasts, osteoblasts and osteocytes derived from the respective stem cell lineage. The differentiation of osteoclasts is stimulated by the receptor activator of nuclear factor kappa-B ligand (RANKL) and macrophage colony-stimulating factor (MSCF) and inhibited by osteoprotegerin (OPG) synthesised by osteoblasts and osteocytes. The osteogenesis of osteoblasts is inhibited by sclerostin and Dickkopf-1 synthesised by osteoblasts. Notes: +, promoting factor; −, inhibiting factor.
The bone remodelling process is coordinated delicately to maintain bone mineral homeostasis and strength. The differentiation of osteoclasts is stimulated by the receptor activator of the nuclear factor kappa-B (NF-kB) ligand (RANKL) and macrophage colony-stimulating factor (MSCF), and inhibited by osteoprotegerin (OPG) synthesised by osteoblasts and osteocytes [13]. The osteocytes synthesised sclerostin and Dickkopf-1 that inhibits the Wnt signalling pathway and osteogenesis by osteoblasts [14]. Bone loss occurs when the rate of bone formation is lower than bone resorption [15].
Many factors can influence the bone remodelling process, skewing it towards a catabolic direction. Estrogen deficiency due to menopause-associated cessation of ovarian function is a well-established cause of bone loss [16]. Recent studies showed that bone loss begins during the menopause transition due to the increase in circulating follicle-stimulating hormone [17]. Estrogen deficiency may even explain age-related bone loss in elderly men since androgens are converted to estrogens via the aromatase enzyme and exert bone protective effects [18][19]. The effects of estrogen deficiency have been replicated consistently in female castrated animals. The effects of estrogen deficiency on bone loss are mediated by the direct modulation of osteoblast, osteoclast, and osteocyte physiology via estrogen receptors on these cells. In particular, estrogen deficiency increases osteoclasts’ differentiation and survival, and causes the opposite effects on osteoblasts and osteocytes [20]. Estrogen deficiency is also linked to increased inflammation and oxidative stress, which further promotes bone loss [21]. Epidemiological studies have shown that estrogen deficiency is associated with increased pro-inflammatory cytokine production by peripheral blood mononuclear cells in women without comorbidities [22].
Recent studies have placed T cells (CD4+) in the central role of inflammation-induced osteoporosis. In particular, Th17 cells secrete several proinflammatory cytokines, such as interleukin (IL)-1, IL-6, IL-17, RANKL and tumour necrosis factor (TNF) and interferon (IFN)-γ, which are pro-osteoclastogenesis. Th17 also facilitates the secretion of RANKL by osteoblasts and osteocytes to support osteoclastogenesis [23]. Regulatory T (Treg) cells, which express transcription factor FOXP3 and are responsible for preventing excessive immune reactions and inflammation, have been shown to have an anti-osteoclastogenic role [24]. In a study, ovariectomised FoxP3-transgenic mice have been reported to be protected from bone loss, and the transfer of Treg to T cell-deficient RAG-1−/− mice improves the bone mass of these mice [25]. These findings showed the importance of Treg in suppressing bone loss independent of other T-cells. Another study showed that IL-15 produced by dendritic cells is critical in activating the synthesis of IL-17A and TNF-α by memory T cells, and contributes to bone loss in ovariectomized mice [26]. Recent studies also revealed that a unique subset of CD4 + CD28− T-cells have higher pro-inflammatory and pro-osteoclastogenic properties than the usual CD28+ T-cells [27]. Apart from T cells, B cells abundantly found in the bone marrow are a significant source of OPG, RANKL and MCSF that regulate osteoclastogenesis [28].
Recent studies also have unveiled the relationship between the gut microbiome and bone health. Compared to normal individuals, patients with osteoporosis show an increased abundance of Actinomyces, Eggerthella, Clostridium Cluster XlVa and Lactobacillus [29]. The gut microbiome could regulate bone remodelling through several mechanisms, such as modulating the activation of lymphocytes and inflammation, influencing hormone and nitric oxide levels, altering the metabolism of vitamin D and calcium absorption, as well as regulating the intestinal-brain axis [30]. Of note, the immune system plays an important role in mediating the gut-bone axis. For instance, lymphocyte-deficient mice did not experience bone loss due to antibiotic-induced dysbiosis [31]. Gut dysbiosis also disrupts the synthesis of anti-inflammatory short-chain fatty acids such as butyrate [32]. Gut dysbiosis also increases intestinal permeability and circulating lipopolysaccharide (LPS) levels [33]. Apart from immune cells, LPS has been shown to stimulate the release of pro-inflammatory cytokines from osteoblasts and fibroblasts [34]. All these changes could induce bone loss.
Oxidative stress represents another significant cause of osteoporosis. Free radicals are generated via aerobic respiration in the body. Under physiological conditions, the antioxidant system protects our body from the harmful effects of free radicals/oxidants. Oxidative stress is generated when the antioxidant system is overwhelmed by these oxidants, leading to the pathogenesis of various diseases, including osteoporosis [35]. The circulating level of endogenous antioxidants, such as uric acid and bilirubin, has been associated with bone mineral density (BMD) in large epidemiological studies [36][37]. Similarly, dietary or circulating antioxidant levels, for example, vitamin E and vitamin C, have been linked positively with BMD in human studies [38][39]. Experimental studies have shown that many risk behaviours for osteoporosis, such as cigarette smoking and alcohol consumption, are linked to increased oxidative stress [40][41]. These associations are contributed by the direct impact of oxidative stress on the physiology of bone cells. Oxidative stress is known to decrease the survival of osteoblasts and osteocytes and increase the differentiation of osteoclasts [42][43].
References
- World Health Organisation. GHE: Life Expectancy and Healthy Life Expectancy. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-life-expectancy-and-healthy-life-expectancy (accessed on 28 September 2022).
- Niccoli, T.; Partridge, L. Ageing as a Risk Factor for Disease. Curr. Biol. 2012, 22, R741–R752.
- Berger, C.; Goltzman, D.; Langsetmo, L.; Joseph, L.; Jackson, S.; Kreiger, N.; Tenenhouse, A.; Davison, K.S.; Josse, R.G.; Prior, J.C.; et al. Peak bone mass from longitudinal data: Implications for the prevalence, pathophysiology, and diagnosis of osteoporosis. J. Bone Miner. Res. 2010, 25, 1948–1957.
- Specker, B.L.; Wey, H.E.; Smith, E.P. Rates of bone loss in young adult males. Int. J. Clin. Rheumtol. 2010, 5, 215–228.
- Alswat, K.A. Gender Disparities in Osteoporosis. J. Clin. Med. Res. 2017, 9, 382–387.
- Lorentzon, M.; Cummings, S.R. Osteoporosis: The evolution of a diagnosis. J. Intern. Med. 2015, 277, 650–661.
- Mitchell, P.J.; Chan, D.D.; Lee, J.K.; Tabu, I.; Alpuerto, B.B. The global burden of fragility fractures—what are the differences, and where are the gaps. Best Pract. Res. Clin. Rheumatol. 2022, 101777.
- Hachuła, M.; Pietrzyk, B.; Gruszka, W.; Cedrych, I.; Chudek, J. High rates of undiagnosed and untreated osteoporosis in postmenopausal women receiving medical services in the area of Upper Silesia. Prz. Menopauzalny 2020, 19, 72–79.
- Clarke, B.L.; Khosla, S. Physiology of bone loss. Radiol. Clin. N. Am. 2010, 48, 483–495.
- Ma, Q.; Liang, M.; Wu, Y.; Luo, F.; Ma, Z.; Dong, S.; Xu, J.; Dou, C. Osteoclast-derived apoptotic bodies couple bone resorption and formation in bone remodeling. Bone Res. 2021, 9, 5.
- Xiao, W.; Wang, Y.; Pacios, S.; Li, S.; Graves, D.T. Cellular and Molecular Aspects of Bone Remodeling. Front. Oral Biol. 2016, 18, 9–16.
- Prideaux, M.; Findlay, D.M.; Atkins, G.J. Osteocytes: The master cells in bone remodelling. Curr. Opin. Pharmacol 2016, 28, 24–30.
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG Pathway: A Mechanism Involved in Exercise-Induced Bone Remodeling. Biomed. Res. Int. 2020, 2020, 6910312.
- Chin, K.Y.; Ekeuku, S.O.; Pang, K.L. Sclerostin in the development of osteoarthritis: A mini review. Malays J. Pathol 2022, 44, 1–18.
- Siddiqui, J.A.; Partridge, N.C. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology 2016, 31, 233–245.
- Ji, M.X.; Yu, Q. Primary osteoporosis in postmenopausal women. Chronic Dis. Transl Med. 2015, 1, 9–13.
- Chin, K.Y. The Relationship between Follicle-stimulating Hormone and Bone Health: Alternative Explanation for Bone Loss beyond Oestrogen? Int. J. Med. Sci. 2018, 15, 1373–1383.
- Chin, K.Y.; Ima-Nirwana, S. Sex steroids and bone health status in men. Int. J. Endocrinol. 2012, 2012, 208719.
- Mohamad, N.V.; Soelaiman, I.N.; Chin, K.Y. A concise review of testosterone and bone health. Clin. Interv. Aging 2016, 11, 1317–1324.
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581.
- Mohamad, N.V.; Ima-Nirwana, S.; Chin, K.Y. Are Oxidative Stress and Inflammation Mediators of Bone Loss Due to Estrogen Deficiency? A Review of Current Evidence. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1478–1487.
- Kim, O.Y.; Chae, J.S.; Paik, J.K.; Seo, H.S.; Jang, Y.; Cavaillon, J.M.; Lee, J.H. Effects of aging and menopause on serum interleukin-6 levels and peripheral blood mononuclear cell cytokine production in healthy nonobese women. Age 2012, 34, 415–425.
- Srivastava, R.K.; Dar, H.Y.; Mishra, P.K. Immunoporosis: Immunology of Osteoporosis-Role of T Cells. Front. Immunol. 2018, 9, 657.
- Bozec, A.; Zaiss, M.M. T Regulatory Cells in Bone Remodelling. Curr. Osteoporos. Rep. 2017, 15, 121–125.
- Zaiss, M.M.; Sarter, K.; Hess, A.; Engelke, K.; Böhm, C.; Nimmerjahn, F.; Voll, R.; Schett, G.; David, J.P. Increased bone density and resistance to ovariectomy-induced bone loss in FoxP3-transgenic mice based on impaired osteoclast differentiation. Arthritis Rheum 2010, 62, 2328–2338.
- Cline-Smith, A.; Axelbaum, A.; Shashkova, E.; Chakraborty, M.; Sanford, J.; Panesar, P.; Peterson, M.; Cox, L.; Baldan, A.; Veis, D.; et al. Ovariectomy Activates Chronic Low-Grade Inflammation Mediated by Memory T Cells, Which Promotes Osteoporosis in Mice. J. Bone Miner. Res. 2020, 35, 1174–1187.
- Fessler, J.; Husic, R.; Schwetz, V.; Lerchbaum, E.; Aberer, F.; Fasching, P.; Ficjan, A.; Obermayer-Pietsch, B.; Duftner, C.; Graninger, W.; et al. Senescent T-Cells Promote Bone Loss in Rheumatoid Arthritis. Front. Immunol. 2018, 9, 95.
- Pietschmann, P.; Mechtcheriakova, D.; Meshcheryakova, A.; Föger-Samwald, U.; Ellinger, I. Immunology of Osteoporosis: A Mini-Review. Gerontology 2016, 62, 128–137.
- Das, M.; Cronin, O.; Keohane, D.M.; Cormac, E.M.; Nugent, H.; Nugent, M.; Molloy, C.; O’Toole, P.W.; Shanahan, F.; Molloy, M.G.; et al. Gut microbiota alterations associated with reduced bone mineral density in older adults. Rheumatology 2019, 58, 2295–2304.
- Ding, K.; Hua, F.; Ding, W. Gut Microbiome and Osteoporosis. Aging Dis. 2020, 11, 438–447.
- Rios-Arce, N.D.; Schepper, J.D.; Dagenais, A.; Schaefer, L.; Daly-Seiler, C.S.; Gardinier, J.D.; Britton, R.A.; McCabe, L.R.; Parameswaran, N. Post-antibiotic gut dysbiosis-induced trabecular bone loss is dependent on lymphocytes. Bone 2020, 134, 115269.
- Chen, J.; Vitetta, L. The Role of Butyrate in Attenuating Pathobiont-Induced Hyperinflammation. Immune Netw. 2020, 20, e15.
- Wang, J.; Gu, X.; Yang, J.; Wei, Y.; Zhao, Y. Gut Microbiota Dysbiosis and Increased Plasma LPS and TMAO Levels in Patients With Preeclampsia. Front. Cell. Infect. Microbiol. 2019, 9, 409.
- Turner, J.D.; Naylor, A.J.; Buckley, C.; Filer, A.; Tak, P.-P. Fibroblasts and Osteoblasts in Inflammation and Bone Damage. In Stromal Immunology; Owens, B.M.J., Lakins, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 37–54.
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763.
- Lee, Y.J.; Hong, J.Y.; Kim, S.C.; Joo, J.K.; Na, Y.J.; Lee, K.S. The association between oxidative stress and bone mineral density according to menopausal status of Korean women. Obstet. Gynecol. Sci. 2015, 58, 46–52.
- Wu, J.; Su, J.; Wang, Y.; Chen, J.; Shang, Y.; Li, J. Association between total bilirubin and bone mineral density level in adolescents. BMC Musculoskelet. Disord. 2022, 23, 639.
- Chin, K.Y.; Ima-Nirwana, S. The effects of alpha-tocopherol on bone: A double-edged sword? Nutrients 2014, 6, 1424–1441.
- Chin, K.Y.; Ima-Nirwana, S. Vitamin C and Bone Health: Evidence from Cell, Animal and Human Studies. Curr. Drug Targets 2018, 19, 439–450.
- Maurel, D.B.; Boisseau, N.; Benhamou, C.L.; Jaffre, C. Alcohol and bone: Review of dose effects and mechanisms. Osteoporos. Int. 2012, 23, 1–16.
- Al-Bashaireh, A.M.; Haddad, L.G.; Weaver, M.; Chengguo, X.; Kelly, D.L.; Yoon, S. The Effect of Tobacco Smoking on Bone Mass: An Overview of Pathophysiologic Mechanisms. J. Osteoporos. 2018, 2018, 1206235.
- Agidigbi, T.S.; Kim, C. Reactive Oxygen Species in Osteoclast Differentiation and Possible Pharmaceutical Targets of ROS-Mediated Osteoclast Diseases. Int. J. Mol. Sci. 2019, 20, 3576.
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216.
More
Information
Subjects:
Endocrinology & Metabolism
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
4.8K
Revisions:
2 times
(View History)
Update Date:
15 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




