
Video Upload Options
Infectious diseases remain the most serious public health issue, which requires the development of more effective strategies for infectious control. As a kind of ultra-trace element, cobalt is essential to the metabolism of different organisms. Nanotechnology has attracted increasing attention worldwide due to its wide application in different areas, including medicine. Based on the important biological roles of cobalt, cobalt nanomaterials have recently been widely developed for their attractive biomedical applications. With advantages such as low costs in preparation, hypotoxicity, photothermal conversion abilities, and high drug loading ability, cobalt nanomaterials have been proven to show promising potential in anticancer and anti-infection treatment.
1. The Biological Activity of the Cobalt Element
1.1. Cobalt Is the Core Element of VB12
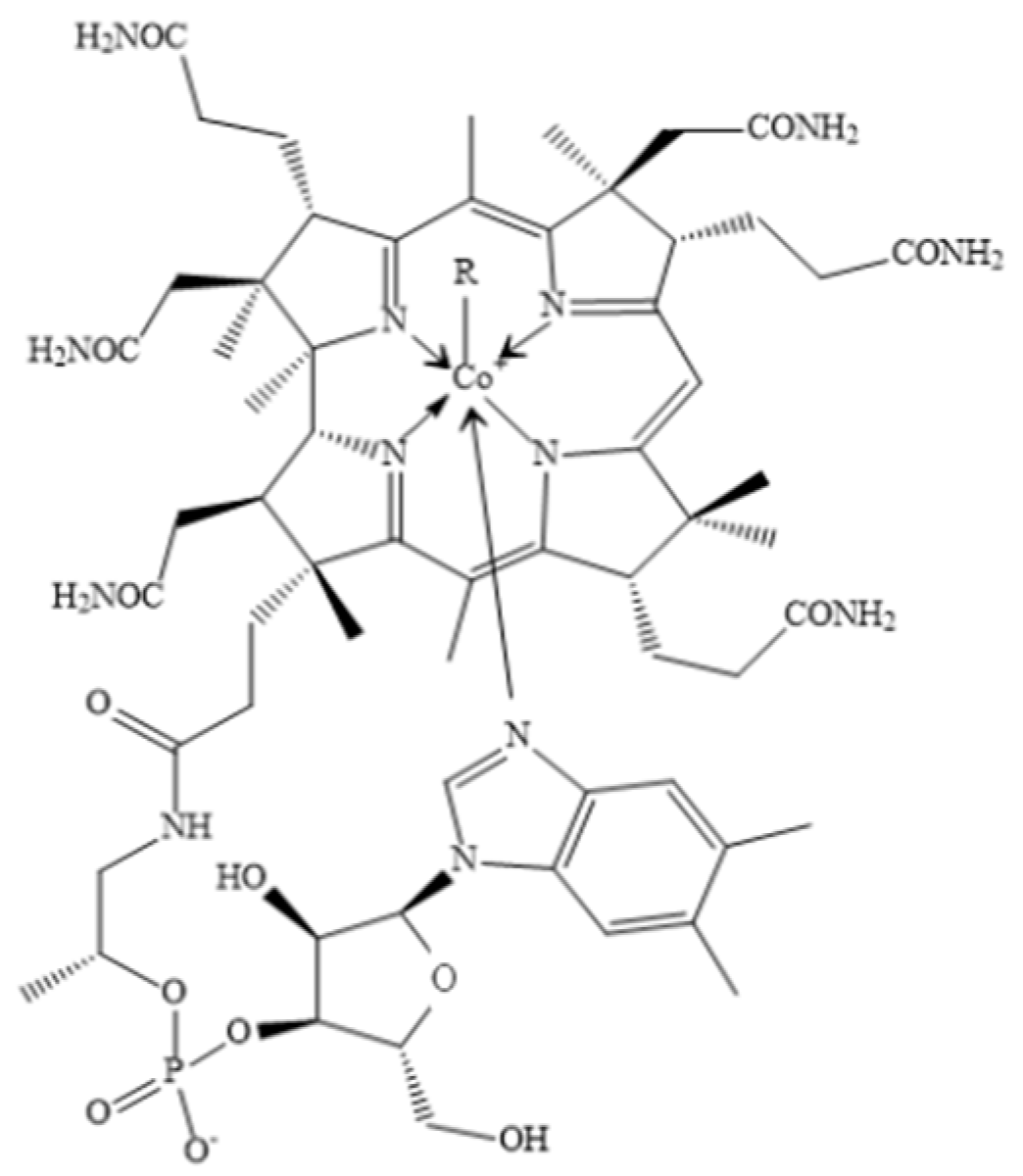
1.2. Physiological Function of Cobalt Based on Their Critical Roles in VB12
1.3. The Physiological Function of the Cobalt in Hematopoiesis
1.4. The Anti-Infective Activity of Cobalt
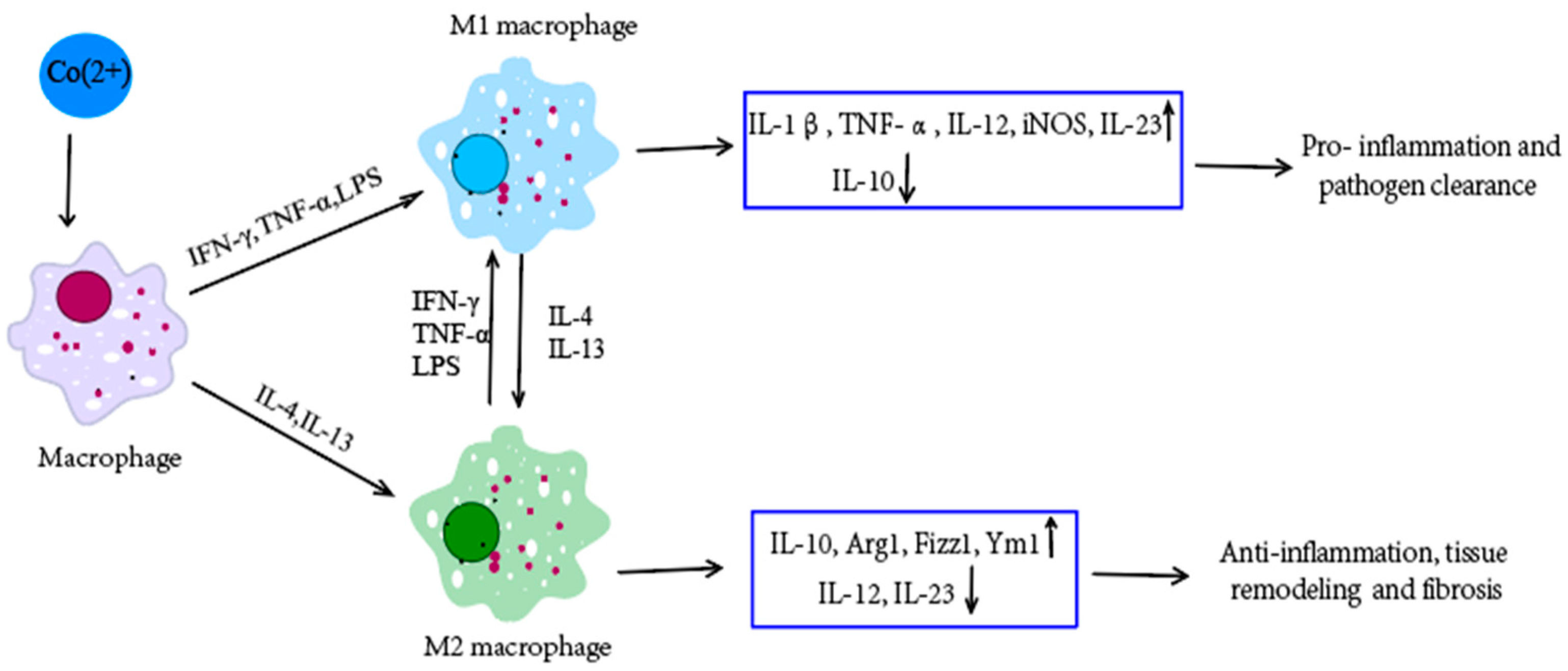
1.5. The Immunoregulatory Role of Cobalt
2. The Synthesis of Cobalt Nanomaterials
3. The Characteristics of Cobalt Nanomaterials
References
- Hu, X.; Wei, X.; Ling, J.; Chen, J. Cobalt: An Essential Micronutrient for Plant Growth? Front. Plant. Sci. 2021, 12, 768523.
- Ammendola, S.; Ciavardelli, D.; Consalvo, A.; Battistoni, A. Cobalt can fully recover the phenotypes related to zinc deficiency in Salmonella Typhimurium. Metallomics 2020, 12, 2021–2031.
- Langan, R.C.; Goodbred, A.J. Vitamin B12 Deficiency: Recognition and Management. Am. Fam. Physician 2017, 96, 384–389.
- Roth, W.; Mohamadzadeh, M. Vitamin B12 and gut-brain homeostasis in the pathophysiology of ischemic stroke. EBioMedicine 2021, 73, 103676.
- Obeid, R.; Heil, S.G.; Verhoeven, M.M.A.; van den Heuvel, E.; de Groot, L.; Eussen, S. Vitamin B12 Intake From Animal Foods, Biomarkers, and Health Aspects. Front. Nutr. 2019, 6, 93.
- Fang, H.; Kang, J.; Zhang, D. Microbial production of vitamin B(12): A review and future perspectives. Microb. Cell Fact. 2017, 16, 15.
- Weerathilake, W.; Brassington, A.H.; Williams, S.J.; Kwong, W.Y.; Sinclair, L.A.; Sinclair, K.D. Added dietary cobalt or vitamin B12, or injecting vitamin B12 does not improve performance or indicators of ketosis in pre- and post-partum Holstein-Friesian dairy cows. Animal 2019, 13, 750–759.
- Allen, L.H.; Miller, J.W.; de Groot, L.; Rosenberg, I.H.; Smith, A.D.; Refsum, H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND): Vitamin B-12 Review. J. Nutr. 2018, 148, 1995S–2027S.
- Guéant, J.L.; Guéant-Rodriguez, R.M.; Alpers, D.H. Vitamin B12 absorption and malabsorption. Vitam. Horm. 2022, 119, 241–274.
- Sugihara, T.; Koda, M.; Okamoto, T.; Miyoshi, K.; Matono, T.; Oyama, K.; Hosho, K.; Okano, J.I.; Isomoto, H.; Murawaki, Y. Falsely Elevated Serum Vitamin B(12) Levels Were Associated with the Severity and Prognosis of Chronic Viral Liver Disease. Yonago Acta Med. 2017, 60, 31–39.
- Dror, D.K.; Allen, L.H. Vitamin B-12 in Human Milk: A Systematic Review. Adv. Nutr. 2018, 9, 358S–366S.
- Wolffenbuttel, B.H.R.; Wouters, H.; Heiner-Fokkema, M.R.; van der Klauw, M.M. The Many Faces of Cobalamin (Vitamin B12) Deficiency. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 200–214.
- Wang, H.; Li, L.; Qin, L.L.; Song, Y.; Vidal-Alaball, J.; Liu, T.H. Oral vitamin B(12) versus intramuscular vitamin B(12) for vitamin B(12) deficiency. Cochrane Database Syst. Rev. 2018, 3, Cd004655.
- Batista, K.S.; Cintra, V.M.; Lucena, P.A.F.; Manhaes-de-Castro, R.; Toscano, A.E.; Costa, L.P.; Queiroz, M.; de Andrade, S.M.; Guzman-Quevedo, O.; Aquino, J.S. The role of vitamin B12 in viral infections: A comprehensive review of its relationship with the muscle-gut-brain axis and implications for SARS-CoV-2 infection. Nutr. Rev. 2022, 80, 561–578.
- Del Bo, C.; Riso, P.; Gardana, C.; Brusamolino, A.; Battezzati, A.; Ciappellano, S. Effect of two different sublingual dosages of vitamin B(12) on cobalamin nutritional status in vegans and vegetarians with a marginal deficiency: A randomized controlled trial. Clin. Nutr. 2019, 38, 575–583.
- Green, R. Vitamin B12 deficiency from the perspective of a practicing hematologist. Blood 2017, 129, 2603–2611.
- Chen, Y.; Huang, H.; He, X.; Duan, W.; Mo, X. Sex differences in the link between blood cobalt concentrations and insulin resistance in adults without diabetes. Environ. Health Prev. Med. 2021, 26, 42.
- Danzeisen, R.; Williams, D.L.; Viegas, V.; Dourson, M.; Verberckmoes, S.; Burzlaff, A. Bioelution, Bioavailability, and Toxicity of Cobalt Compounds Correlate. Toxicol. Sci. 2020, 174, 311–325.
- Tjong, E.; Dimri, M.; Mohiuddin, S.S. Biochemistry, Tetrahydrofolate. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Chen, R.; Jiang, T.; She, Y.; Xu, J.; Li, C.; Zhou, S.; Shen, H.; Shi, H.; Liu, S. Effects of Cobalt Chloride, a Hypoxia-Mimetic Agent, on Autophagy and Atrophy in Skeletal C2C12 Myotubes. BioMed Res. Int. 2017, 2017, 7097580.
- Francis, W.R.; Liu, Z.; Owens, S.E.; Wang, X.; Xue, H.; Lord, A.; Kanamarlapudi, V.; Xia, Z. Role of hypoxia inducible factor 1alpha in cobalt nanoparticle induced cytotoxicity of human THP-1 macrophages. Biomater. Transl. 2021, 2, 143–150.
- Skalny, A.V.; Zaitseva, I.P.; Gluhcheva, Y.G.; Skalny, A.A.; Achkasov, E.E.; Skalnaya, M.G.; Tinkov, A.A. Cobalt in athletes: Hypoxia and doping—New crossroads. J. Appl. Biomed. 2019, 17, 28.
- Ssempijja, F.; Iceland Kasozi, K.; Daniel Eze, E.; Tamale, A.; Ewuzie, S.A.; Matama, K.; Ekou, J.; Bogere, P.; Mujinya, R.; Musoke, G.H.; et al. Consumption of Raw Herbal Medicines Is Associated with Major Public Health Risks amongst Ugandans. J. Environ. Public Health 2020, 2020, 8516105.
- Abass, A.A.; Abdulridha, W.A.M.; Alaarage, W.K.; Abdulrudha, N.H.; Haider, J. Evaluating the antibacterial effect of cobalt nanoparticles against multi-drug resistant pathogens. J. Med. Life 2021, 14, 823–833.
- Kumar, R.; Badogu, K.; Kour, K.; Farooq, S.; Singh, R. Hydrogel-Nanofiber Composites for Tissue Reconstruction Applications: A State of the Art Review. Encycl. Mater. Plast. Polym. 2022, 3, 306–316.
- Bhattacharjee, A.; Hassan, R.; Gupta, A.; Verma, M.; Murugan, P.A.; Sengupta, P.; Matheshwaran, S.; Manna, I.; Balani, K. Effect of Zn and Co Doping on Antibacterial Efficacy and Cytocompatibility of Spark Plasma Sintered Hydroxyapatite. J. Am. Ceram. Soc. 2020, 103, 4090–4100.
- Zhang, E.; Zhao, X.; Hu, J.; Wang, R.; Fu, S.; Qin, G. Antibacterial metals and alloys for potential biomedical implants. Bioact. Mater. 2021, 6, 2569–2612.
- Kumar, V.; Mishra, R.K.; Kaur, G.; Dutta, D. Cobalt and nickel impair DNA metabolism by the oxidative stress independent pathway. Metallomics 2017, 9, 1596–1609.
- Fernandes, L.P.; Silva, J.M.B.; Martins, D.O.S.; Santiago, M.B.; Martins, C.H.G.; Jardim, A.C.G.; Oliveira, G.S.; Pivatto, M.; Souza, R.A.C.; Franca, E.F.; et al. Fragmentation Study, Dual Anti-Bactericidal and Anti-Viral Effects and Molecular Docking of Cobalt(III) Complexes. Int. J. Mol. Sci. 2020, 21, 8355.
- Díez-Tercero, L.; Delgado, L.M.; Bosch-Rué, E.; Perez, R.A. Evaluation of the immunomodulatory effects of cobalt, copper and magnesium ions in a pro inflammatory environment. Sci. Rep. 2021, 11, 11707.
- Maggini, S.; Pierre, A.; Calder, P.C. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients 2018, 10, 1531.
- Wu, D.; Lewis, E.D.; Pae, M.; Meydani, S.N. Nutritional Modulation of Immune Function: Analysis of Evidence, Mechanisms, and Clinical Relevance. Front. Immunol. 2018, 9, 3160.
- Wang, C.; Zhang, R.; Wei, X.; Lv, M.; Jiang, Z. Metalloimmunology: The metal ion-controlled immunity. Adv. Immunol. 2020, 145, 187–241.
- Luo, Y.; Fu, Y.; Huang, Z.; Li, M. Transition metals and metal complexes in autophagy and diseases. J. Cell. Physiol. 2021, 236, 7144–7158.
- Yu, Y.; Li, W.; Ren, L.; Yang, C.; Li, D.; Han, X.; Sun, Y.; Lv, C.; Han, F. Inhibition of autophagy enhanced cobalt chloride-induced apoptosis in rat alveolar type II epithelial cells. Mol. Med. Rep. 2018, 18, 2124–2132.
- Salloum, Z.; Lehoux, E.A.; Harper, M.E.; Catelas, I. Effects of cobalt and chromium ions on glycolytic flux and the stabilization of hypoxia-inducible factor-1alpha in macrophages in vitro. J. Orthop. Res. 2021, 39, 112–120.
- Turecka, K.; Chylewska, A.; Rychłowski, M.; Zakrzewska, J.; Waleron, K. Antibacterial Activity of Co(III) Complexes with Diamine Chelate Ligands against a Broad Spectrum of Bacteria with a DNA Interaction Mechanism. Pharmaceutics 2021, 13, 946.
- Jonitz-Heincke, A.; Sellin, M.L.; Seyfarth, A.; Peters, K.; Mueller-Hilke, B.; Fiedler, T.; Bader, R.; Klinder, A. Analysis of Cellular Activity Short-Term Exposure to Cobalt and Chromium Ions in Mature Human Osteoblasts. Materials 2019, 12, 2771.
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt toxicity in humans—A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56.
- Dolomatov, S.; Sataeva, T.P.; Zukow, W. Modern aspects of regulatory, pathophysiological and toxic effects of cobalt ions during oral intake in the human body. Health Risk Anal. 2019, 2019, 161–174.
- Salloum, Z.; Lehoux, E.A.; Harper, M.E.; Catelas, I. Effects of cobalt and chromium ions on oxidative stress and energy metabolism in macrophages in vitro. J. Orthop. Res. 2018, 36, 3178–3187.
- Kassapidou, M.; Stenport, V.F.; Johansson, C.B.; Ostberg, A.K.; Johansson, P.H.; Hjalmarsson, L. Inflammatory Response to Cobalt-Chromium Alloys Fabricated With Different Techniques. J. Oral Maxillofac. Res. 2021, 12, e3.
- Xu, J.; Yang, J.; Nyga, A.; Ehteramyan, M.; Moraga, A.; Wu, Y.; Zeng, L.; Knight, M.M.; Shelton, J.C. Cobalt (II) ions and nanoparticles induce macrophage retention by ROS-mediated down-regulation of RhoA expression. Acta Biomater. 2018, 72, 434–446.
- Riedel, F.; Aparicio-Soto, M.; Curato, C.; Thierse, H.J.; Siewert, K.; Luch, A. Immunological Mechanisms of Metal Allergies and the Nickel-Specific TCR-pMHC Interface. Int. J. Environ. Res. Public Health 2021, 18, 10867.
- Riedel, F.; Aparicio-Soto, M.; Curato, C.; Munch, L.; Abbas, A.; Thierse, H.J.; Peitsch, W.K.; Luch, A.; Siewert, K. Unique and common TCR repertoire features of Ni2+-, Co2+-, and Pd2+-specific human CD154 + CD4+ T cells. Allergy 2022.
- Baskey, S.J.; Lehoux, E.A.; Catelas, I. Effects of cobalt and chromium ions on lymphocyte migration. J. Orthop. Res. 2017, 35, 916–924.
- Xu, J.; Nyga, A.; Li, W.; Zhang, X.; Gavara, N.; Knight, M.M.; Shelton, J. Cobalt ions stimulate a fibrotic response through matrix remodelling, fibroblast contraction and release of pro-fibrotic signals from macrophages. Eur. Cells Mater. 2018, 36, 142–155.
- Tsui, H.C.; Decaesteker, T.; Jonckheere, A.C.; Vande Velde, G.; Cremer, J.; Verbeken, E.; Hoet, P.H.M.; Nemery, B.; Vanoirbeek, J.A.J. Cobalt exposure via skin alters lung immune cells and enhances pulmonary responses to cobalt in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L641–L651.
- Esa, Y.A.; Sapawe, N. A short review on biosynthesis of cobalt metal nanoparticles. Mater. Today Proc. 2020, 31, 378–385.
- Vishwanath, R.; Negi, B. Conventional and green methods of synthesis of silver nanoparticles and their antimicrobial properties. Curr. Res. Green Sustain. Chem. 2021, 4, 100205.
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174.
- Vijayanandan, A.S.; Balakrishnan, R.M. Biosynthesis of cobalt oxide nanoparticles using endophytic fungus Aspergillus nidulans. J. Environ. Manag. 2018, 218, 442–450.
- Khusnuriyalova, A.; Caporali, M.; Hey-Hawkins, E.; Sinyashin, O.; Yakhvarov, D. Preparation of Cobalt Nanoparticles. Eur. J. Inorg. Chem. 2021, 2021, 3023–3047.
- Qasim, S.; Zafar, A.; Saif, M.S.; Ali, Z.; Nazar, M.; Waqas, M.; Haq, A.U.; Tariq, T.; Hassan, S.G.; Iqbal, F.; et al. Green synthesis of iron oxide nanorods using Withania coagulans extract improved photocatalytic degradation and antimicrobial activity. J. Photochem. Photobiol. B 2020, 204, 111784.
- Tripathi, D.; Modi, A.; Narayan, G.; Rai, S.P. Green and cost effective synthesis of silver nanoparticles from endangered medicinal plant Withania coagulans and their potential biomedical properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 152–164.
- Iravani, S.; Varma, R. Sustainable synthesis of cobalt and cobalt oxide nanoparticles and their catalytic and biomedical applications. Green Chem. 2020, 22, 2643–2661.
- Ahmad, S.; Munir, S.; Zeb, N.; Ullah, A.; Khan, B.; Ali, J.; Bilal, M.; Omer, M.; Alamzeb, M.; Salman, S.M.; et al. Green nanotechnology: A review on green synthesis of silver nanoparticles—An ecofriendly approach. Int. J. Nanomed. 2019, 14, 5087–5107.
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84.
- Singh, A.K. A review on plant extract-based route for synthesis of cobalt nanoparticles: Photocatalytic, electrochemical sensing and antibacterial applications. Curr. Res. Green Sustain. Chem. 2022, 5, 100270.
- Bibi, I.; Nazar, N.; Iqbal, M.; Kamal, S.; Nawaz, H.; Nouren, S.; Safa, Y.; Jilani, K.; Sultan, M.; Ata, S.; et al. Green and eco-friendly synthesis of cobalt-oxide nanoparticle: Characterization and photo-catalytic activity. Adv. Powder Technol. 2017, 28, 2035–2043.
- Krishna, P.G.; Chandra Mishra, P.; Naika, M.M.; Gadewar, M.; Ananthaswamy, P.P.; Rao, S.; Boselin Prabhu, S.R.; Yatish, K.V.; Nagendra, H.G.; Moustafa, M.; et al. Photocatalytic Activity Induced by Metal Nanoparticles Synthesized by Sustainable Approaches: A Comprehensive Review. Front. Chem. 2022, 10, 917831.
- Manzanares, D.; Ceña, V. Endocytosis: The Nanoparticle and Submicron Nanocompounds Gateway into the Cell. Pharmaceutics 2020, 12, 371.
- Jahan, S.T.; Sadat, S.M.A.; Walliser, M.; Haddadi, A. Targeted Therapeutic Nanoparticles: An Immense Promise to Fight against Cancer. J. Drug Deliv. 2017, 2017, 9090325.
- de la Harpe, K.M.; Kondiah, P.P.D.; Choonara, Y.E.; Marimuthu, T.; du Toit, L.C.; Pillay, V. The Hemocompatibility of Nanoparticles: A Review of Cell-Nanoparticle Interactions and Hemostasis. Cells 2019, 8, 1209.
- Janagam, D.R.; Wu, L.; Lowe, T.L. Nanoparticles for drug delivery to the anterior segment of the eye. Adv. Drug Deliv. Rev. 2017, 122, 31–64.
- Yuan, D.; He, H.; Wu, Y.; Fan, J.; Cao, Y. Physiologically Based Pharmacokinetic Modeling of Nanoparticles. J. Pharm. Sci. 2019, 108, 58–72.
- Dzulkharnien, N.S.F.; Rohani, R. A Review on Current Designation of Metallic Nanocomposite Hydrogel in Biomedical Applications. Nanomaterials 2022, 12, 1629.
- Lin, S.; Dong, J.; Zhang, B.; Yuan, Z.; Lu, C.; Han, P.; Xu, J.; Jia, L.; Wang, L. Synthesis of bifunctional fluorescent nanohybrids of carbon dots-copper nanoclusters via a facile method for Fe3+ and Tb3+ ratiometric detection. Anal. Methods 2021, 13, 3577–3584.
- Han, X.; Xu, K.; Taratula, O.; Farsad, K. Applications of nanoparticles in biomedical imaging. Nanoscale 2019, 11, 799–819.
- Islam, T.; Hasan, M.M.; Awal, A.; Nurunnabi, M.; Ahammad, A.J.S. Metal Nanoparticles for Electrochemical Sensing: Progress and Challenges in the Clinical Transition of Point-of-Care Testing. Molecules 2020, 25, 5787.
- Sondhi, P.; Maruf, M.H.U.; Stine, K.J. Nanomaterials for Biosensing Lipopolysaccharide. Biosensors 2019, 10, 2.
- Arikan, K.; Burhan, H.; Bayat, R.; Sen, F. Glucose nano biosensor with non-enzymatic excellent sensitivity prepared with nickel-cobalt nanocomposites on f-MWCNT. Chemosphere 2022, 291, 132720.
- Waris, A.; Din, M.; Ali, A.; Afridi, S.; Baset, A.; Khan, A.U.; Ali, M. Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review. Open Life Sci. 2021, 16, 14–30.
- Rozina; Ahmad, M.; Alruqi, M.; Zafar, M. Cleaner production of biodiesel from novel and non-edible seed oil of Chamaerops humilis using recyclable cobalt oxide nanoparticles: A contribution to resilient and sustainable world. J. Clean. Prod. 2022, 369, 133378.
- Maksoud, M.; El-Sayyad, G.S.; Ashour, A.H.; El-Batal, A.I.; Elsayed, M.A.; Gobara, M.; El-Khawaga, A.M.; Abdel-Khalek, E.K.; El-Okr, M.M. Antibacterial, antibiofilm, and photocatalytic activities of metals-substituted spinel cobalt ferrite nanoparticles. Microb. Pathog. 2019, 127, 144–158.
- Rauwel, E.; Al-Arag, S.; Salehi, H.; Amorim, C.O.; Cuisinier, F.; Guha, M.; Rosario, M.S.; Rauwel, P. Assessing Cobalt Metal Nanoparticles Uptake by Cancer Cells Using Live Raman Spectroscopy. Int. J. Nanomed. 2020, 15, 7051–7062.
- Khannanov, A.A.; Rossova, A.A.; Ignatyeva, K.A.; Ulakhovich, N.A.; Gerasimov, A.V.; Boldyrev, A.E.; Evtugyn, V.G.; Rogov, A.M.; Cherosov, M.A.; Gilmutdinov, I.F.; et al. Superparamagnetic cobalt nanoparticles in hyperbranched polyester polyol matrix with anti-protease activity. J. Magn. Magn. Mater. 2022, 547, 168808.
- Farkas, B.; Terranova, U.; de Leeuw, N.H. Binding modes of carboxylic acids on cobalt nanoparticles. Phys. Chem. Chem. Phys. 2020, 22, 985–996.
- Kong, I.C.; Ko, K.S.; Koh, D.C.; Chon, C.M. Comparative Effects of Particle Sizes of Cobalt Nanoparticles to Nine Biological Activities. Int. J. Mol. Sci. 2020, 21, 6767.
- Nagababu, U.; Shanmukha Kumar, J.V.; Rafi Shaik, M.; Sharaf, M.A.F. Facile synthesis, physiochemical characterization and bio evaluation of sulfadimidine capped cobalt nanoparticles. Saudi J. Biol. Sci. 2021, 28, 2168–2174.
- Shahzadi, T.; Zaib, M.; Riaz, T.; Shehzadi, S.; Abbasi, M.; Shahid, M. Synthesis of Eco-friendly Cobalt Nanoparticles Using Celosia argentea Plant Extract and Their Efficacy Studies as Antioxidant, Antibacterial, Hemolytic and Catalytical Agent. Arab. J. Sci. Eng. 2019, 44, 6435–6444.





