Elementary silver nanoparticles (AgNPs) are of great interest because of their various probable medical application. Due to their antimicrobial activity, low toxicity and inexpensive cost, the mechanism of silver nanoparticles biosynthesis in bacterial, fungal and plant cells is extremely exciting from the point of view of the different cellular compounds participation in it: proteins, enzymes, acids, etc.
- AgNPs
- silver nanoparticles
- Biosynthesis
1. Introduction
In the modern world, “green technologies” are gaining more and more popularity due to their effectiveness, non-toxicity, and “eco-friendly”. One of the directions of “green synthesis” is the production of elementary silver nanoparticles (AgNPs) for use in various areas of human activity, primarily in medicine. It should be noted that mankind has been familiar with the bactericidal effects of Ag+ ions from time immemorial. The presence of both silver ions and AgNPs was established by the research in the solution named “Holy water”, known from the beginning of the first Millennium as a protection tool against infection by microorganisms [1]. It is thanks to the silver ions and AgNP suspension that it can have a bactericidal, bacteriostatic, antiviral, and antifungal effect on a large number of pathogenic microorganisms, yeast fungi, and viruses. In addition, the inhibitory effect can sometimes be expressed even slightly stronger in comparison with penicillin, biomycin, and other “classic” antibiotics due to the resistance of many strains of microorganisms to antibiotics [2,3]. This circumstance, together with their low toxicity, almost complete absence of allergic reactions, and good tolerance, has made AgNPs a very popular survey object. Moreover, the high interest in silver nanoparticles over the past decades has allowed for not only the confirmation of their antibacterial activity, but also to discover new properties worthy of application.
Anticipating the story of the use of AgNPs with various properties used in practice, it is necessary to note the wide and diverse forms of nanoparticles obtained during their synthesis by both physico-chemical and biological methods. The variety of objects used for the synthesis of nanoparticles inevitably leads to a variety of AgNPs forms: these can be “nanowires”, tabular prisms, cubes, octahedra, and pyramids [4,5]. The different studies devoted to silver nanoparticles showed that the shape and size of the resulting AgNPs largely depended on experimental parameters such as temperature, concentration of the Ag(I) compound, pH solution, and in the case of biological synthesis, on the direct object used to produce AgNPs [5]. As the most striking characteristic of AgNPs, their shape also largely determines their properties including the material features that these nanoparticles are part of. Despite the huge number of publications devoted to AgNP biosynthesis in bacterial, fungal, and plant cells, a more detailed approach is required not only to the synthesis itself, but also to its mechanism, the participation of various cellular compounds: proteins, enzymes, acids, etc.
In addition, an important aspect to be considered in the future practical application of AgNPs are the interaction mechanism of nanoparticles directly with the cell, and the processes occurring inside it. This serious fact is extremely important, whereas the currently fashionable prefix “bio” should reflect not only the method of obtaining a practically significant substance, but also the application safety, especially used in medicine. In addition, considering silver nanoparticles as a potential medical agent, we should not forget about their potential toxicity. A large number of publications on this topic have shown that the toxic potential of nanoparticles is determined by factors such as size, shape, surface area, aggregation or agglomeration, and dose [6,7,8,9]. It is generally believed that easily ionized silver particles can affect the cell by the Trojan horse mechanism. Phagocytosis of AgNPs stimulates inflammatory signaling through the generation of reactive oxygen species (ROS) in macrophage cells, after which activated macrophage cells induce TNF-α secretion. Increased levels of TNF-α lead to cell membrane damage and apoptosis [10,11]. It should be noted that a number of studies have shown the toxicity of AgNPs (for example, in studies of rat hepatocytes and neuronal cells, mouse stem cells, and human lung epithelial cells) in relation to cells, and its absence (in studies of healthy mammalian cells) [12,13,14,15,16,17]. In this regard, the study of toxicity is extremely important using in vivo and in vitro assays as well as in silico models [18].
2. Mechanism of Silver Nanoparticles (AgNPs) Biosynthesis
Notwithstanding an incredibly large number of publications describing the AgNP synthesis via various groups of organisms such as bacteria, fungi, lichens, algae, and higher plants, the mechanism of this process still remains completely unexplored.
The AgNP synthesis results using a variety of microorganisms demonstrated that the process of AgNP formation can occur both inside and outside the cell. Extracellular synthesis involves the presence of proteins–enzymes present on the cell wall of bacteria and secreted proteins, thanks to which Ag+ is reduced to Ag0. It was shown that AgNP extracellular synthesis is typical for both Gram-positive bacteria genus Bacillus, in particular, for B. pumilus, B. persicus, and B. licheniformis, B. indicus and B. cecembensis as well as Planomicrobium sp., Streptomyces sp., Rhodococcus sp., and for Gram-negative bacteria such as Klebsiella pneumoniae, Escherichia coli, and Acinetobacter calcoaceticus [19,20,21,22,23,24,25]. This mechanism of synthesis has also been determined for a number of other microorganisms such as the fungi Rhizopus stolonifer, Aspergillus niger, Fusarium oxysporum, Fusarium sp., and A. flavus [26,27,28,29].
Nevertheless, the nanoparticle synthesis by microorganisms has been shown in a number of studies as intracellular. This mechanism is represented in Gram-negative bacteria and is associated with membrane proteins transporting the silver ions into the cell. For example, the intracellular nature of AgNP synthesis was established for Enterobacter cloacae by El-Baghdady et al. [30]. Similar results were demonstrated for Pseudomonas stutzeri [31]. This mechanism was also shown for Gram-positive bacteria Corynebacterium sp. [32] as well as for various bacteria of the genus Streptomyces [33]. Among the representatives of the fungi kingdom, acidophilus Verticillium sp. can be highlighted [34]. Moreover, some microorganisms are able to perform AgNP biosynthesis both intracellularly and extracellularly including Bacillus strain CS 11 and Proteus mirablis [35,36].
Whether AgNP biosynthesis is intracellular or extracellular, the fundamental factor in this process is enzymes. Most researchers agree that it has a leading role in the AgNP formation of NADH-dependent nitrate reductase, which acts as an electron shuttle, taking electrons from the nitrate molecule and transferring it to the metal ion for the formation of nanoparticles, which is clearly shown for F. oxysporum, Ps. aeruginosa, and others [37,38,39,40,41]. The supposed mechanism of this process in shown in Figure 1. The information about respiratory [42] and periplasmic nitrate reductases [43] is also in the literature. In some experiments, it was found that proteins and sugars of the cell wall, where the bioreduction process can occur, can participate in the silver ion grab [32,44]. In addition, it is believed that the presence of a carboxylate group on the bacterial cell surface, which causes its mostly negative charge, provides an electrostatic interaction between this group and positively charged silver ions, which helps capture silver ions [45].
Some amino acids such as arginine, aspartic acid, cysteine, glutamic acid, lysine, and methionine are also implicated in the reduction of silver ions or silver nanocrystals, which act as catalysts, producing a hydroxyl ion that reacts with reducing agents such as aldehyde [46,47]. It is shown by Graf et al. that peptides containing disulfide bonds can also participate in the reduction of Ag+ to Ag0 [48]. The reaction conditions also make an important contribution: for example, a high pH plays an important role in the subunit activation of the oxidoreductase enzyme, and promotes the conversion of tryptophan into a transition tryptophil radical, which gives electrons to silver ions and leads to a reduction to elementary silver [49,50]. An important peculiarity is the intensification of AgNP biosynthesis by light. This effect may be associated with the activation of reducing agents in the culture of the supernatant, which for their part, causes the release of electrons to reduce Ag+ to Ag0 nanoparticles [51,52,53]. The other hypothetical mechanism of nanoparticle synthesis is based on the fact that certain bacteria generate the trans-membrane proton gradient, which is broken down by the active symport of Na+ ions along with Ag ions from the extracellular environment [54,55]. Several silver-binding membrane proteins attract silver ions and by deriving energy from ATP hydrolysis, results in the uptake of silver ions inside the cells and initiate synthesis of AgNPs [55].
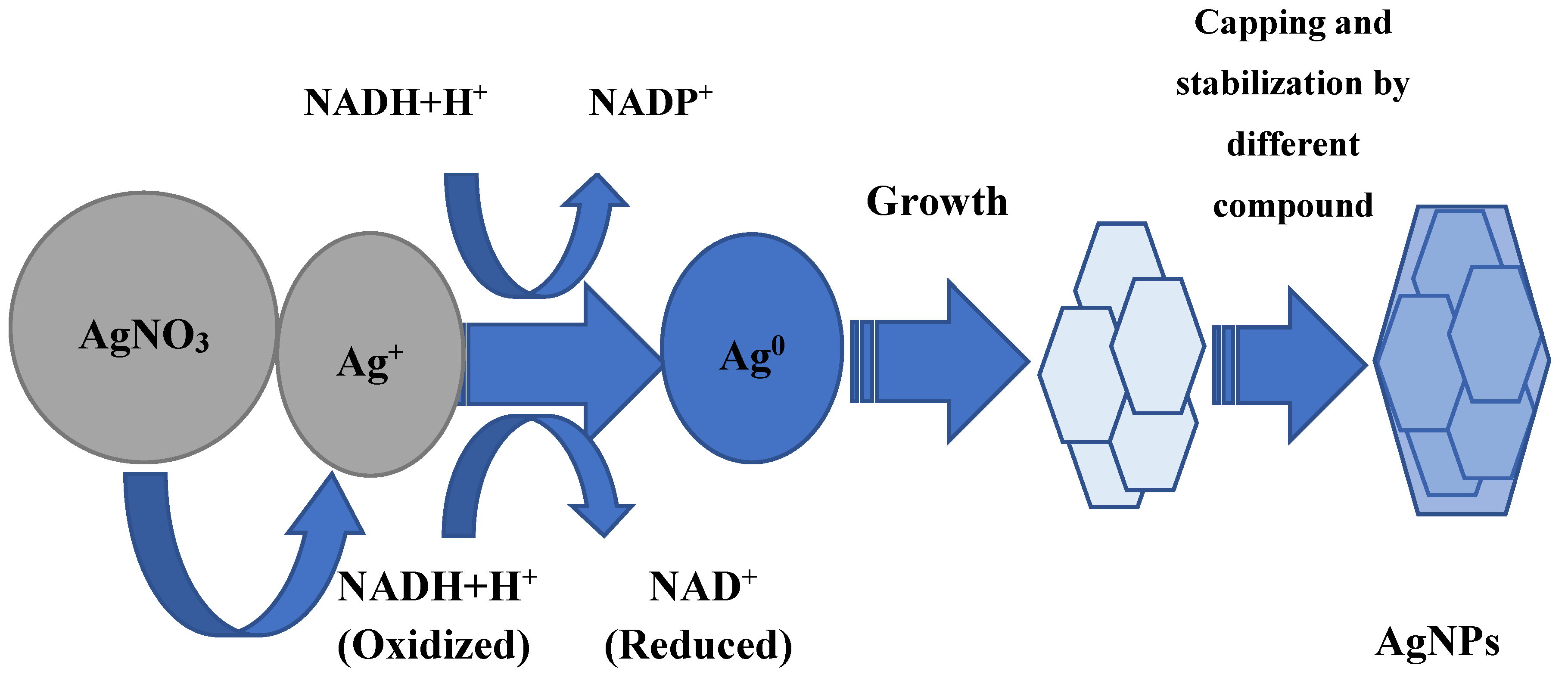
An interesting fact is that the production of silver nanoparticles is possible not only with the help of nitrate reductase, but also with a completely different class enzyme: extracellular keratinase B. safensis plays a crucial role in AgNP biosynthesis [56,57].
Fourier-transform infrared spectroscopy (FTIR) analysis is studied for synthesized AgNPs to find out the possible reducing bio-molecules that can stabilize nanoparticles, prevent agglomeration, and create their capping in an aqueous solution. It should be noted that the final packaging of microbial nanoparticles involves a very large number of different compounds. These can be peptides, enzymes, carboxylic acids, aldehydes, ketones, rhamnose sugar, and rhamnolipids [40,58,59,60,61,62,63,64,65,66,67,68,69,70,71]. It is assumed that the enzymes can bind to silver nanoparticles using free amino and cysteine groups of proteins.
The number of publications on AgNP synthesis with the assistance of various plant extracts (leaves, stems, roots, etc.) is incalculable. A wide diversity of plants including medicinal herbs are used as “factories” for the production of silver nanoparticles. The prospective mechanism of AgNP synthesis is generally similar to that of microorganisms and is enzymatic in nature. However, the compounds for the nanoparticles’ stabilization and final capping are different and specific from those for microorganisms, because plant cells contain a complex of diversified antioxidant metabolites preventing the oxidation and damage of cellular components [72]. Therefore, enzymes, glycosides, saponins, and other biomolecules can participate in the nanoparticle’s stabilization [73,74]. They are especially important in terms of further practical applications of AgNPs, seeing that they have anti-inflammatory, antioxidant, antitumor, and other effects [75,76]. The literature data indicate that when metal salts are added to the plant extract, silver ions bind to proteins and water-soluble compounds using –OH and –COOH groups, leading to conformational changes in the protein molecule, which contribute to the captured metal ion transformation into a silver nanoparticle [77,78]. In addition, amino groups and cysteine residues of proteins take part in the silver reduction process and the formation of AgNPs [79,80]. Alkanes, amines, phenols, polyphenols, arabinose and galactose, aldehydes and ketones, alcohols, alkaloids, lignans, terpenoids, and flavonoids can act as “capping” agents for the formation of silver nanoparticles [81,82,83,84,85,86,87].
Flavonoids are particularly interesting in this case due to their high antioxidant activity for medical purposes. Hydrophilic functional groups of various compounds surrounding nanoparticles make them colloid-stable in an aqueous medium [88]. Other interesting substances that act as reducing and stabilizing substances are the sucrose and fructose of garlic extract [89]. Furthermore, polyols are responsible for the reduction of Ag+ into silver nanoparticles in the Dioscorea bulbifera tuber extract [90]. It is supposed that terpenoids are surface-active molecules that adsorb on the AgNPs surface for stabilizing nanoparticles and preventing the AgNPs from agglomeration [91]. The reduction from Ag+ ions to silver nanoparticles (Ag0) with terpenoids may involve the conversion of C–O group of the terpenes to the –C–O group [92]. It is likely that the terpenoids play a role in the reduction of metal ions by the oxidation of aldehydic groups in the molecules to carboxylic acids [93]. Apparently “capping” agents have the possibility of selective binding to different types of facets on a nanocrystal to change their specific surface free energies and thus their area proportions [94]. Thus, nanoparticle “capping” can perform several important functions, namely prevent the agglomeration of nanoparticles, reduce toxicity, and improve antimicrobial properties; additionally these molecules can enhance the affiliation possibility and action of AgNPs on the bacterial cells. [95,96]. Remarkably, plant “capping” agents frequently have their own antimicrobial activity that can increase the activity of AgNPs.
This entry is adapted from the peer-reviewed paper 10.3390/jfb11040084
