
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ekaterina Mikhailova | + 1940 word(s) | 1940 | 2020-12-03 08:01:46 | | | |
| 2 | Vicky Zhou | Meta information modification | 1940 | 2020-12-09 04:17:13 | | | | |
| 3 | Vicky Zhou | Meta information modification | 1940 | 2020-12-15 06:55:21 | | |
Video Upload Options
Elementary silver nanoparticles (AgNPs) are of great interest because of their various probable medical application. Due to their antimicrobial activity, low toxicity and inexpensive cost, the mechanism of silver nanoparticles biosynthesis in bacterial, fungal and plant cells is extremely exciting from the point of view of the different cellular compounds participation in it: proteins, enzymes, acids, etc.
1. Introduction
In the modern world, “green technologies” are gaining more and more popularity due to their effectiveness, non-toxicity, and “eco-friendly”. One of the directions of “green synthesis” is the production of elementary silver nanoparticles (AgNPs) for use in various areas of human activity, primarily in medicine. It should be noted that mankind has been familiar with the bactericidal effects of Ag+ ions from time immemorial. The presence of both silver ions and AgNPs was established by the research in the solution named “Holy water”, known from the beginning of the first Millennium as a protection tool against infection by microorganisms [1]. It is thanks to the silver ions and AgNP suspension that it can have a bactericidal, bacteriostatic, antiviral, and antifungal effect on a large number of pathogenic microorganisms, yeast fungi, and viruses. In addition, the inhibitory effect can sometimes be expressed even slightly stronger in comparison with penicillin, biomycin, and other “classic” antibiotics due to the resistance of many strains of microorganisms to antibiotics [2][3]. This circumstance, together with their low toxicity, almost complete absence of allergic reactions, and good tolerance, has made AgNPs a very popular survey object. Moreover, the high interest in silver nanoparticles over the past decades has allowed for not only the confirmation of their antibacterial activity, but also to discover new properties worthy of application.
Anticipating the story of the use of AgNPs with various properties used in practice, it is necessary to note the wide and diverse forms of nanoparticles obtained during their synthesis by both physico-chemical and biological methods. The variety of objects used for the synthesis of nanoparticles inevitably leads to a variety of AgNPs forms: these can be “nanowires”, tabular prisms, cubes, octahedra, and pyramids [4][5]. The different studies devoted to silver nanoparticles showed that the shape and size of the resulting AgNPs largely depended on experimental parameters such as temperature, concentration of the Ag(I) compound, pH solution, and in the case of biological synthesis, on the direct object used to produce AgNPs [5]. As the most striking characteristic of AgNPs, their shape also largely determines their properties including the material features that these nanoparticles are part of. Despite the huge number of publications devoted to AgNP biosynthesis in bacterial, fungal, and plant cells, a more detailed approach is required not only to the synthesis itself, but also to its mechanism, the participation of various cellular compounds: proteins, enzymes, acids, etc.
In addition, an important aspect to be considered in the future practical application of AgNPs are the interaction mechanism of nanoparticles directly with the cell, and the processes occurring inside it. This serious fact is extremely important, whereas the currently fashionable prefix “bio” should reflect not only the method of obtaining a practically significant substance, but also the application safety, especially used in medicine. In addition, considering silver nanoparticles as a potential medical agent, we should not forget about their potential toxicity. A large number of publications on this topic have shown that the toxic potential of nanoparticles is determined by factors such as size, shape, surface area, aggregation or agglomeration, and dose [6][7][8][9]. It is generally believed that easily ionized silver particles can affect the cell by the Trojan horse mechanism. Phagocytosis of AgNPs stimulates inflammatory signaling through the generation of reactive oxygen species (ROS) in macrophage cells, after which activated macrophage cells induce TNF-α secretion. Increased levels of TNF-α lead to cell membrane damage and apoptosis [10][11]. It should be noted that a number of studies have shown the toxicity of AgNPs (for example, in studies of rat hepatocytes and neuronal cells, mouse stem cells, and human lung epithelial cells) in relation to cells, and its absence (in studies of healthy mammalian cells) [12][13][14][15][16][17]. In this regard, the study of toxicity is extremely important using in vivo and in vitro assays as well as in silico models [18].
2. Mechanism of Silver Nanoparticles (AgNPs) Biosynthesis
Notwithstanding an incredibly large number of publications describing the AgNP synthesis via various groups of organisms such as bacteria, fungi, lichens, algae, and higher plants, the mechanism of this process still remains completely unexplored.
The AgNP synthesis results using a variety of microorganisms demonstrated that the process of AgNP formation can occur both inside and outside the cell. Extracellular synthesis involves the presence of proteins–enzymes present on the cell wall of bacteria and secreted proteins, thanks to which Ag+ is reduced to Ag0. It was shown that AgNP extracellular synthesis is typical for both Gram-positive bacteria genus Bacillus, in particular, for B. pumilus, B. persicus, and B. licheniformis, B. indicus and B. cecembensis as well as Planomicrobium sp., Streptomyces sp., Rhodococcus sp., and for Gram-negative bacteria such as Klebsiella pneumoniae, Escherichia coli, and Acinetobacter calcoaceticus [19][20][21][22][23][24][25]. This mechanism of synthesis has also been determined for a number of other microorganisms such as the fungi Rhizopus stolonifer, Aspergillus niger, Fusarium oxysporum, Fusarium sp., and A. flavus [26][27][28][29].
Nevertheless, the nanoparticle synthesis by microorganisms has been shown in a number of studies as intracellular. This mechanism is represented in Gram-negative bacteria and is associated with membrane proteins transporting the silver ions into the cell. For example, the intracellular nature of AgNP synthesis was established for Enterobacter cloacae by El-Baghdady et al. [30]. Similar results were demonstrated for Pseudomonas stutzeri [31]. This mechanism was also shown for Gram-positive bacteria Corynebacterium sp. [32] as well as for various bacteria of the genus Streptomyces [33]. Among the representatives of the fungi kingdom, acidophilus Verticillium sp. can be highlighted [34]. Moreover, some microorganisms are able to perform AgNP biosynthesis both intracellularly and extracellularly including Bacillus strain CS 11 and Proteus mirablis [35][36].
Whether AgNP biosynthesis is intracellular or extracellular, the fundamental factor in this process is enzymes. Most researchers agree that it has a leading role in the AgNP formation of NADH-dependent nitrate reductase, which acts as an electron shuttle, taking electrons from the nitrate molecule and transferring it to the metal ion for the formation of nanoparticles, which is clearly shown for F. oxysporum, Ps. aeruginosa, and others [37][38][39][40][41]. The supposed mechanism of this process in shown in Figure 1. The information about respiratory [42] and periplasmic nitrate reductases [43] is also in the literature. In some experiments, it was found that proteins and sugars of the cell wall, where the bioreduction process can occur, can participate in the silver ion grab [32][44]. In addition, it is believed that the presence of a carboxylate group on the bacterial cell surface, which causes its mostly negative charge, provides an electrostatic interaction between this group and positively charged silver ions, which helps capture silver ions [45].
Some amino acids such as arginine, aspartic acid, cysteine, glutamic acid, lysine, and methionine are also implicated in the reduction of silver ions or silver nanocrystals, which act as catalysts, producing a hydroxyl ion that reacts with reducing agents such as aldehyde [46][47]. It is shown by Graf et al. that peptides containing disulfide bonds can also participate in the reduction of Ag+ to Ag0 [48]. The reaction conditions also make an important contribution: for example, a high pH plays an important role in the subunit activation of the oxidoreductase enzyme, and promotes the conversion of tryptophan into a transition tryptophil radical, which gives electrons to silver ions and leads to a reduction to elementary silver [49][50]. An important peculiarity is the intensification of AgNP biosynthesis by light. This effect may be associated with the activation of reducing agents in the culture of the supernatant, which for their part, causes the release of electrons to reduce Ag+ to Ag0 nanoparticles [51][52][53]. The other hypothetical mechanism of nanoparticle synthesis is based on the fact that certain bacteria generate the trans-membrane proton gradient, which is broken down by the active symport of Na+ ions along with Ag ions from the extracellular environment [54][55]. Several silver-binding membrane proteins attract silver ions and by deriving energy from ATP hydrolysis, results in the uptake of silver ions inside the cells and initiate synthesis of AgNPs [55].
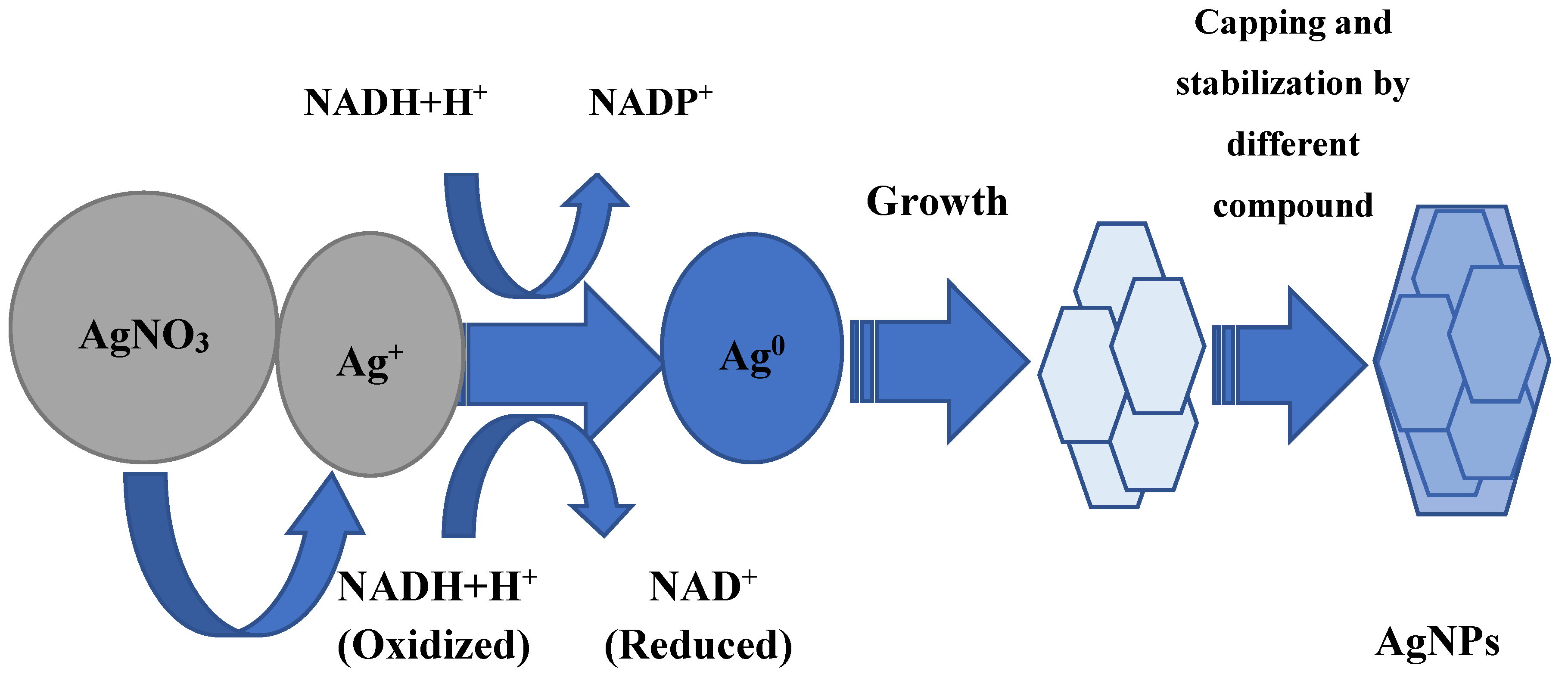
An interesting fact is that the production of silver nanoparticles is possible not only with the help of nitrate reductase, but also with a completely different class enzyme: extracellular keratinase B. safensis plays a crucial role in AgNP biosynthesis [56][57].
Fourier-transform infrared spectroscopy (FTIR) analysis is studied for synthesized AgNPs to find out the possible reducing bio-molecules that can stabilize nanoparticles, prevent agglomeration, and create their capping in an aqueous solution. It should be noted that the final packaging of microbial nanoparticles involves a very large number of different compounds. These can be peptides, enzymes, carboxylic acids, aldehydes, ketones, rhamnose sugar, and rhamnolipids [40][58][59][60][61][62][63][64][65][66][67][68][69][70][71]. It is assumed that the enzymes can bind to silver nanoparticles using free amino and cysteine groups of proteins.
The number of publications on AgNP synthesis with the assistance of various plant extracts (leaves, stems, roots, etc.) is incalculable. A wide diversity of plants including medicinal herbs are used as “factories” for the production of silver nanoparticles. The prospective mechanism of AgNP synthesis is generally similar to that of microorganisms and is enzymatic in nature. However, the compounds for the nanoparticles’ stabilization and final capping are different and specific from those for microorganisms, because plant cells contain a complex of diversified antioxidant metabolites preventing the oxidation and damage of cellular components [72]. Therefore, enzymes, glycosides, saponins, and other biomolecules can participate in the nanoparticle’s stabilization [73][74]. They are especially important in terms of further practical applications of AgNPs, seeing that they have anti-inflammatory, antioxidant, antitumor, and other effects [75][76]. The literature data indicate that when metal salts are added to the plant extract, silver ions bind to proteins and water-soluble compounds using –OH and –COOH groups, leading to conformational changes in the protein molecule, which contribute to the captured metal ion transformation into a silver nanoparticle [77][78]. In addition, amino groups and cysteine residues of proteins take part in the silver reduction process and the formation of AgNPs [79][80]. Alkanes, amines, phenols, polyphenols, arabinose and galactose, aldehydes and ketones, alcohols, alkaloids, lignans, terpenoids, and flavonoids can act as “capping” agents for the formation of silver nanoparticles [81][82][83][84][85][86][87].
Flavonoids are particularly interesting in this case due to their high antioxidant activity for medical purposes. Hydrophilic functional groups of various compounds surrounding nanoparticles make them colloid-stable in an aqueous medium [88]. Other interesting substances that act as reducing and stabilizing substances are the sucrose and fructose of garlic extract [89]. Furthermore, polyols are responsible for the reduction of Ag+ into silver nanoparticles in the Dioscorea bulbifera tuber extract [90]. It is supposed that terpenoids are surface-active molecules that adsorb on the AgNPs surface for stabilizing nanoparticles and preventing the AgNPs from agglomeration [91]. The reduction from Ag+ ions to silver nanoparticles (Ag0) with terpenoids may involve the conversion of C–O group of the terpenes to the –C–O group [92]. It is likely that the terpenoids play a role in the reduction of metal ions by the oxidation of aldehydic groups in the molecules to carboxylic acids [93]. Apparently “capping” agents have the possibility of selective binding to different types of facets on a nanocrystal to change their specific surface free energies and thus their area proportions [94]. Thus, nanoparticle “capping” can perform several important functions, namely prevent the agglomeration of nanoparticles, reduce toxicity, and improve antimicrobial properties; additionally these molecules can enhance the affiliation possibility and action of AgNPs on the bacterial cells. [95][96]. Remarkably, plant “capping” agents frequently have their own antimicrobial activity that can increase the activity of AgNPs.
References
- Henglein, A. Small-particle research: Physicochemical properties of extremely small colloidal metal and semiconductor particles. Chem. Rev. 1989, 89, 1861–1873.
- Shahverdy, A.R.; Fakhimi, A.; Minaian, S. Synthesis and effect of silver nanopracles on the antibacterial activity of different antibiotics against Staphylococcus and Escherichia coli. Nanomedicine 2007, 3, 168–171.
- Landsdown, A.B.G. Silver in Healthcare: Its Antimicrobial Efficacy and Safety in Use; Royal Society of Chemistry: Cambridge, UK, 2010; p. 217.
- Krutyakov, Y.A.; Kudrinskiy, A.A.; Olenin, A.Y.; Lisichkin, G.V. Synthesis and properties of silver nanoparticles: Advances and prospects. Russ. Chem. Rev. 2008, 77, 233–257.
- Mikhailov, O.V.; Mikhailova, E.O. Elemental silver nanoparticles. Biosynthesis and bio application. Materials 2019, 12, 3177.
- Karlsson, H.L.; Gustafsson, J.; Cronholm, P.; Möller, L. Size-dependent toxicity of metal oxide particles—A comparison between nano-and micrometer size. Toxicol. Lett. 2009, 188, 112–118.
- Zhao, X.; Lu, D.; Hao, F.; Liu, R. Exploring the diameter and surface dependent conformational changes in carbon nanotube-protein corona and the related cytotoxicity. J. Hazard. Mater. 2015, 292, 98–107.
- Abdelmonem, A.M. Charge and agglomeration dependent in vitro uptake and cytotoxicity of zinc oxide nanoparticles. J. Inorg. Biochem. 2015, 153, 334–338.
- Tiwari, D.K.; Jin, T.; Behari, J. Dose-dependent in-vivo toxicity assessment of silver nanoparticle in Wistar rats. Toxicol. Mech. Methods 2011, 21, 13–24.
- Park, E.-J. Silver nanoparticles induce cytotoxicity by a Trojan-horse type mechanism. Toxicol. Vitr. 2010, 24, 872–878.
- Luoma, S.N. Silver nanotechnologies and the environment. Proj. Emerg. Nanotechnol. Rep. 2008, 15, 12–13.
- Mohamed El Mahdy, M.; Salah, T.; Sayed Aly, H.; Mohammed, F.; Shaalan, M. Evaluation of hepatotoxic and genotoxic potential of silver nanoparticles in albino rats. Exp. Toxicol. Pathol. 2015, 67, 21–29.
- Pinzaru, I.; Coricovac, D.; Dehelean, C.; Moaca, E.A.; Mioc, M.; Baderca, F.; Sizemore, I.; Brittle, S.; Marti, D.; Calina, C.D.; et al. Stable peg-coated silver nanoparticles—A comprehensive toxicological profile. Food Chem. Toxicol. 2018, 111, 546–556.
- Vandebriel, R.J.; Tonk, E.C.M.; de la Fonteyne-Blankestijn, L.J.; Gremmer, E.R.; Verharen, H.W.; van der Ven, L.T.; van Loveren, H.; de Jong, W.H. Immunotoxicity of silver nanoparticles in an intravenous 28-day repeated-dose toxicity study in rats. Part. Fibre Toxicol. 2014, 11, 21.
- Boudreau, M.D.; Imam, M.S.; Paredes, A.M.; Bryant, M.S.; Cunningham, C.K.; Felton, R.P.; Jones, M.Y.; Davis, K.J.; Olson, G.R. Differential effects of silver nanoparticles and silver ions on tissue accumulation, distribution, and toxicity in the Sprague Dawley rat following daily oral gavage administration for 13 weeks. Toxicol. Sci. 2016, 150, 131–160.
- Stensberg, M.C.; Wei, Q.; McLamore, E.S.; Porterfield, D.M.; Wei, A.; Sepúlveda, M.S. Toxicological studies on silver nanoparticles: Challenges and opportunities in assessment, monitoring and imaging. Nanomedicine 2011, 6, 879–898.
- Burdus, C.; Gherasim, O.; Grumezescu, A.M.; Mogoanta, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681.
- Raies, A.B.; Bajic, V.B. In silico toxicology: Computational methods for the prediction of chemical toxicity. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2016, 6, 147–172.
- Elbeshehy, E.K.; Elazzazy, A.M.; Aggelis, G. Silver nanoparticles synthesis mediated by new isolates of Bacillus spp., nanoparticle characterization and their activity against bean yellow mosaic virus and human pathogens. Front. Microbiol. 2015, 6, 453.
- Mokhtari, N.; Daneshpajouh, S.; Seyedbagheri, S.; Atashdehghan, R.; Abdi, K.; Sarkar, S.; Minaian, S.; Shahverdi, H.R.; Shahverdi, A.R. Biological synthesis of very small silver nanoparticles by culture supernatant of Klebsiella pneumonia: The effects of visible-light irradiation and the liquid mixing process. Mater. Res. Bull. 2009, 44, 1415–1421.
- Chauhan, R.; Kumar, A.; Abraham, J. A biological approach to synthesis of silver nanoparticles with Streptomyces sp. JAR1 and its antimicrobial activity. Sci. Pharm. 2013, 81, 607–621.
- Otari, S.; Patil, R.; Nadaf, N.; Ghosh, S.; Pawar, S. Green biosynthesis of silver nanoparticles from an actinobacteria Rhodococcus sp. Mater. Lett. 2012, 72, 92–94.
- Natarajan, K.; Selvaraj, S.; Murty, V.R. Microbial production of silver nanoparticles. Dig. J. Nanomater. Biostr. 2010, 5, 135–140.
- Rajeshkumar, S.; Malarkodi, C. In Vitro Antibacterial Activity and Mechanism of Silver Nanoparticles against Foodborne Pathogens. Bioinorg. Chem. Appl. 2014, 4, 581890.
- Singh, R.; Wagh, P.; Wadhwani, S.; Gaidhani, S.; Kumbhar, A.; Bellare, J.; Chopade, B.A. Synthesis, optimization, and characterization of silver nanoparticles from Acinetobacter calcoaceticus and their enhanced antibacterial activity when combined with antibiotics. Int. J. Nanomed. 2013, 8, 4277–4290.
- Ninganagouda, S.; Rathod, V.; Singh, D.; Hiremath, J.; Singh, A.K.; Mathew, J.; Ul-Haq, M. Growth Kinetics and Mechanistic Action of Reactive Oxygen Species Released by Silver Nanoparticles from Aspergillus niger on Escherichia coli. Biomed. Res. Int. 2014, 2014, 753419.
- Rathod, V.; Banu, A.; Ranganath, E. Biosynthesis of highly stabilized silver nanoparticles by Rhizopus stolonifer and their Anti-fungal efficacy. Int. J. Mol. Clin. Microbiol. 2011, 1, 65–70.
- Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B Biointerfaces 2003, 28, 313–318.
- Abdel Hafez, E.H.; Ahmed, E.A.; Abbas, H.S.; Salah El Din, R.A. Efficacy of Antibiotics Combined with Biosynthesized Silver Nanoparticles on some Pathogenic Bacteria. IJSR 2017, 6, 1294–1303.
- El-Baghdady, K.Z.; El-Shatoury, E.H.; Abdullah, O.M.; Khalil, M.M.H. Biogenic production of silver nanoparticles by Enterobacter cloacae Ism26. Turk. J. Biol. 2018, 42, 319–328.
- Klaus, T.; Joerger, R.; Olsson, E.; Granqvist, C.-G. Silver-based crystalline nanoparticles, microbially fabricated. Proc. Natl. Acad. Sci. USA 1999, 96, 13611–13614.
- Zhang, H.; Li, Q.; Lu, Y.; Sun, D.; Lin, X.; Deng, X.; He, N.; Zheng, S. Biosorption and bioreduction of diamine silver complex by Corynebacterium. J. Chem. Technol. Biotechnol. 2005, 80, 285–290.
- Alani, F.; Moo-Young, M.; Anderson, W. Biosynthesis of silver nanoparticles by a new strain of Streptomyces sp. compared with Aspergillus fumigatus. World J. Microbiol. Biotechnol. 2012, 28, 1081–1086.
- Sastry, M.; Ahmad, A.; Islam, N.I.; Kumar, R. Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr. Sci. 2003, 85, 162–170.
- Das, V.L.; Thomas, R.; Varghese, R.T.; Soniya, E.V.; Mathew, J.; Radhakrishnan, E.K. Extracellular synthesis of silver nanoparticles by the Bacillus strain CS 11 isolated from industrialized area. 3 Biotech 2013, 4, 121–126.
- Samadi, N.; Golkaran, D.; Eslamifar, A.; Jamalifar, H.; Fazeli, M.R.; Mohseni, F.A. Intra/extracellular biosynthesis of silver nanoparticles by an autochthonous strain of Proteus mirabilis isolated from photographic waste. J. Biomed. Nanotechnol. 2009, 5, 247–253.
- Jeevan, P.; Ramya, K.; Edith Rena, A. Extracellular biosynthesis of silver nanoparticles by culture supernatant of Pseudomonas Aeruginosa. Indian J. Biotechnol. 2012, 11, 72–76.
- Cho, K.-H.; Park, J.-E.; Osaka, T.; Park, S.-G. The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim. Acta 2005, 51, 956–960.
- Ali, J.; Hameed, A.; Ahmed, S.; Ali, M.I.; Zainab, S.; Ali, N. Role of catalytic protein and stabilising agents in the transformation of Ag ions to nanoparticles by Pseudomonas aeruginosa. IET Nanobiotechnol. 2016, 10, 295–300.
- Kumar, S.A.; Abyaneh, M.K.; Gosavi, S.; Kulkarni, S.K.; Pasricha, R.; Ahmad, A.; Khan, M.I. Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. Biotechnol. Lett. 2007, 29, 439–445.
- Zomorodian, K.; Pourshahid, S.; Sadatsharifi, A. Biosynthesis and characterization of silver nanoparticles by Aspergillus species. Biomed Res. Int. 2016, 2016, 5435397.
- Deepak, V.; Kalishwaralal, K.; Pandian, S.R.K. An insight into the bacterial biogenesis of silver nanoparticles, industrial production and scale-up. In Metal Nanoparticles in Microbiology; Rai, M., Duran, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 17–35.
- Stewart, V.; Lu, Y.; Darwin, A.J. Periplasmic nitrate reductase (Napabc eEnzyme) supports anaerobic respiration by Escherichia coli K-12. J. Bacteriol. 2002, 184, 1314–1323.
- Gordon, O.; Vig Slenters, T.N.; Brunetto, P.S.; Villaruz, A.E.; Sturdevant, D.E.; Otto, M.; Landmann, R.; Fromm, K.M. Silver coordination polymers for prevention of implant infection: Thiol interaction, impact on respiratory chain enzymes, and hydroxyl radical induction. Antimicrob. Agents Chemother. 2009, 54, 4208–4218.
- Wang, F.; Liu, B.; Huang, P.J.; Liu, J. Rationally designed nucleobase and nucleotide coordinated nanoparticles for selective DNA adsorption and detection. Anal. Chem. 2013, 85, 12144–12151.
- Naik, R.R.; Stringer, S.J.; Agarwal, G.; Jones, S.E.; Stone, M.O. Biomimetic synthesis and patterning of silver nanoparticles. Nat. Mater. 2002, 1, 169–172.
- Nam, H.Y.; Hahn, H.J.; Nam, K.; Choi, W.H.; Jeong, Y.; Kim, D.E.; Park, J.S. Evaluation of generations 2, 3 and 4 arginine modified PAMAM dendrimers for gene delivery. Int. J. Pharm. 2008, 363, 199–205.
- Graf, P.; Mantion, A.; Foelske, A.; Shkilnyy, A.; Masic, A.; Thunemann, A.F.; Taubert, A. Peptide-coated silver nanoparticles: Synthesis, surface chemistry, and pH-triggered, reversible assembly into particle assemblies. Chemistry 2009, 15, 5831–5844.
- Prasad, G.K.; Ramacharyulu, P.V.; Merwyn, S.; Agarwal, G.S.; Srivastava, A.R.; Singh, B.; Rai, G.P.; Vijayaraghavan, R. Photocatalytic inactivation of spores of Bacillus anthracis using titania nanomaterials. J. Hazard. Mater. 2010, 185, 977–982.
- Si, S.; Mandal, T.K. Tryptophan-based peptides to synthesize gold and silver nanoparticles: A mechanistic and kinetic study. Chemistry 2007, 13, 3160–3168.
- Nam, K.T.; Lee, Y.J.; Krauland, E.M.; Kottmann, S.T.; Belcher, A.M. Peptide-mediated reduction of silver ions on engineered biological scaffold. ACS Nano 2008, 2, 1480–1486.
- Wei, X.; Luo, M.; Li, W.; Yang, L.; Liang, X.; Xu, L. Bioresource technology synthesis of silver nanoparticles by solar irradiation of cell-free Bacillus amyloliquefaciens extracts and AgNO3. Bioresour. Technol. 2012, 103, 273–278.
- Eby, D.M.; Luckarift, H.R.; Johnson, G.R. Hybrid antimicrobial enzyme and silver nanoparticle coatings for medical instruments. ACS Appl. Mater. Inter. 2009, 1, 1553–1560.
- Javaid, A.; Folarin Oloketuyi, S.; Khan, M.M.; Khan, F. Diversity of bacterial synthesis of silver nanoparticles. BioNanoScience 2018, 8, 43–59.
- Prakash, A.; Sharma, S.; Ahmad, N.; Ghosh, A.; Sinha, P. Synthesis of AgNPs by Bacillus cereus bacteria and their antimicrobial potential. JBNB 2011, 2, 155–161.
- Revathi, K.; Shaifali, S.; Mohd, A.K.; Suneetha, V. A potential strain of keratinolytic bacteria VIT RSAS2 from katpadi and its pharmacological benefits. Int. J. Pharm. Sci. Res. 2013, 20, 89–92.
- Lateef, A.; Adelere, I.A.; Gueguim-Kana, E.B.; Asafa, T.B.; Beukes, L.S. Green synthesis of silver nanoparticles using keratinase obtained from a strain of Bacillus safensis LAU 13 A. Int. Nano Lett. 2015, 5, 29–35.
- Saha, S.; Sarkar, J.; Chattopadhayay, D.; Patra, S.; Chakraborty, A.; Acharya, K. Production of silver nanoparticles by a phytopathogenic fungus Bipolaris nodulosa and its antimicrobial activity. Dig. J. Nanomater. Biostruct. 2010, 5, 887–895.
- Jain, N.; Bhargava, A.; Majumdar, S.; Tarafdar, J.C.; Panwar, J. Extracellular biosynthesis and characterization of silver nanoparticles using Aspergillus flavus NJP08: A mechanism perspective. Nanoscale 2011, 3, 635–641.
- Kumar, C.G.; Mamidyala, S.K.; Das, B.; Sridhar, B.; Sarala Devi, G.; Karuna, M.S. Synthesis of biosurfactant-based silver nanoparticles with purified rhamnolipids isolated from Pseudomonas aeruginosa Bs-161R. J. Microbiol. Biotechnol. 2010, 20, 1061–1068.
- Mandal, S.; Phadtare, S.; Sastry, M. Interfacing biology with nanoparticles. Curr. Appl. Phys. 2005, 5, 118–127.
- Jayalakashmi, S.; Dinakaran, S. Biosynthesis and characterization of silver nanoparticles by a marine strain Escherichia coli SJ101. Int. J. Innov. Res. 2013, 1, 1–35.
- Kumar, C.G.; Mamidyala, S.K. Extracellular synthesis of silver nanoparticles using culture supernatant of Pseudomonas aeruginosa. Colloids Surf. B Biointerfaces 2011, 84, 462–466.
- Parashar, U.K.; Kumar, V.; Bera, T.; Saxena, P.S.; Nath, G.; Srivastava, S.K.; Giri, R.; Srivastava, A. Study of mechanism of enhanced antibacterial activity by green synthesis of silver nanoparticles. Nanotechnology 2011, 22, 415104.
- Carmel Mary, R.; Panneerselvam, A.; Arokia Vijaya Anand, M.; Ernest, D. Mycosynthesized Silver Nanoparticles from marine derived Aspergillus terreus and its bactericidal activity against clinical pathogens. Int. J. S. Res. Sci. Tech. 2018, 4, 922–932.
- Abdel Ghany, T.M.; Kasem, W.T.; Nabih, M.A.; Mabrouk, A.S. Dual synergistic actions of silver nanoparticles with natural products on Ochratoxin A production. Life Sci. J. 2017, 14, 65–71.
- Paul, D.; Sinha, S.N. Extracellular synthesis of silver nanoparticles using Pseudomonas aeruginosa KUPSB12 and its antibacterial activity. JJBS 2014, 7, 245–250.
- Busi, S.; Rajkumari, J.; Ranjan, B.; Karuganti, S. Green rapid biogenic synthesis of bioactive silver nanoparticles (AgNPs) using Pseudomonas aeruginosa. IET Nanobiotechnol. 2014, 8, 267–274.
- Majeed, S.; Danish, M.; Binti Zahrudin, A.; Dash, G. Biosynthesis and characterization of silver nanoparticles from fungal species and its antibacterial and anticancer effect. Karbala Int. J. Mod. Sci. 2018, 4, 86–92.
- Sarsar, V.; Selwal, M.K.; Selwal, K.K. Biofabrication, characterization and antibacterial efficacy of extracellular silver nanoparticles using novel fungal strain of Penicillium atramentosum. J. Saudi Chem. Soc. 2015, 19, 682–688.
- Kumar, P.S.; Balachandran, C.; Duraipandiyan, V.; Ramasamy, D.; Ignacimuthu, S.; Al-Dhabi, N.A. Extracellular biosynthesis of silver nanoparticle using Streptomyces sp. 09 PBT 005 and its antibacterial and cytotoxic properties. Appl. Nanosci. 2015, 5, 169–180.
- Prasad, R. Synthesis of Silver Nanoparticles in Photosynthetic Plants. J. Nanoparticles 2014, 2014, 963961.
- Abd-Elsalam, K.A.; Prasad, R. Nanobiotechnology Applications in Plant Protection; Springer International Publishing: Basel, Switzerland, 2015; pp. 271–288.
- Tripathi, R.M.; Rana, D.; Shrivastava, A.; Singh, R.P.; Shrivastav, B.R. Biogenic synthesis of silver nanoparticles using Saraca indica leaf extract and evaluation of their antibacterial activity. Nano Biomed. Eng. 2013, 5, 50–56.
- Mashwani, Z.R.; Khan, T.; Khan, M.A.; Nadhman, A. Synthesis in plants and plant extracts of silver nanoparticles with potent antimicrobial properties: Current status and future prospects. Appl. Microbiol. Biotechnol. 2015, 99, 9923–9934.
- Bharathi, A.; Vigneshwaran, K.; Loganathan, K. Green synthesis of silver nanoparticles from Ficus racemosa. L extract and their antifungal activity. Nano Biomed. Eng. 2014, 10, 93–96.
- Huang, J.; Lin, L.; Sun, D.; Chen, H.; Yang, D.; Li, Q. Bio-inspired synthesis of metal nanomaterials and applications. Chem. Soc. Rev. 2015, 44, 6330–6374.
- Singh, P.; Pandit, S.; Beshay, M.; Mokkapati, V.R.S.S.; Garnaes, J.; Olsson, M.E.; Sultan, A.; Mackevica, A.; Mateiu, R.V.; Lütken, H.; et al. Anti-biofilm effects of gold and silver nanoparticles synthesized by the Rhodiola rosea rhizome extracts. Artif. Cells Nanomed. Biotechnol. 2018, 46, 886–899.
- Kasithevar, M.; Saravanan, M.; Prakash, P.; Kumar, H.; Ovais, M.; Barabadi, H.; Shinwari, Z.K. Green synthesis of silver nanoparticles using Alysicarpus monilifer leaf extract and its antibacterial activity against MRSA and CoNS isolates in HIV patients. J. Interdiscip. Nanomed. 2017, 2, 131–141.
- Benakashani, F.; Allafchian, A.R.; Jalali, S.A.H. Biosynthesis of silver nanoparticles using Capparis spinosa L. leaf extract and their antibacterial activity. Karbala Int. J. Mod. Sci. 2016, 2, 251–258.
- Mandal, P.; Babu, S.S.; Mandal, N. Antimicrobial activity of saponins from Acacia auriculiformis. Fitoterapia 2005, 76, 462–465.
- Ahmad, N.; Sharma, S. Green synthesis of silver nanoparticles using extracts of Ananas comosus. Green Sustain. Chem. 2012, 2, 141–147.
- Marimuthu, S.; Rahuman, A.A.; Rajakumar, G.; Santhoshkumar, T.; Kirthi, A.V.; Jayaseelan, C.; Bagavan, A.; Zahir, A.A.; Elango, G.; Kamaraj, C. Evaluation of green synthesized silver nanoparticles against parasites. Parasitol. Res. 2011, 108, 1541–1549.
- Elavazhagan, T.; Arunachalam, K.D. Memecylon edule leaf extract mediated green synthesis of silver and gold nanoparticles. Int. J. Nanomed. 2011, 6, 1265–1278.
- Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics 2014, 4, 316–335.
- Patra, S.; Mukherjee, S.; Barui, A.K.; Ganguly, A.; Sreedhar, B.; Patra, C.R. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater. Sci. Eng. C 2015, 53, 298–309.
- Khan, M.A.; Khan, T.; Nadhman, A. Applications of plant terpenoids in the synthesis of colloidal silver nanoparticles. Adv. Colloid Interface Sci. 2016, 234, 132–141.
- Ebrahiminezhad, A.; Taghizadeh, S.; Ghasemi, Y. Green synthesis of silver nanoparticles using mediterranean cypress (Cupressus sempervirens) Leaf Extract. Am. J. Biochem. Biotechnol. 2017, 13, 1–6.
- VonWhite, G.; Kerscher, P.; Brown, R.M.; Morella, J.D.; McAllister, W.; Dean, D.; Kitchens, C.L. Green Synthesis of Robust, Biocompatible Silver Nanoparticles Using Garlic Extract. J. Nanomater. 2012, 2012, 730746.
- Ghosh, S.; Patil, S.; Ahire, M.; Kitture, R.; Kale, S.; Pardesi, K.; Cameotra, S.S.; Bellare, J.; Dhavale, D.D.; Jabgunde, A.; et al. Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents. Int. J. Nanomed. 2012, 7, 483–496.
- Parlinska-Wojtan, M.; Kus-Liskiewicz, M.; Depciuch, J.; Sadik, O. Green synthesis and antibacterial effects of aqueous colloidal solutions of silver nanoparticles using camomile terpenoids as a combined reducing and capping agent. Bioprocess. Biosyst. Eng. 2016, 39, 1213–1223.
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 2004, 275, 496–502.
- Chitte, H.K.; Bhat, N.V.; Karmakar, N.S.; Kothari, B.C.; Shinde, G.N. Tuning of refractive index of poly(vinyl alcohol): Effect of embedding Cu and Ag nanoparticles tuning of refractive index of poly(vinyl alcohol): Effect of embedding Cu and Ag nanoparticles. World J. Nanosci. Eng. 2012, 2, 19–24.
- Xia, Y.; Xia, X.; Peng, H.C. Shape-controlled synthesis of colloidal metal nanocrystals: Thermodynamic versus kinetic products. J. Am. Chem. Soc. 2015, 137, 7947–7966.
- Roy, A.; Bulut, O.; Some, S.; Kumar Mandal, A.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702.
- El-Rafie, M.H.; El-Naggar, M.E.; Ramadan, M.A.; Fouda, M.M.G.; Al-Dey, S.S.; Hebeish, A. Environmental synthesis of silver nanoparticles using hydroxypropyl starch and their characterization. Carbohydr. Polym. 2011, 86, 630–635.




