
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bruno Moreira Silva | -- | 2399 | 2022-11-11 13:09:32 | | | |
| 2 | Bruno Moreira Silva | Meta information modification | 2399 | 2022-11-11 13:15:48 | | | | |
| 3 | Amina Yu | Meta information modification | 2399 | 2022-11-14 02:36:40 | | | | |
| 4 | Amina Yu | -4 word(s) | 2395 | 2022-11-18 05:10:41 | | |
Video Upload Options
Obtaining perceived exertion is relatively easy, but practitioners often neglect some critical methodological issues extensively addressed in its inception (e.g., construct definition), which can subsequently compromise the validity of perceived exertion assessment. In addition, several theoretical models have proposed that perceived exertion plays a role in explaining endurance exercise performance. These models rely on assumptions about the origin of the neural signals responsible for generating the perceived exertion. Although the scientific knowledge about central and peripheral signals involved in the perceived exertion genesis has notably progressed, the scenario is complex, and some caveats remain, requiring an integrative physiological interpretation to advance the field further. Lastly, practitioners have extensively applied perceived exertion to prescribe exercise intensity and monitor training responses.
1. Available Perceived Exertion Definitions
2. Neurophysiological Mechanisms Associated with Perceived Exertion
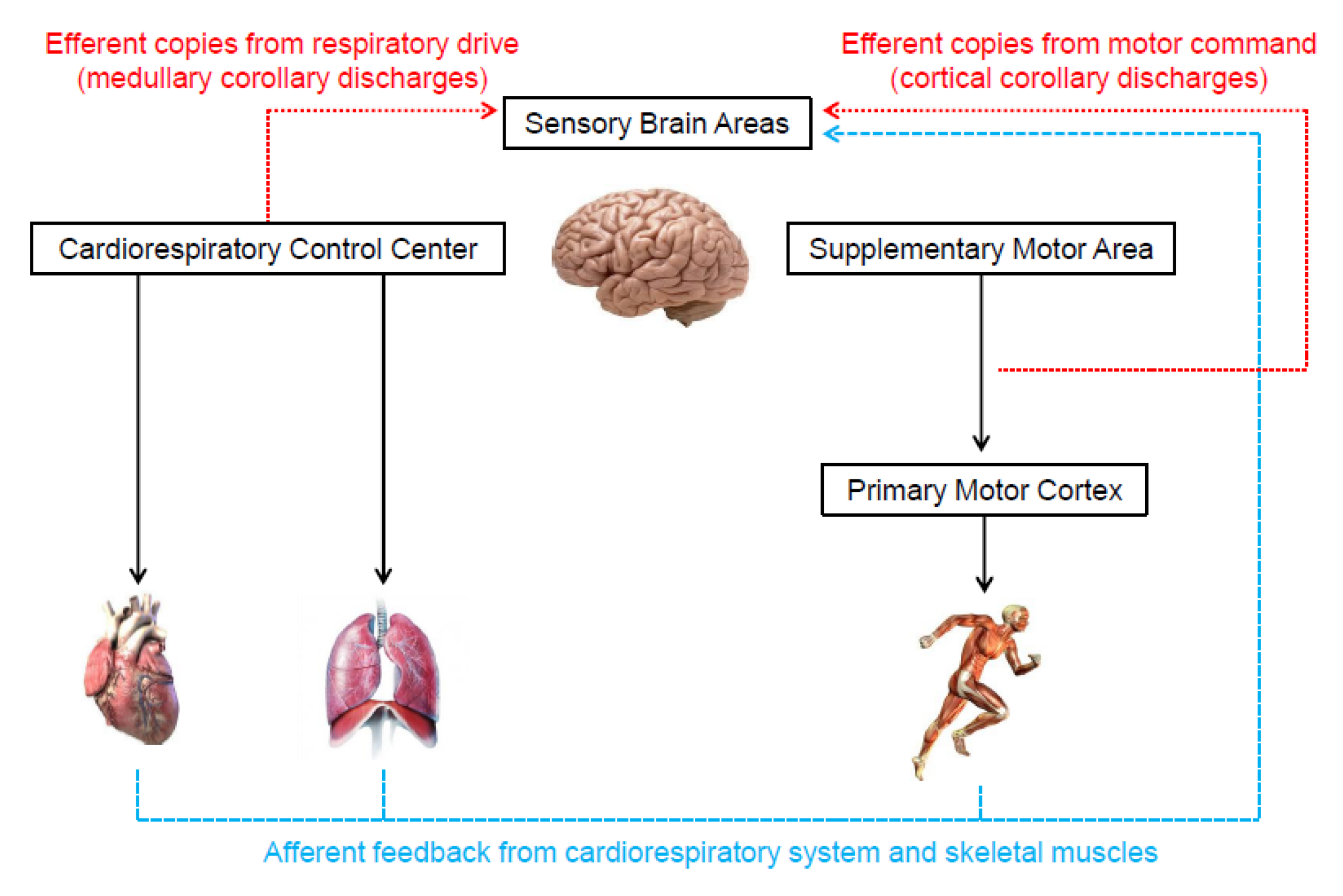
3. Methodological Issues to Quantify Perceived Exertion
4. Practical Applications of Perceived Exertion Measurement
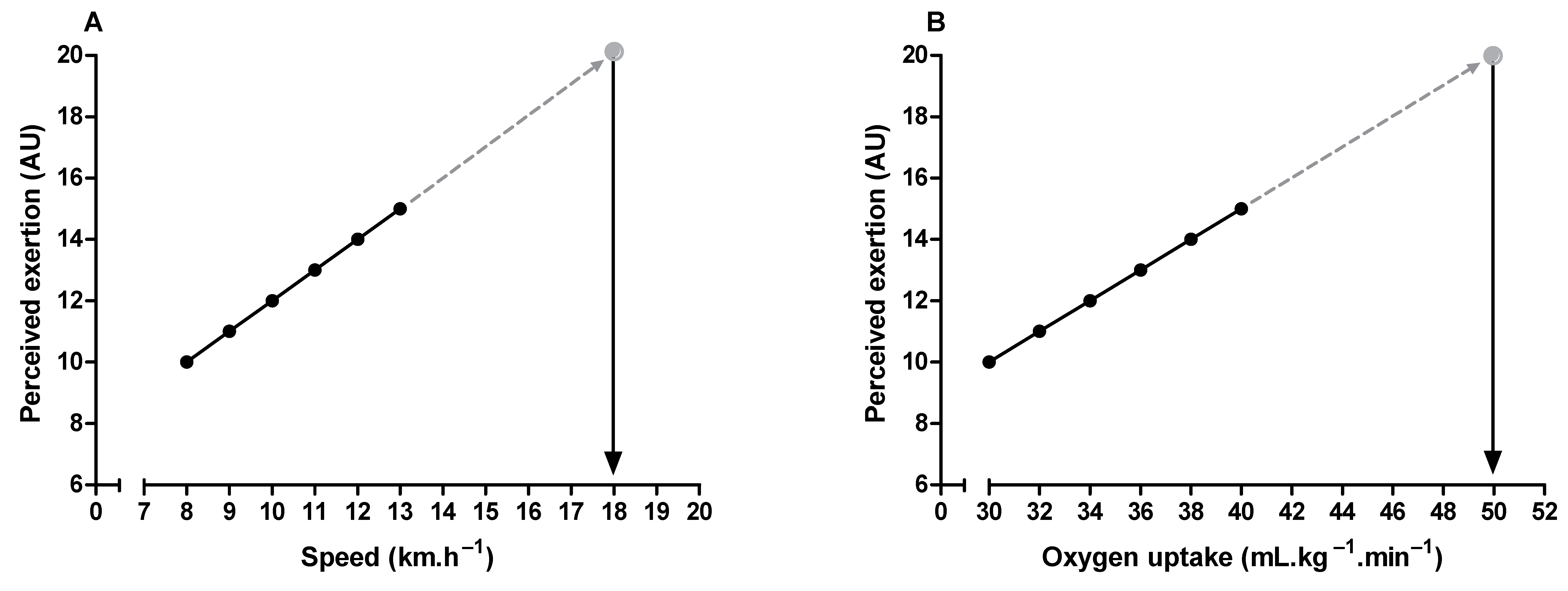
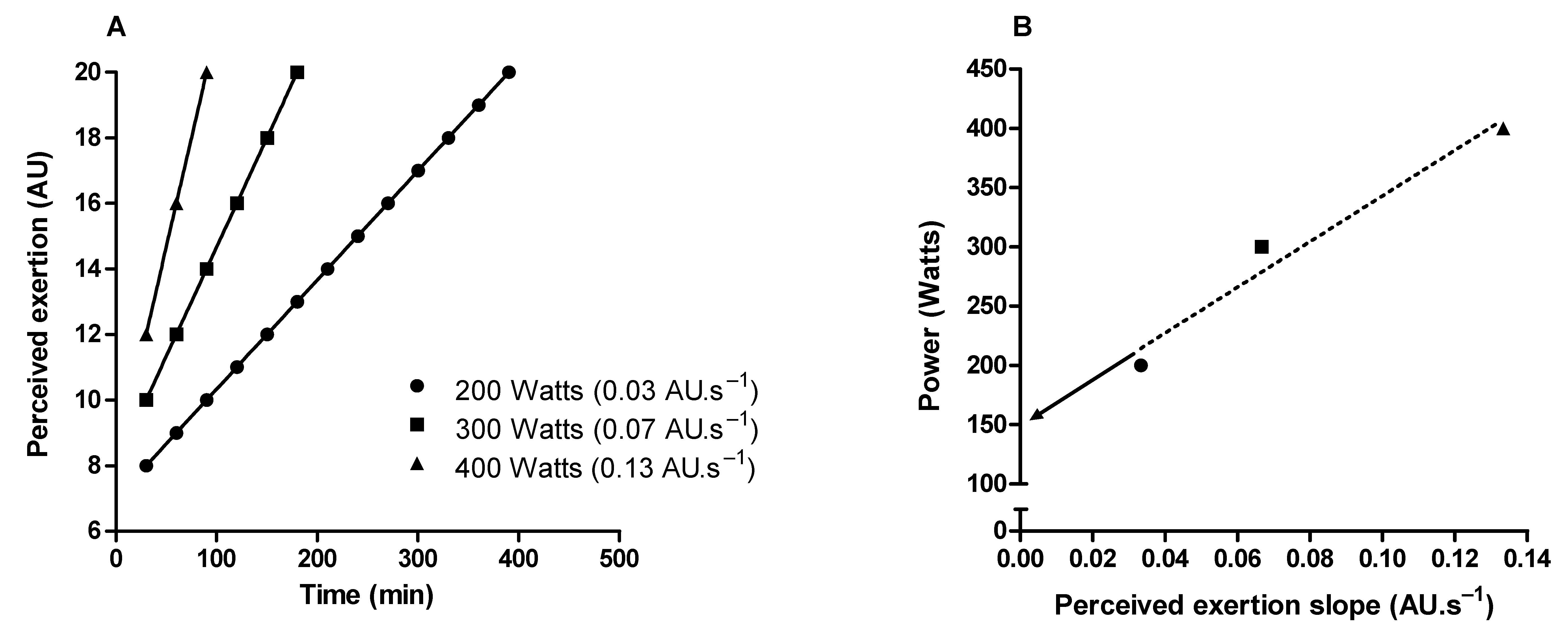
References
- Pageaux, B. Perception of effort in Exercise Science: Definition, measurement and perspectives. Eur. J. Sport. Sci. 2016, 16, 885–894.
- Abbiss, C.R.; Peiffer, J.J.; Meeusen, R.; Skorski, S. Role of Ratings of Perceived Exertion during Self-Paced Exercise: What are We Actually Measuring? Sport. Med. 2015, 45, 1235–1243.
- Halperin, I.; Emanuel, A. Rating of Perceived Effort: Methodological Concerns and Future Directions. Sport. Med. 2020, 50, 679–687.
- Borg, G.; Noble, B.J. Perceived exertion. Exerc. Sport. Sci. Rev. 1974, 2, 131–154.
- Borg, G. Borg’s Perceived Exertion and Pain Scales, 1st ed.; Human Kinetics: Champaign, IL, USA, 1998.
- Borg, G. Psychophysical bases of perceived exertion. Med. Sci. Sport. Exerc. 1982, 14, 377–381.
- Morgan, W.P. Psychological components of effort sense. Med. Sci. Sport. Exerc. 1994, 26, 1071–1077.
- Robertson, R.J.; Noble, B.J. Perception of physical exertion: Methods, mediators, and applications. Exerc. Sport. Sci. Rev. 1997, 25, 407–452.
- Christian, R.J.; Bishop, D.J.; Billaut, F.; Girard, O. The role of sense of effort on self-selected cycling power output. Front. Physiol. 2014, 5, 115.
- Hamilton, A.L.; Killian, K.J.; Summers, E.; Jones, N.L. Quantification of intensity of sensations during muscular work by normal subjects. J. Appl. Physiol. 1996, 81, 1156–1161.
- Lansing, R.W.; Im, B.S.; Thwing, J.I.; Legedza, A.T.; Banzett, R.B. The perception of respiratory work and effort can be independent of the perception of air hunger. Am. J. Respir. Crit. Care Med. 2000, 162, 1690–1696.
- Jones, L.A.; Hunter, I.W. Effect of fatigue on force sensation. Exp. Neurol. 1983, 81, 640–650.
- Demediuk, B.H.; Manning, H.; Lilly, J.; Fencl, V.; Weinberger, S.E.; Weiss, J.W.; Schwartzstein, R.M. Dissociation between dyspnea and respiratory effort. Am. Rev. Respir. Dis. 1992, 146, 1222–1225.
- Zhang, J.; Schaeffer, M.R.; Mitchell, R.A.; Boyle, K.G.; Hutchinson, O.N.; Puyat, J.H.; Guenette, J.A. A multidimensional assessment of dyspnoea in healthy adults during exercise. Eur. J. Appl. Physiol. 2020, 120, 2533–2545.
- Smith, S.A.; Micklewright, D.; Winter, S.L.; Mauger, A.R. Muscle pain induced by hypertonic saline in the knee extensors decreases single-limb isometric time to task failure. Eur. J. Appl. Physiol. 2020, 120, 2047–2058.
- Cook, D.B.; O’Connor, P.J.; Eubanks, S.A.; Smith, J.C.; Lee, M. Naturally occurring muscle pain during exercise: Assessment and experimental evidence. Med. Sci. Sport. Exerc. 1997, 29, 999–1012.
- Jones, L.A. Perception of force and weight: Theory and research. Psychol. Bull. 1986, 100, 29–42.
- Smirmaul, B.P.C. Sense of effort and other unpleasant sensations during exercise: Clarifying concepts and mechanisms. Br. J. Sport. Med. 2012, 46, 308–311.
- de Morree, H.M.; Marcora, S.M. Psychobiology of Perceived Effort During Physical Tasks. In Handbook of Biobehavioral Approaches to Self-Regulation; Gendolla, G.H.E., Tops, M., Koole, S.L., Eds.; Springer: New York, NY, USA, 2015; pp. 255–270.
- Marcora, S.M. Psychobiology of fatigue during endurance exercise. In Endurance Performance in Sports Psychological Theory and Interventions; Meijen, C., Ed.; Routledge: London, UK, 2019; pp. 12–32.
- Marcora, S.M. Effort: Perception of. In Encyclopedia of Perception; Goldstein, E., Ed.; Sage: Thousaand Oaks, CA, USA, 2010; pp. 380–383.
- Marcora, S.M. Perception of effort during exercise is independent of afferent feedback from skeletal muscles, heart, and lungs. J. Appl. Physiol. 2009, 106, 2060–2062.
- Hureau, T.J.; Romer, L.M.; Amann, M. The ‘sensory tolerance limit’: A hypothetical construct determining exercise performance? Eur. J. Sport. Sci. 2018, 18, 13–24.
- Hampson, D.B.; St Clair Gibson, A.; Lambert, M.I.; Noakes, T.D. The influence of sensory cues on the perception of exertion during exercise and central regulation of exercise performance. Sport. Med. 2001, 31, 935–952.
- Nobrega, A.C.L.; O’Leary, D.; Silva, B.M.; Marongiu, E.; Piepoli, M.F.; Crisafulli, A. Neural regulation of cardiovascular response to exercise: Role of central command and peripheral afferents. Biomed. Res. Int. 2014, 2014, 478965.
- Silva, T.M.; Aranda, L.C.; Paula-Ribeiro, M.; Oliveira, D.M.; Medeiros, W.M.; Vianna, L.C.; Nery, L.E.; Silva, B.M. Hyperadditive ventilatory response arising from interaction between the carotid chemoreflex and the muscle mechanoreflex in healthy humans. J. Appl. Physiol. 2018, 125, 215–225.
- Mihevic, P.M. Sensory cues for perceived exertion: A review. Med. Sci. Sport. Exerc. 1981, 13, 150–163.
- Scherr, J.; Wolfarth, B.; Christle, J.W.; Pressler, A.; Wagenpfeil, S.; Halle, M. Associations between Borg’s rating of perceived exertion and physiological measures of exercise intensity. Eur. J. Appl. Physiol. 2013, 113, 147–155.
- Ekblom, B.; Goldbarg, A.N. The influence of physical training and other factors on the subjective rating of perceived exertion. Acta Physiol. Scand. 1971, 83, 399–406.
- Lopes, T.R.; Oliveira, D.M.; Simurro, P.B.; Akiba, H.T.; Nakamura, F.Y.; Okano, A.H.; Dias, Á.M.; Silva, B.M. No Sex Difference in Mental Fatigue Effect on High-Level Runners’ Aerobic Performance. Med. Sci. Sport. Exerc. 2020, 52, 2207–2216.
- O’Donnell, D.E.; Banzett, R.B.; Carrieri-Kohlman, V.; Casaburi, R.; Davenport, P.W.; Gandevia, S.C.; Gelb, A.F.; Mahler, D.A.; Webb, K.A. Pathophysiology of dyspnea in chronic obstructive pulmonary disease: A roundtable. Proc. Am. Thorac. Soc. 2007, 4, 145–168.
- Amann, M.; Proctor, L.T.; Sebranek, J.J.; Pegelow, D.F.; Dempsey, J.A. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J. Physiol. 2009, 587, 271–283.
- Kjaer, M.; Hanel, B.; Worm, L.; Perko, G.; Lewis, S.F.; Sahlin, K.; Galbo, H.; Secher, N.H. Cardiovascular and neuroendocrine responses to exercise in hypoxia during impaired neural feedback from muscle. Am. J. Physiol. 1999, 277, R76–R85.
- Smith, S.A.; Querry, R.G.; Fadel, P.J.; Gallagher, K.M.; Strømstad, M.; Ide, K.; Raven, P.B.; Secher, N.H. Partial blockade of skeletal muscle somatosensory afferents attenuates baroreflex resetting during exercise in humans. J. Physiol. 2003, 551, 1013–1021.
- de Morree, H.M.; Klein, C.; Marcora, S.M. Perception of effort reflects central motor command during movement execution. Psychophysiology 2012, 49, 1242–1253.
- de Morree, H.M.; Klein, C.; Marcora, S.M. Cortical substrates of the effects of caffeine and time-on-task on perception of effort. J. Appl. Physiol. 2014, 117, 1514–1523.
- Zénon, A.; Sidibé, M.; Olivier, E. Disrupting the supplementary motor area makes physical effort appear less effortful. J. Neurosci. 2015, 35, 8737–8744.
- Gandevia, S.C.; Killian, K.; McKenzie, D.K.; Crawford, M.; Allen, G.M.; Gorman, R.B.; Hales, J.P. Respiratory sensations, cardiovascular control, kinaesthesia and transcranial stimulation during paralysis in humans. J. Physiol. 1993, 470, 85–107.
- Takarada, Y.; Mima, T.; Abe, M.; Nakatsuka, M.; Taira, M. Inhibition of the primary motor cortex can alter one’s “sense of effort”: Effects of low-frequency rTMS. Neurosci. Res. 2014, 89, 54–60.
- Machado, A.C.; Vianna, L.C.; Gomes, E.A.C.; Teixeira, J.A.C.; Ribeiro, M.L.; Villacorta, H.; Nobrega, A.C.L.; Silva, B.M. Carotid chemoreflex and muscle metaboreflex interact to the regulation of ventilation in patients with heart failure with reduced ejection fraction. Physiol. Rep. 2020, 8, 14361.
- Amann, M.; Wan, H.Y.; Thurston, T.S.; Georgescu, V.P.; Weavil, J.C. On the Influence of Group III/IV Muscle Afferent Feedback on Endurance Exercise Performance. Exerc. Sport Sci. Rev. 2020, 48, 209–216.
- Sidhu, S.K.; Lauber, B.; Cresswell, A.G.; Carroll, T.J. Sustained cycling exercise increases intracortical inhibition. Med. Sci. Sport. Exerc. 2013, 45, 654–662.
- Sidhu, S.K.; Weavil, J.C.; Mangum, T.S.; Jessop, J.E.; Richardson, R.S.; Morgan, D.E.; Amann, M. Group III/IV locomotor muscle afferents alter motor cortical and corticospinal excitability and promote central fatigue during cycling exercise. Clin. Neurophysiol. 2017, 128, 44–55.
- Sidhu, S.K.; Weavil, J.C.; Thurston, T.S.; Rosenberger, D.; Jessop, J.E.; Wang, E.; Richardson, R.S.; McNeil, C.J.; Amann, M. Fatigue-related group III/IV muscle afferent feedback facilitates intracortical inhibition during locomotor exercise. J. Physiol. 2018, 596, 4789–4801.
- Weavil, J.C.; Amann, M. Corticospinal excitability during fatiguing whole body exercise. Prog. Brain Res. 2018, 240, 219–246.
- Sharples, S.A.; Gould, J.A.; Vandenberk, M.S.; Kalmar, J.M. Cortical Mechanisms of Central Fatigue and Sense of Effort. PLoS ONE 2016, 11, e0149026.
- Romer, L.M.; Polkey, M.I. Exercise-induced respiratory muscle fatigue: Implications for performance. J. Appl. Physiol. 2008, 104, 879–888.
- Romer, L.M.; Lovering, A.T.; Haverkamp, H.C.; Pegelow, D.F.; Dempsey, J.A. Effect of inspiratory muscle work on peripheral fatigue of locomotor muscles in healthy humans. J. Physiol. 2006, 571, 425–439.
- Dempsey, J.A.; Romer, L.; Rodman, J.; Miller, J.; Smith, C. Consequences of exercise-induced respiratory muscle work. Respir. Physiol. Neurobiol. 2006, 151, 242–250.
- Ramsook, A.H.; Schaeffer, M.R.; Mitchell, R.A.; Dhillon, S.S.; Milne, K.M.; Ferguson, O.N.; Puyat, J.H.; Koehle, M.S.; Sheel, A.W.; Guenette, J.A. Sex Differences in Diaphragm Voluntary Activation after Exercise. Med. Sci. Sport. Exerc. 2022, 54, 1167–1175.
- Borg, G.; Borg, E. A new generation of scaling methods: Level-anchored ratio scaling. Psychologica 2001, 28, 15–45.
- Cabral, L.L.; Nakamura, F.Y.; Stefanello, J.M.F.; Pessoa, L.C.V.; Smirmaul, B.P.C.; Pereira, G. Initial Validity and Reliability of the Portuguese Borg Rating of Perceived Exertion 6–20 Scale. Meas. Phys. Educ. Exerc. Sci. 2020, 24, 103–114.
- Lopes, T.R.; De Almeida, A.A.; Da Silva, A.C.; Silva, B.M. Are heart rate deflection point and peak velocity determined in the Université of Montréal Track Test valid to approximate aerobic parameters measured in the laboratory? J. Sport. Med. Phys. Fit. 2016, 56, 510–519.
- Rossi Neto, J.M.; Tebexreni, A.S.; Alves, A.N.F.; Smanio, P.E.P.; de Abreu, F.B.; Thomazi, M.C.; Nishio, P.A.; Cuninghant, I.A. Cardiorespiratory fitness data from 18,189 participants who underwent treadmill cardiopulmonary exercise testing in a Brazilian population. PLoS ONE 2019, 14, e0209897.
- Eston, R. Use of ratings of perceived exertion in sports. Int. J. Sport. Physiol. Perform. 2012, 7, 175–182.
- Eston, R.G.; Thompson, M. Use of ratings of perceived exertion for predicting maximal work rate and prescribing exercise intensity in patients taking atenolol. Br. J. Sport. Med. 1997, 31, 114–119.
- Faulkner, J.; Eston, R. Overall and peripheral ratings of perceived exertion during a graded exercise test to volitional exhaustion in individuals of high and low fitness. Eur. J. Appl. Physiol. 2007, 101, 613–620.
- Lambrick, D.M.; Faulkner, J.A.; Rowlands, A.V.; Eston, R.G. Prediction of maximal oxygen uptake from submaximal ratings of perceived exertion and heart rate during a continuous exercise test: The efficacy of RPE 13. Eur. J. Appl. Physiol. 2009, 107, 1–9.
- Marcora, S.M.; Staiano, W. The limit to exercise tolerance in humans: Mind over muscle? Eur. J. Appl. Physiol. 2010, 109, 763–770.
- Horstman, D.H.; Morgan, W.P.; Cymerman, A.; Stokes, J. Perception of effort during constant work to self-imposed exhaustion. Percept. Mot. Ski. 1979, 48, 1111–1126.
- de Koning, J.J.; Foster, C.; Bakkum, A.; Kloppenburg, S.; Thiel, C.; Joseph, T.; Cohen, J.; Porcari, J.P. Regulation of pacing strategy during athletic competition. PLoS ONE 2011, 6, 0015863.
- Jones, A.M.; Burnley, M.; Black, M.I.; Poole, D.C.; Vanhatalo, A. The maximal metabolic steady state: Redefining the ‘gold standard’. Physiol. Rep. 2019, 7, 14098.
- Burnley, M.; Jones, A.M. Power-duration relationship: Physiology, fatigue, and the limits of human performance. Eur. J. Sport Sci. 2018, 18, 1–12.
- Jones, A.M.; Wilkerson, D.P.; DiMenna, F.; Fulford, J.; Poole, D.C. Muscle metabolic responses to exercise above and below the “critical power” assessed using 31P-MRS. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, 5.
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal muscle fatigue: Cellular mechanisms. Physiol. Rev. 2008, 88, 287–332.
- Vanhatalo, A.; Jones, A.M.; Burnley, M. Application of critical power in sport. Int. J. Sport. Physiol. Perform. 2011, 6, 128–136.
- Mielke, M.; Housh, T.J.; Malek, M.H.; Beck, T.W.; Schmidt, R.J.; Johnson, G.O. The development of rating of perceived exertion-based tests of physical working capacity. J. Strength Cond. Res. 2008, 22, 293–302.
- Nakamura, F.Y.; Pereira, G.; Chimin, P.; Siqueira-Pereira, T.A.; Simões, H.G.; Bishop, D.J. Estimating the perceived exertion threshold using the OMNI scale. J. Strength Cond. Res. 2010, 24, 1602–1608.
- Nakamura, F.Y.; Okuno, N.M.; Perandini, L.A.; LF, S.C.; Simões, H.G.; Cardoso, J.R.; Bishop, D.J. Critical power can be estimated from nonexhaustive tests based on rating of perceived exertion responses. J. Strength Cond. Res. 2008, 22, 937–943.
- Coutts, A.J.; Crowcroft, S.; Kempton, T. Developing athlete monitoring systems: Theoretical basis and practical applications. In Sport, Recovery and Performance: Interdisciplinary Insights, 1st ed.; Kellmann, M., Beckmann, J., Eds.; Routledge: Abingdon, UK, 2017; pp. 19–32.
- Impellizzeri, F.M.; Marcora, S.M.; Coutts, A.J. Internal and External Training Load: 15 Years On. Int. J. Sport. Physiol. Perform. 2019, 14, 270–273.
- Siegl, A.; Elisa, M.K.; Tam, N.; Koschnick, S.; Langerak, N.G.; Skorski, S.; Meyer, T.; Lamberts, R.P. Submaximal Markers of Fatigue and Overreaching; Implications for Monitoring Athletes. Int. J. Sport. Med. 2017, 38, 675–682.
- Roete, A.J.; Elferink-Gemser, M.T.; Otter, R.T.A.; Stoter, I.K.; Lamberts, R.P. A Systematic Review on Markers of Functional Overreaching in Endurance Athletes. Int. J. Sport. Physiol. Perform. 2021, 8, 1065–1073.
- Buchheit, M.; Chivot, A.; Parouty, J.; Mercier, D.; Al Haddad, H.; Laursen, P.B.; Ahmaidi, S. Monitoring endurance running performance using cardiac parasympathetic function. Eur. J. Appl. Physiol. 2010, 108, 1153–1167.
- Buchheit, M.; Simpson, M.B.; Al Haddad, H.; Bourdon, P.C.; Mendez-Villanueva, A. Monitoring changes in physical performance with heart rate measures in young soccer players. Eur. J. Appl. Physiol. 2012, 112, 711–723.
- Eston, R.G.; Lamb, K.L.; Parfitt, G.; King, N. The validity of predicting maximal oxygen uptake from a perceptually-regulated graded exercise test. Eur. J. Appl. Physiol. 2005, 94, 221–227.
- Truong, P.; Millet, G.P.; Gojanovic, B. Perceptually Regulated Exercise Test Allows Determination of VO2max and Ventilatory Threshold But Not Respiratory Compensation Point In Trained Runners. Int. J. Sport. Med. 2018, 39, 304–313.
- Coquart, J.; Tabben, M.; Farooq, A.; Tourny, C.; Eston, R. Submaximal, Perceptually Regulated Exercise Testing Predicts Maximal Oxygen Uptake: A Meta-Analysis Study. Sport. Med. 2016, 46, 885–897.
- Dunbar, C.C.; Goris, C.; Michielli, D.W.; Kalinski, M.I. Accuracy and reproducibility of an exercise prescription based on Ratings of Perceived Exertion for treadmill and cycle ergometer exercise. Percept. Mot. Ski. 1994, 78, 1335–1344.
- Dunbar, C.C.; Robertson, R.J.; Baun, R.; Blandin, M.F.; Metz, K.; Burdett, R.; Goss, F.L. The validity of regulating exercise intensity by ratings of perceived exertion. Med. Sci. Sport. Exerc. 1992, 24, 94–99.
- Glass, S.C.; Knowlton, R.G.; Becque, M.D. Accuracy of RPE from graded exercise to establish exercise training intensity. Med. Sci. Sport. Exerc. 1992, 24, 1303–1307.




