Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pooja Chawla | -- | 1356 | 2022-11-07 19:16:48 | | | |
| 2 | Catherine Yang | Meta information modification | 1356 | 2022-11-08 03:06:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Virendra, S.A.; Kumar, A.; Chawla, P.A.; Mamidi, N. Heterocyclic PPAR Ligands for Potential Therapeutic Applications. Encyclopedia. Available online: https://encyclopedia.pub/entry/33376 (accessed on 08 February 2026).
Virendra SA, Kumar A, Chawla PA, Mamidi N. Heterocyclic PPAR Ligands for Potential Therapeutic Applications. Encyclopedia. Available at: https://encyclopedia.pub/entry/33376. Accessed February 08, 2026.
Virendra, Sharma Arvind, Ankur Kumar, Pooja A. Chawla, Narsimha Mamidi. "Heterocyclic PPAR Ligands for Potential Therapeutic Applications" Encyclopedia, https://encyclopedia.pub/entry/33376 (accessed February 08, 2026).
Virendra, S.A., Kumar, A., Chawla, P.A., & Mamidi, N. (2022, November 07). Heterocyclic PPAR Ligands for Potential Therapeutic Applications. In Encyclopedia. https://encyclopedia.pub/entry/33376
Virendra, Sharma Arvind, et al. "Heterocyclic PPAR Ligands for Potential Therapeutic Applications." Encyclopedia. Web. 07 November, 2022.
Copy Citation
The family of nuclear peroxisome proliferator-activated receptors (PPARα, PPARβ/δ, and PPARγ) is a set of ligand-activated transcription factors that regulate different functions in the body. Whereas activation of PPARα is known to reduce the levels of circulating triglycerides and regulate energy homeostasis, the activation of PPARγ brings about insulin sensitization and increases the metabolism of glucose. On the other hand, PPARβ when activated increases the metabolism of fatty acids. Further, these PPARs have been claimed to be utilized in various metabolic, neurological, and inflammatory diseases, neurodegenerative disorders, fertility or reproduction, pain, and obesity.
peroxisome proliferator-activated receptors
ligands
a thiazolidinedione
1. Introduction
Nuclear receptors are a type of protein that recognize steroid and thyroid hormones in the body [1]. Nuclear receptors tend to influence the growth, homeostasis, and metabolism of organisms by binding to DNA and regulating the expression of specific genes. As a result of their ability to modulate transcription, these are known as transcription factors [2][3]. The nuclear receptor superfamily’s peroxisome proliferator-activated receptor (PPAR) is a ligand-dependent transcription factor [4]. To build a heterodimer, all PPARs interact with the retinoid X receptor [5][6][7]. In 1990, PPARs were first observed in rodents [8]. PPARs are receptors associated with a superfamily of nuclear receptors that also contain steroids, retinoid receptors [9], thyroid hormone receptors, and Vit. D receptors [10]. They are multiple and persuasive regulators of various cellular functions and metabolic functions such as glucose and lipid homeostasis, cholesterol, and energy balance [11][12]. Peroxisome proliferator-activated receptors are a subgroup of the ligand-dependent transcription factor that contains peroxisome proliferator response elements as transcription factors to regulate transcriptional activity [13][14][15].
2. Structure of PPAR
The three-dimensional structure of PPARs includes the N-terminus and C-terminus where DNA binding domain and ligand-binding domain are attached, respectively, [16]. They are translocated to the nucleus and heterodimerized with retinoid X receptors (RXR) [17]. In targeted genes, the PPREs (peroxisome proliferator hormone response elements) are particular DNA areas that interact with PPARs [18][19]. They are connate in the fatty acid-binding protein like PPAR-responsive gene promoters that activate transcription of multiple genes involved in various physiological processes shown in Figure 1 [20][21]. The protein structure of various PPARs is fetched from the protein data bank using three PDB ids such as 2P54 (PPARα), 2A5G (PPA, Rβ,), and 3VI8 (PPARγ), shown in Figure 2 [22][23][24][25][26].
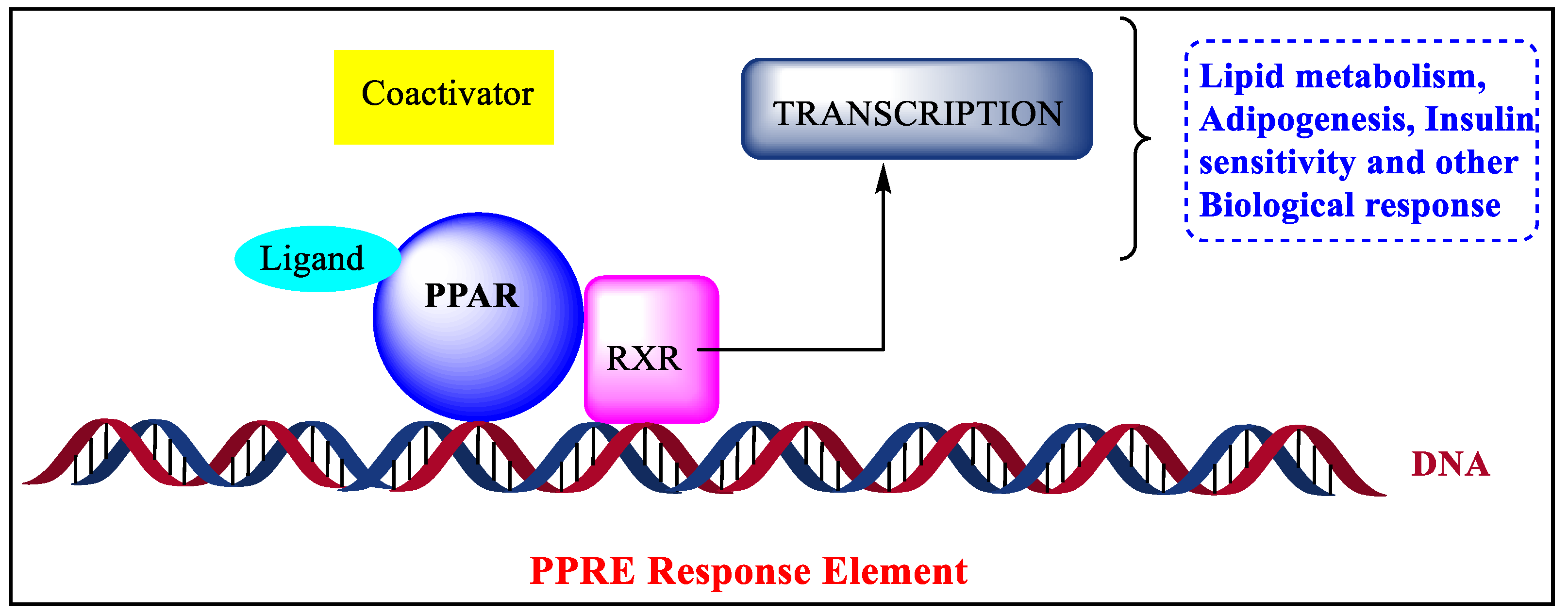
Figure 1. Mechanism of biological responses through PPAR.
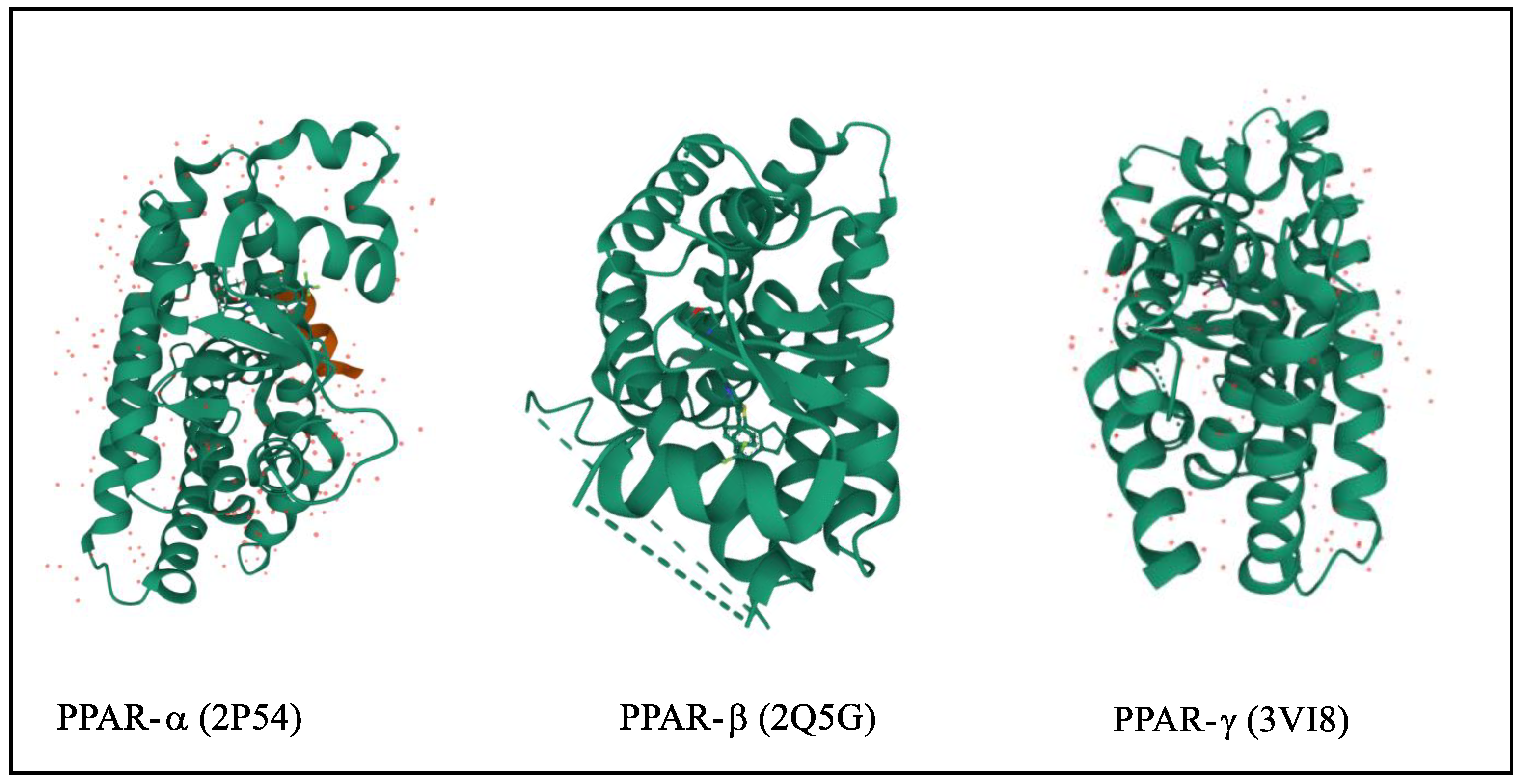
Figure 2. 3D structure of PPARα/β/γ.
3. Types and Expressions of PPARs
The transcriptional factors of peroxisome proliferator-activated receptors (PPARs) contain three different isoforms, namely nuclear receptor subfamily PPARα or NR1C1, PPARβ/δ or NR1C2, and PPARγ or NR1C3 [27][28][29]. These isoforms are expressed in multiple tissue and organs with identical character and ligand specificity [30]. The PPAR-α is expressed mainly in the liver but is also present in muscle, bone, and heart. The PPAR-δ is expressed in most parts of the body and regulates energy expenditure [31][32]. The PPAR-γ is expressed in vascular smooth muscle cells, and endothelial cells [33][34]. The expression of various types of PPARs is shown in Figure 3 [35][36][37].
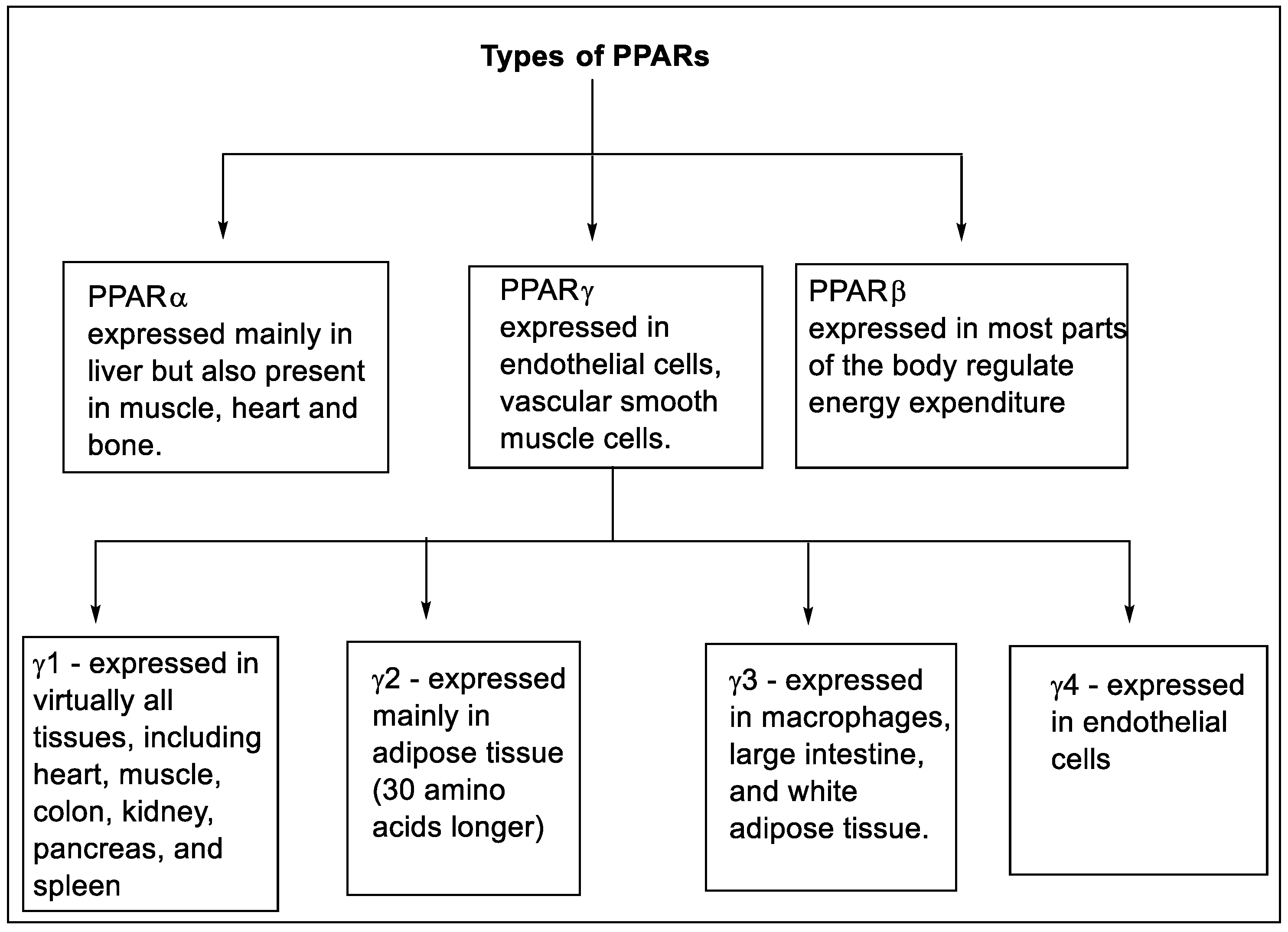
Figure 3. Types and expression of PPARs.
4. Functions of PPARs
In the human body, the three forms of PPARs are responsible for the activation of most of the enzymes that are required for fatty acid oxidation, glucose metabolism, and lipid metabolism [38]. These PPARs perform specific or distinct functions such as cellular energy balancing, and maintenance of energy homeostasis on their own or by helping each other [39]. Energy metabolism at a molecular level through PPARs is still not explained but when ligands are attached to these receptors, they transcript the genes involved in regulatory energy functions [40]. The PPARα is involved in the activation of gene-encoding enzymes such as carnitine palmityl transferase 1 (CPT1), fatty acid transport protein, acyl-CoA dehydrogenase or oxidase, or synthase required in the fatty acid oxidation pathway [41]. It also reduces plasma triglyceride levels and increases high-density lipoprotein, which gives benefits such as ketogenesis [42]. The PPARβ controls fatty acid metabolism, increases insulin sensitivity, obesity resistance, suppression of macrophage-derived inflammation, and also the formation of oxidative muscle fibers. The PPARγ promotes fatty acid uptake, and adipokine production raises insulin sensitivity, also in adipogenesis [43][44][45][46]. Other functions associated with PPARs are described in Figure 4 [47][48][49].
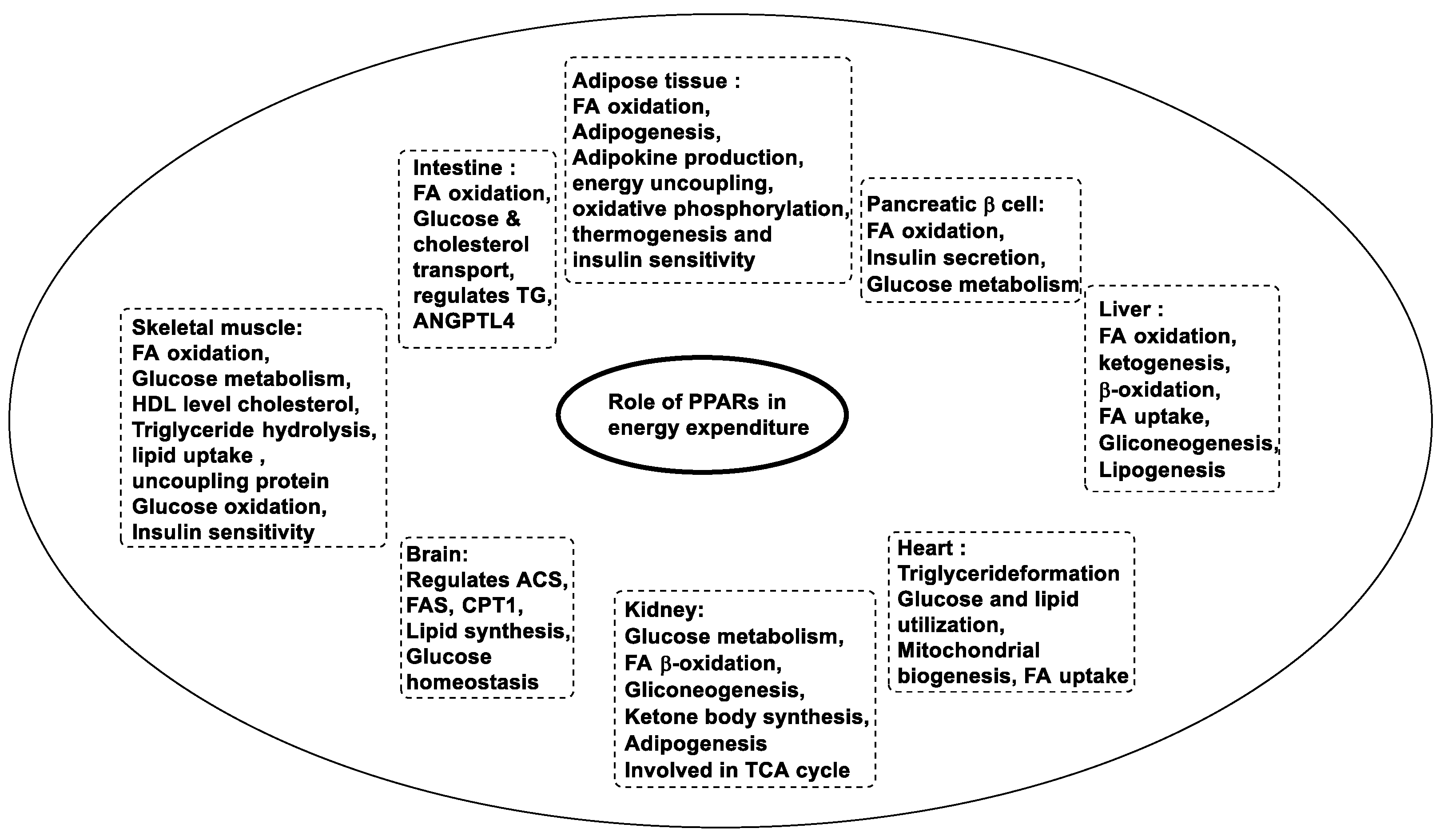
Figure 4. Functions of PPARs.
In several areas of medicinal chemistry, heterocyclic molecules are indispensable [50][51]. These compounds constitute nitrogen, sulfur, and oxygen atoms with various positional combinations in the cyclic ring [52]. Medicinal chemists and researchers always explore heterocycles due to their vital contribution to drug discovery and design by boosting the novel lead moieties with potential biological action in medicinal chemistry [53]. Based on statistics, more than 85% of biologically potential chemical moieties contain heterocyclic motifs [54]. Some of the most common heterocycles are pyrimidine, imidazole, oxazole, tetrazole, triazine, triazle, thiazole, indole, pyridine, thiophene, pyrazole, coumarin, oxindole, furan, etc. [55][56][57][58]. These heterocyclic nuclei possess a broad spectrum of medicinal potential such as an anti-microbial [59], antimalarial [60], anti-anxiety [61], anti-cancer [62], anti-depressant [63], anti-tubercular [64], anti-virus [65], anti-protozoal [66], and anti-convulsant, etc. Several heterocyclic analogs have been reported to affect PPAR in different diseases [67][68][69][70][71][72]. Apart from commercially available marketed drugs, Table 1. depicts the patented drugs acting on PPARs.
Table 1. Various patents on Peroxisome proliferator-activated receptor.
| Sr No | Date | Patent Number | Description | Ref. |
|---|---|---|---|---|
| 1. | 19 August 2021 | WO2021161218 | Sulfinic acid and sulfonic acid compounds for use in modulating peroxisome proliferator-activated receptors | [73] |
| 2. | 23 December 2020 | US20210238125 | Diabetes and Metabolic Syndrome Treatment with a Novel Dual Modulator of Soluble Epoxide Hydrolase and Peroxisome Proliferator-Activated Receptors | [74] |
| 3. | 18 July 2018 | US20190077774A1 | Peroxisome proliferator-activated receptor agonists | [75] |
| 4. | 28 July 2018 | US20180305403A1 | PPAR agonists and methods of use thereof | [76] |
| 5. | 01 July 2018 | US20190000790A1 | PPAR-γ activators and their therapeutical usage | [77] |
| 6. | 13 December 2017 | US20190060269A1 | Brain-Derived PPARα Ligands | [78] |
| 7. | 07 April 2017 | US20180015062A1 | PPAR-α Activator, Pharmaceutical Composition, Food and Drink, Food Additive, Supplement, and Method of Manufacturing the Same | [79] |
| 8. | 13 April 2017 | WO2017180818A1 | PPAR agonists, compounds, pharmaceutical compositions, and methods of use thereof | [80] |
| 9. | 07 April 2017 | US20170304255A1 | PPAR agonists, compounds, pharmaceutical compositions, and methods of use thereof | [81] |
| 10. | 30 January 2017 | US20170210711A1 | Competitive PPAR-α antagonists | [82] |
The SAR of these derivatives will be discussed to pave the way for the development of new, safe, and economical ligands shortly. Various heterocyclic moieties that act on the PPAR receptors have been summarized and displayed in Table 2.
Table 2. Summary of various heterocycles with the different substitutions for Peroxisome proliferator-activated receptor.
| Heterocycles | Most Potent Substitution | Activity | Reference |
|---|---|---|---|
| Thiazolidinediones | Allyl derivative (1a) | EC50 of −4.95 μM in the human PPAR-γ transactivation assay | [83] |
| Amino derivative (3b) | 1.7 times more than reference compounds in glucose uptake assay | [84] | |
| Chloro derivative (8a) | 63.15%, PPAR-γ transactivation | [85] | |
| Dichloro derivative (9a) | (−11.6930) Docking score |
[86] | |
| Nitro and carboxylic acid derivative (10d) | (−17.44) Docking score | [87] | |
| Flouro derivative (12e) | Reduced 0.09-fold the expression gene of PPARγ in cultured adipocytes compared to the control group | [88] | |
| Oxadiazole | No substitution (13d) | EC50 = −0.15 for PPARα EC50 = −0.29 for PPARδ |
[89] |
| ADAM (14) | 2.5-fold greater efficacy in activating PPARα | [90] | |
| Bromo derivative (15a) | Potency and selectivity towards PPARα/δ receptors with PPARα/δ/γ EC50, EC50 γ/α ratio, and EC50 γ/δ ratio value was 8/5/2939 nM, 367, 588 respectively | [91] | |
| Flouro derivative (16d) | PPARα −0.06 ± 0.0005, PPARγ −0.07 ± 0.0006 | [92] | |
| Bemzoimidazole | Chloro derivative | EC50 = −0.19 ± 0.01 | [93] |
| Flouro and chloro derivative | −68 ± 28j | [94] | |
| (20c) | −10.6 ± 0.4 | [95] | |
| Chloro and iodo derivative | Ki = 1023 µM for PPARγ and Ki = 0.106 µM for PPARδ | [96] | |
| Chloro derivative (22b) | PPARα 307 PPARγ 2052 PPARδ 214 | [97] | |
| (23) | Kd value = −2.8 ± 0.8 nM | [98] | |
| Methoxy derivative (24a) | [99] | ||
| Thiazole | - | - | [100] |
| (26) | EC50 > 10 µM | [101] | |
| Phenyl derivative (27b) | PPARα Imax% 22 ± 16 |
[102] | |
| p-halogenated phenyl substituted thiazole derivative (28f) | Increased PPARγ activity by almost 4- to 5-fold while rosiglitazone exhibited approximately 10-fold activation | [103] | |
| fluoro and nitro derivative (29b) | - | [104] | |
| indole | 2,4 dimethoxy derivative 30d | - | [105] |
| Chloro and phenyl derivative (31a) | PPARα/δ/γ profile at potency and efficacy level.(31a, Emax = 50%) | [106] | |
| Cyclohexyl derivative | 24.43% Reduction in blood glucose level | [107] | |
| Furan | phenyl derivative 33h | hPPARα (LBD)-GAL4 EC50 (µM) −7.31 hPPARγ (LBD)-GAL4 EC50 (µM) −2.97 hPPARδ (LBD)-GAL4 EC50 (µM) −1.98 |
[108] |
| Triflouro carbon phenyl derivative (34a) | 16.2-fold and 8.4-fold more PPAR-α/δ agonistic activity than PPARγ | [109] | |
| Triflouro carbon phenyl derivative (35a) | High potency toward PPAR- α/δ (0.26 ± 0.08 µM 0.50 ± 0.10 µM) and higher selectivity against PPARγ (4.22 ± 0.18 M) than that of GFT505 | [110] | |
| Benzopyran | Nitro derivative (36b) | EC50 −0.91 µM | [111] |
| Butane derivative (37c) | hPPARα and γ with % values of 91% and 88% using a transactivation assay | [112] | |
| Di-hydroxyl deritive (38b) | Ki value 1.41 μM | [113] | |
| 39 | hPPARα with high selectivity (123 % and 38% for α and γ, respectively). | [114] | |
| Bavachinin | (40) | PPARα agonistic activity EC50 −0.43 | [115] |
| 41 | 21 (EC50 = −22.28 μM) | [116] | |
| Miscellaneous | 42 | - | [117] |
| Cyanophenyl (43a) | IC50 6.5 µM time-resolved FRET technique | [118] | |
| 44 | EC50 (nM) = −170 ± 10 | [119] | |
| 45a | HepG2 and A549 cell growth with IC50 values of −0.54 and −0.47 μM, respectively, CCK-8 assay | [120] | |
| 46 | - | [121] | |
| Methoxy and cyclohexyl derivative | IC50 values −0.350 fluorescence polarization assay |
[122] | |
| 50a | [123] | ||
| o-Tolyl (51a1–5) | glucose uptake activity in vivo evaluation, with −37.05 ± 0.44 |
[124] | |
| 52 | - | [125] | |
| 53a | - | [126] | |
| Ethylcyclopropane derivative (54a) | - | [127] |
References
- Lee, S.J.; Samala, M.; Woo, S.Y.; Hahn, D.; Kim, D.; Kadayat, T.M.; Jung, K.; Kim, J.; Kim, D.S.; Kwon, S.; et al. Enantioselective Synthesis of a Novel Thiazoline Core as a Potent Peroxisome Proliferator-Activated Receptor δ Agonist. ACS Omega 2018, 3, 1970–1976.
- Kadayat, T.M.; Lee, G.; Jung, K.; Hwang, H.J.; Joo, J.; Hahn, D.; Hwang, H.; Park, K.G.; Cho, S.J.; Kim, K.H.; et al. Synthesis of a unique dimethyl thiazoline containing intermediate of novel peroxisome proliferator-activated receptors(PPAR)δ agonists. Tetrahedron Lett. 2018, 59, 4384–4386.
- Miyamoto, T.; Kaneko, A.; Kakizawa, T.; Yajima, H.; Kamijo, K.; Sekine, R.; Hiramatsu, K.; Nishii, Y.; Hashimoto, T.; Hashizume, K. Inhibition of Peroxisome Proliferator Signaling Pathways by Thyroid Hormone Receptor Competitive Binding To The Response Element*. J. Biol. Chem. 1997, 272, 7752–7758.
- Shi, Y.; Li, J.; Kennedy, L.J.; Tao, S.; Hernández, A.S.; Lai, Z.; Chen, S.; Wong, H.; Zhu, J.; Trehan, A.; et al. Discovery and Preclinical Evaluation of BMS-711939, an Oxybenzylglycine Based PPARα Selective Agonist. ACS Med. Chem. Lett. 2016, 7, 590–594.
- Lee, Y.; Cho, J.H.; Lee, S.; Lee, W.; Chang, S.C.; Chung, H.Y.; Moon, H.R.; Lee, J. Neuroprotective effects of MHY908, a PPAR α/γ dual agonist, in an MPTP-induced Parkinson’s disease model. Brain Res. 2019, 1704, 47–58.
- Mirza, R.; Sharma, B. A selective peroxisome proliferator-activated receptor-γ agonist benefited propionic acid-induced autism-like behavioral phenotypes in rats by attenuation of neuroinflammation and oxidative stress. Chem.-Biol. Interact. 2019, 311, 108758.
- Locci, A.; Pinna, G. Stimulation of Peroxisome Proliferator-Activated Receptor-α by N-Palmitoylethanolamine Engages Allopregnanolone Biosynthesis to Modulate Emotional Behavior. Biol. Psychiatry 2019, 85, 1036–1045.
- Ohura, A.; Itoh, T.; Ishida, H.; Saito, A.; Yamamoto, K. Three-Component Regioselective Synthesis of Tetrahydrofuro oxazoles and Their Efficient Conversion to Oxazoles. Asian J. Org. Chem. 2017, 6, 673–676.
- Li, Y.; Chen, H.; Ke, Z.; Huang, J.; Huang, L.; Yang, B.; Fan, S.; Huang, C. Identification of isotschimgine as a novel farnesoid X receptor agonist with potency for the treatment of obesity in mice. Biochem. Biophys. Res. Commun. 2020, 521, 639–645.
- Fan, S.; Tong, T.; Fang, L.; Wu, J.; Li, E.; Kang, H.; Lv, X.; Wang, X. A facile one-pot synthesis of 2-o-cyanoaryl oxazole derivatives mediated by CuCN. Tetrahedron Lett. 2018, 59, 1409–1413.
- Giampietro, L.; Gallorini, M.; de Filippis, B.; Amoroso, R.; Cataldi, A.; di Giacomo, V. PPAR-γ agonist GL516 reduces oxidative stress and apoptosis occurrence in a rat astrocyte cell line. Neurochem. Int. 2019, 126, 239–245.
- Choudhary, N.S.; Kumar, N.; Duseja, A. Peroxisome Proliferator-Activated Receptors and Their Agonists in Nonalcoholic Fatty Liver Disease. J. Clin. Exp. Hepatol. 2019, 9, 731–739.
- Shioi, R.; Okazaki, S.; Noguchi-Yachide, T.; Ishikawa, M.; Makishima, M.; Hashimoto, Y.; Yamaguchi, T. Switching subtype-selectivity: Fragment replacement strategy affords novel class of peroxisome proliferator-activated receptor α/δ (PPARα/δ) dual agonists. Bioorgan. Med. Chem. Lett. 2017, 27, 3131–3134.
- Mirza, A.Z.; Althagafi, I.I.; Shamshad, H. Role of PPAR receptor in different diseases and their ligands: Physiological importance and clinical implications. Eur. J. Med. Chem. 2019, 166, 502–513.
- Takada, I.; Makishima, M. Peroxisome proliferator-activated receptor agonists and antagonists: A patent review (2014-present). Expert Opin. Ther. Pat. 2020, 30, 1–13.
- Zoete, V.; Grosdidier, A.; Michielin, O. Peroxisome proliferator-activated receptor structures: Ligand specificity, molecular switch and interactions with regulators. Biochim. Et Biophys. Acta—Mol. Cell Biol. Lipids 2007, 1771, 915–925.
- Christofides, A.; Konstantinidou, E.; Jani, C.; Boussiotis, V.A. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metab. Clin. Exp. 2021, 114, 154338.
- D’Aniello, E.; Fellous, T.; Iannotti, F.A.; Gentile, A.; Allarà, M.; Balestrieri, F.; Gray, R.; Amodeo, P.; Vitale, R.M.; di Marzo, V. Identification and characterization of phytocannabinoids as novel dual PPARα/γ agonists by a computational and in vitro experimental approach. Biochim. Biophys. Acta—Gen. Subj. 2019, 1863, 586–597.
- Dou, X.Z.; Nath, D.; Shin, Y.; Ma, J.X.; Duerfeldt, A.S. Structure-guided evolution of a 2-phenyl-4-carboxyquinoline chemotype into PPARα selective agonists: New leads for oculovascular conditions. Bioorgan. Med. Chem. Lett. 2018, 28, 2717–2722.
- Cam, M.E.; Hazar-Yavuz, A.N.; Yildiz, S.; Keles, R.; Ertas, B.; Kabasakal, L. Dapagliflozin attenuates depressive-like behavior of male rats in the forced swim test. Eur. Neuropsychopharmacol. 2019, 29, S262–S263.
- Feng, X.Y.; Jia, W.Q.; Liu, X.; Jing, Z.; Liu, Y.Y.; Xu, W.R.; Cheng, X.C. Identification of novel PPARα/γ dual agonists by pharmacophore screening, docking analysis, ADMET prediction, and molecular dynamics simulations. Comput. Biol. Chem. 2019, 78, 178–189.
- Wang, Y.; Nakajima, T.; Gonzalez, F.J.; Tanaka, N. PPARs as metabolic regulators in the liver: Lessons from liver-specific PPAR-null mice. Int. J. Mol. Sci. 2020, 21, 2061.
- Lamichane, S.; Lamichane, B.D.; Kwon, S.M. Pivotal roles of peroxisome proliferator-activated receptors (PPARs) and their signal cascade for cellular and whole-body energy homeostasis. Int. J. Mol. Sci. 2018, 19, 949.
- Tyagi, S.; Gupta, P.; Saini, A.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240.
- Popeijus, H.E. Peroxisome Proliferator-Activated Receptor Alpha (PPAR-Alpha). In Encyclopedia of Signaling Molecules; Springer: New York, NY, USA, 2016; pp. 1–6.
- Popeijus, H.E.; van Otterdijk, S.D.; van der Krieken, S.E.; Konings, M.; Serbonij, K.; Plat, J.; Mensink, R.P. Fatty acid chain length and saturation influences PPARα transcriptional activation and repression in HepG2 cells. Mol. Nutr. Food Res. 2014, 58, 2342–2349.
- Wirth, M.; Benson, G.; Schwarz, C.; Obe, T.K.; Stekovic, S.; Madeo, F.; Oel, A.F. O1-12-03 Spermidine Supplementation To Improve Episodic Memory In Aging Adults At Risk Of Dementia. Alzheimer’s Dement. 2018, 14, 250.
- Kim, D.S.; Lee, J.; Londhe, A.M.; Kadayat, T.M.; Joo, J.; Hwang, H.; Kim, K.H.; Pae, A.N.; Chin, J.; Cho, S.J.; et al. Synthesis and evaluation of an orally available “Y”-shaped biaryl peroxisome proliferator-activated receptor δ agonist. Bioorgan. Med. Chem. 2018, 26, 4382–4389.
- Rohini, A.; Agrawal, N.; Kumar, H.; Nath, V.; Kumar, V. Norbixin, an apocarotenoid derivative activates PPARγ in cardiometabolic syndrome: Validation by in silico and in vivo experimental assessment. Life Sci. 2018, 209, 69–77.
- Grygiel-Górniak, B. Peroxisome Proliferator-Activated Receptors and Their Ligands: Nutritional and Clinical Implications-a Review. 2014. Available online: http://www.nutritionj.com/content/13/1/17 (accessed on 15 July 2022).
- Govindarajulu, M.Y. P3-040: Novel Ppar-Γ Agonist Improves Pathologies And Memory Deficits In In Vitro And In Vivo Models Of Alzheimer’s Disease. Alzheimer’s Dementia 2018, 14, 1079.
- Li, Y.; Xu, L.; Zeng, K.; Xu, Z.; Suo, D.; Peng, L.; Ren, T.; Sun, Z.; Yang, W.; Jin, X.; et al. Propane-2-sulfonic acid octadec-9-enyl-amide, a novel PPARα/γ dual agonist, protects against ischemia-induced brain damage in mice by inhibiting inflammatory responses. Brain Behav. Immun. 2017, 66, 289–301.
- Stebbins, K.J.; Broadhead, A.R.; Cabrera, G.; Correa, L.D.; Messmer, D.; Bundey, R.; Baccei, C.; Bravo, Y.; Chen, A.; Stock, N.S.; et al. In vitro and in vivo pharmacology of NXT629, a novel, and selective PPARα antagonist. Eur. J. Pharmacol. 2017, 809, 130–140.
- Hirschfield, G.M.; Galambos, M.R.; Bowlus, C.L.; Swain, M.G.; Doerffel, Y.; Steinberg, A.S.; Varga, M.; Choi, Y.-J.; Martin, R.L.; Chera, H.; et al. Proof of efficacy for Seladelpar, a selective PPAR-δ agonist, in patients with primary biliary cholangitis non-responsive to ursodeoxycholic acid: Results of an international Phase 2 randomized controlled clinical study. J. Hepatol. 2017, 66, S357.
- Wang, Y.; Guan, X.; Gao, C.L.; Ruan, W.; Zhao, S.; Kai, G.; Li, F.; Pang, T. Medioresinol as a novel PGC-1α activator prevents pyroptosis of endothelial cells in ischemic stroke through PPARα-GOT1 axis. Pharmacol. Res. 2021, 169, 105640.
- Huang, F.; Zeng, Z.; Zhang, W.; Yan, Z.; Chen, J.; Yu, L.; Yang, Q.; Li, Y.; Yu, H.; Chen, J.; et al. Design, synthesis, and biological evaluation of novel sulindac derivatives as partial agonists of PPARγ with potential anti-diabetic efficacy. Eur. J. Med. Chem. 2021, 222, 113542.
- Zhou, Y.; Guo, Y.; Zhu, Y.; Sun, Y.; Li, W.; Li, Z.; Wei, L. Dual PPARγ/ɑ agonist oroxyloside suppresses cell cycle progression by glycolipid metabolism switch-mediated increase of reactive oxygen species levels. Free Radic. Biol. Med. 2021, 167, 205–217.
- Decara, J.; Rivera, P.; López-Gambero, A.J.; Serrano, A.; Pavón, F.J.; Baixeras, E.; de Fonseca, F.R.; Suárez, J. Peroxisome Proliferator-Activated Receptors: Experimental Targeting for the Treatment of Inflammatory Bowel Diseases. Front. Pharmacol. 2020, 11, 730.
- Ferré, P. The Biology of Peroxisome Proliferator-Activated Receptors Relationship With Lipid Metabolism and Insulin Sensitivity. Diabetes 2004, 53, S43–S50.
- Tinto, F.; Archambault, A.S.; Dumais, É.; Rakotoarivelo, V.; Kostrzewa, M.; Martin, C.; Plante, P.L.; Desjardins, Y.; Simard, M.; Pouliot, R.; et al. Synthesis and molecular targets of N-13-hydroxy-octadienoyl-ethanolamine, a novel endogenous bioactive 15-lipoxygenase-derived metabolite of N-linoleoyl-ethanolamine found in the skin and saliva. Biochim. Biophys. Acta—Mol. Cell Biol. Lipids 2021, 1866, 158954.
- DeFronzo, R.A. Chiglitazar: A novel pan-PPAR agonist. Sci. Bull. 2021, 66, 1497–1498.
- Dharavath, R.N.; Arora, S.; Kondepudi, K.K.; Bishnoi, M.; Chopra, K. Saroglitazar, a novel dual PPAR-α/γ agonist, reverses high fat-low protein diet-induced metabolic and cognitive aberrations in C57BL/6J male mice. Life Sci. 2021, 271, 119191.
- Rzemieniec, J.; Litwa, E.; Wnuk, A.; Lason, W.; Kajta, M. Bazedoxifene and raloxifene protect neocortical neurons undergoing hypoxia via targeting ERα and PPAR-γ. Mol. Cell. Endocrinol. 2018, 461, 64–78.
- Usui-Ouchi, A.; Ouchi, Y.; Ebihara, N. The peroxisome proliferator-activated receptor pan-agonist bezafibrate suppresses microvascular inflammatory responses of retinal endothelial cells and vascular endothelial growth factor production in retinal pigmented epithelial cells. Int. Immunopharmacol. 2017, 52, 70–76.
- Takei, K.; Nakagawa, Y.; Wang, Y.; Han, S.I.; Satoh, A.; Sekiya, M.; Matsuzaka, T.; Shimano, H. Effects of K-877, a novel selective PPARα modulator, on small intestine contribute to the amelioration of hyperlipidemia in low-density lipoprotein receptor knockout mice. J. Pharmacol. Sci. 2017, 133, 214–222.
- Piemontese, L.; Cerchia, C.; Laghezza, A.; Ziccardi, P.; Sblano, S.; Tortorella, P.; Iacobazzi, V.; Infantino, V.; Convertini, P.; Piaz, F.D.; et al. New diphenylmethane derivatives as peroxisome proliferator-activated receptor alpha/gamma dual agonists endowed with anti-proliferative effects and mitochondrial activity. Eur. J. Med. Chem. 2017, 127, 379–397.
- Rodríguez-Pascau, L.; Britti, E.; Calap-Quintana, P.; Dong, Y.N.; Vergara, C.; Delaspre, F.; Medina-Carbonero, M.; Tamarit, J.; Pallardó, F.v.; Gonzalez-Cabo, P.; et al. PPAR gamma agonist leriglitazone improves frataxin-loss impairments in cellular and animal models of Friedreich Ataxia. Neurobiol. Dis. 2021, 148, 105162.
- Ji, L.; Song, W.; Fang, H.; Li, W.; Geng, J.; Wang, Y.; Guo, L.; Cai, H.; Yang, T.; Li, H.; et al. Efficacy and safety of chiglitazar, a novel peroxisome proliferator-activated receptor pan-agonist, in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled, phase 3 trial (CMAP). Sci. Bull. 2021, 66, 1571–1580.
- Barak, Y.; Liao, D.; He, W.; Ong, E.S.; Nelson, M.C.; Olefsky, J.M.; Boland, R.; Evans, R.M. Effects of peroxisome proliferator-activated receptor δ on placentation, adiposity, and colorectal cancer. Proc. Natl. Acad. Sci. USA 2002, 99(1), 303–308.
- Boyer-Diaz, Z.; Aristu-Zabalza, P.; Andrés-Rozas, M.; Robert, C.; Ortega-Ribera, M.; Fernández-Iglesias, A.; Broqua, P.; Junien, J.L.; Wettstein, G.; Bosch, J.; et al. Pan-PPAR agonist lanifibranor improves portal hypertension and hepatic fibrosis in experimental advanced chronic liver disease. J. Hepatol. 2021, 74, 1188–1199.
- Abdel-Razek, E.A.N.; Abo-Youssef, A.M.; Azouz, A.A. Benzbromarone mitigates cisplatin nephrotoxicity involving enhanced peroxisome proliferator-activated receptor-alpha (PPAR-α) expression. Life Sci. 2020, 243, 117272.
- Tutunchi, H.; Ostadrahimi, A.; Saghafi-Asl, M.; Hosseinzadeh-Attar, M.J.; Shakeri, A.; Asghari-Jafarabadi, M.; Roshanravan, N.; Farrin, N.; Naemi, M.; Hasankhani, M. Oleoylethanolamide supplementation in obese patients newly diagnosed with non-alcoholic fatty liver disease: Effects on metabolic parameters, anthropometric indices, and expression of PPAR-α, UCP1, and UCP2 genes. Pharmacol. Res. 2020, 156, 104770.
- Kim, D.H.; Kim, D.H.; Heck, B.E.; Shaffer, M.; Yoo, K.H.; Hur, J. PPAR-δ agonist affects adipo-chondrogenic differentiation of human mesenchymal stem cells through the expression of PPAR-γ. Regen. Ther. 2020, 15, 103–111.
- Horikawa, T.; Kawanami, T.; Hamaguchi, Y.; Tanaka, Y.; Kita, S.; Ryorin, R.; Takashi, Y.; Takahashi, H.; Tanabe, M.; Yanase, T.; et al. Pemafibrate, a PPAR alpha agonist, attenuates neointima formation after vascular injury in mice fed normal chow and a high-fat diet. Heliyon 2020, 6, e05431.
- Sanchez, M.B.; Miranda-Perez, E.; Verjan, J.C.G.; Barrera, M.d.F.; Perez-Ramos, J.; Alarcon-Aguilar, F.J. Potential of the chlorogenic acid as multitarget agent: Insulin-secretagogue and PPAR α/γ dual agonist. Biomed. Pharmacother. 2017, 94, 169–175.
- Bernardo, A.; Giammarco, M.L.; de Nuccio, C.; Ajmone-Cat, M.A.; Visentin, S.; de Simone, R.; Minghetti, L. Docosahexaenoic acid promotes oligodendrocyte differentiation via PPAR-γ signaling and prevents tumor necrosis factor-α-dependent maturational arrest. Biochim. Biophys. Acta—Mol. Cell Biol. Lipids 2017, 1862, 1013–1023.
- Song, G.J.; Nam, Y.; Jo, M.; Jung, M.; Koo, J.Y.; Cho, W.; Koh, M.; Park, S.B.; Suk, K. A novel small-molecule agonist of PPAR-γ potentiates an anti-inflammatory M2 glial phenotype. Neuropharmacology 2016, 109, 159–169.
- Okazaki, S.; Shioi, R.; Noguchi-Yachide, T.; Ishikawa, M.; Makishima, M.; Hashimoto, Y.; Yamaguchi, T. Structure-activity relationship studies of non-carboxylic acid peroxisome proliferator-activated receptor α/δ (PPARα/δ) dual agonists. Bioorgan. Med. Chem. 2016, 24, 5455–5461.
- Kinarivala, N.; Suh, J.H.; Botros, M.; Webb, P.; Trippier, P.C. Pharmacophore elucidation of phosphoiodyn A—Potent and selective peroxisome proliferator-activated receptor β/δ agonists with neuroprotective activity. Bioorgan. Med. Chem. Lett. 2016, 26, 1889–1893.
- Lund, J.; Stensrud, C.; Rajender; Bohov, P.; Thoresen, G.H.; Berge, R.K.; Wright, M.; Kamal, A.; Rustan, A.C.; Miller, A.D.; et al. The molecular structure of thioether fatty acids influences PPAR-dependent regulation of lipid metabolism. Bioorgan. Med. Chem. 2016, 24, 1191–1203.
- Agarwal, S.; Yadav, A.; Chaturvedi, R.K. Peroxisome proliferator-activated receptors (PPARs) as therapeutic target in neurodegenerative disorders. Biochem. Biophys. Res. Commun. 2017, 483, 1166–1177.
- Behl, T.; Kaur, I.; Goel, H.; Kotwani, A. Implications of the endogenous PPAR-gamma ligand, 15-deoxy-delta-12, 14-prostaglandin J2, in diabetic retinopathy. Life Sci. 2016, 153, 93–99.
- Ishibashi, S.; Yamashita, S.; Arai, H.; Araki, E.; Yokote, K.; Suganami, H.; Fruchart, J.C.; Kodama, T. Effects of K-877, a novel selective PPARα modulator (SPPARMα), in dyslipidaemic patients: A randomized, double-blind, active- and placebo-controlled, phase 2 trial. Atherosclerosis 2016, 249, 36–43.
- Meyer, M.; Foulquier, S.; Dupuis, F.; Flament, S.; Grimaud, L.; Henrion, D.; Lartaud, I.; Monard, G.; Grillier-Vuissoz, I.; Boisbrun, M. Synthesis and evaluation of new designed multiple ligands directed towards both peroxisome proliferator-activated receptor-γ and angiotensin II type 1 receptor. Eur. J. Med. Chem. 2018, 158, 334–352.
- Jacintho, J.D.; Baccei, C.S.; Bravo, Y.; Broadhead, A.; Chen, A.; Correa, L.; Fischer, K.; Laffitte, B.; Lee, C.; Lorrain, D.S.; et al. Discovery of potent and selective PPARα/δ dual antagonists and initial biological studies. Bioorgan. Med. Chem. Lett. 2019, 29, 503–508.
- Chen, D.; Jia, D.; Wu, X.; Shi, K.; Ren, C.; Dou, Y.; Guo, M.; Wang, J.; Ma, M.; Wu, Z.; et al. A novel metformin derivative showed improvement of lipid metabolism in obese rats with type 2 diabetes. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1382–1392.
- Lin, L.; Metherel, A.H.; di Miceli, M.; Liu, Z.; Sahin, C.; Fioramonti, X.; Cummins, C.L.; Layé, S.; Bazinet, R.P. Tetracosahexaenoylethanolamide, a novel N-acylethanolamide, is elevated in ischemia and increases neuronal output. J. Lipid Res. 2020, 61, 1480–1490.
- Laghezza, A.; Piemontese, L.; Tortorella, P.; Loiodice, F. An update about the crucial role of stereochemistry on the effects of Peroxisome Proliferator-Activated Receptor ligands. Eur. J. Med. Chem. 2019, 176, 326–342.
- Wada, Y.; Maekawa, M.; Ohnishi, T.; Balan, S.; Matsuoka, S.; Iwamoto, K.; Iwayama, Y.; Ohba, H.; Watanabe, A.; Hisano, Y.; et al. Peroxisome proliferator-activated receptor α as a novel therapeutic target for schizophrenia. EBioMedicine 2020, 62, 103130.
- Li, Z.; Zhou, Z.; Hu, L.; Deng, L.; Ren, Q.; Zhang, L. ZLY032, the first-in-class dual FFA1/PPARδ agonist, improves glucolipid metabolism and alleviates hepatic fibrosis. Pharmacol. Res. 2020, 159, 105035.
- Lim, J.J.; Dutta, M.; Dempsey, J.L.; Lehmler, H.J.; MacDonald, J.; Bammler, T.; Walker, C.; Kavanagh, T.J.; Gu, H.; Mani, S.; et al. Neonatal exposure to BPA, BDE-99, and PCB produces persistent changes in hepatic transcriptome associated with gut dysbiosis in adult mouse livers. Toxicol. Sci. 2021, 184, 83–103.
- Eeda, V.; Wu, D.; Lim, H.Y.; Wang, W. Design, synthesis, and evaluation of potent novel peroxisome proliferator-activated receptor γ indole partial agonists. Bioorgan. Med. Chem. Lett. 2019, 29, 126664.
- Judkins, R.; Benthem, L. Sulfinic Acid and Sulfonic Acid Compounds for Use in Modulating Peroxisome Proliferator-Activated Receptors. WO2021161218, 19 August 2021. Available online: https://patents.google.com/patent/WO2021161218A1 (accessed on 15 July 2022).
- Imig, J.D.; Khan, M.A.H.; Proschak, E.; Blocher, R. Diabetes and Metabolic Syndrome Treatment with a Novel Dual Modulator of Soluble Epoxide Hydrolase and Peroxisome Proliferator-Activated Receptors. U.S. Patent 20210238125, 8 May 2021.
- Yu, D.D.; Forman, B.M.; Li, H. Peroxisome Proliferator-Activated Receptor Agonists. U.S. Patent 20190077774A1, 14 March 2019.
- Evans, R.M.; Downes, M. PPAR Agonists and Methods of Use Thereof. U.S. Patent 20180305403A1, 4 February 2020.
- Antkaly, T. PPAR-γ Activators and Their Therapeutic Usages. U.S. Patent 2020190000790A1, 3 January 2019.
- Pahan, K.; Roy, A. Brain PPARα Ligands. U.S. Patent 20190060269A1, 28 February 2019.
- Uji-Shi, T.K.; Uji-Shi, T.G.; Uji-Shi, H.T.; Uji-Shi, H.C.; Ichip, N.; Nakata, K. PPARα Activator, Pharmaceutical Composition, Food and Drink, Food Additive, Supplement and Method of Manufacturing the Same. U.S. Patent 20180015062A1, 2017. Available online: https://patents.google.com/patent/US20180015062A1 (accessed on 15 July 2022).
- Legu, B.; Senaiar, R. PPAR Agonist, Compounds, Pharmaceutical Composition, and Method of Use Thereof. WO2017180818A1, 2017. Available online: https://patents.google.com/patent/WO2017180818A1 (accessed on 15 July 2022).
- Baiga, T.; Downes, M.; Evans, R.; Kluge, A.; Laga, B.; Miura, M.; Panograhi, S.K. PPAR Agonist, Compounds, Pharmaceutical Composition, and Method of Use Thereof. US20170304255A1, 26 October 2017. Available online: https://patents.google.com/patent/US20170304255A1 (accessed on 15 July 2022).
- Knape, T.; Knethen, A.V.; Parnham, M.J.; Schubert-Zsilavecz, M.; Wurglics, M.; Flesch, D. Comoetative PPAR-α Antagonista. U.S. Patent 20170210711A1, 27 July 2017.
- Darwish, K.M.; Ismail, S.; Samia, M.; Mohamed, S.G.; Mohamed, A.H. Design, synthesis, and biological evaluation of novel thiazolidinediones as PPARγ/FFAR1 dual agonists. Eur. J. Med. Chem. 2016, 109, 157–172.
- Chhajed, S.S.; Chaskar, S.; Kshirsagar, S.K.; Haldar, G.M.A.; Mahapatra, D.K. Rational design and synthesis of some PPAR-γ agonists: Substituted benzylideneamino-benzylidene-thiazolidine-2,4-diones. Comput. Biol. Chem. 2017, 67, 260–265.
- Abdellatif, K.R.A.; Fadaly, W.A.A.; Kamel, G.M.; Elshaier, Y.A.M.M.; El-Magd, M.A. Design, synthesis, modeling studies and biological evaluation of thiazolidine derivatives containing pyrazole core as potential anti-diabetic PPAR-γ agonists and anti-inflammatory COX-2 selective inhibitors. Bioorgan. Chem. 2019, 82, 86–99.
- Srivastava, A.R.; Bhatia, R.; Chawla, P. Synthesis, biological evaluation and molecular docking studies of novel 3,5-disubstituted 2,4-thiazolidinediones derivatives. Bioorgan. Chem. 2019, 89, 102993.
- Dai, L.; Feng, Z.; Zha, R.; Cheng, K.; Wen, X.; Sun, H.; Yuan, H. Discovery of Novel Peroxisome Proliferator-Activated Receptor α (PPARα) Agonists by Virtual Screening and Biological Evaluation. J. Chem. Inf. Model. 2020, 60, 1717–1727.
- Shakour, N.; Sahebkar, A.; Karimi, G.; Paseban, M.; Tasbandi, A.; Mosaffa, F.; Tayarani-Najaran, Z.; Ghodsi, R.; Hadizadeh, F. Design, synthesis and biological evaluation of novel 5-(imidazolyl-methyl) thiazolidinediones as antidiabetic agents. Bioorgan. Chem. 2021, 115, 105162.
- Okazaki, S.; Noguchi-Yachide, T.; Sakai, T.; Ishikawa, M.; Makishima, M.; Hashimoto, Y.; Yamaguchi, T. Discovery of N-(1-(3-(4-phenoxyphenyl)-1,2,4-oxadiazol-5-yl)ethyl)acetamides as novel acetyl-CoA carboxylase 2 (ACC2) inhibitors with peroxisome proliferator-activated receptor α/δ (PPARα/δ) dual agonistic activity. Bioorgan. Med. Chem. 2016, 24, 5258–5269.
- Arnesen, H.; Haj-Yasein, N.N.; Tungen, J.E.; Soedling, H.; Matthews, J.; Paulsen, S.M.; Nebb, H.I.; Sylte, I.; Hansen, T.V.; Sæther, T. Molecular modeling, synthesis, and biological evaluations of a 3,5-disubstituted isoxazole fatty acid analog as a PPARα-selective agonist. Bioorgan. Med. Chem. 2019, 27, 4059–4068.
- Jiang, Z.; Liu, X.; Yuan, Z.; He, H.; Wang, J.; Zhang, X.; Gong, Z.; Hou, L.; Shen, L.; Guo, F.; et al. Discovery of a Novel Selective Dual Peroxisome Proliferator-Activated Receptor α/δAgonist for the Treatment of Primary Biliary Cirrhosis. ACS Med. Chem. Lett. 2019, 10, 1068–1073.
- Kaur, P.; Bhat, Z.R.; Bhat, S.; Kumar, R.; Kumar, R.; Tikoo, K.; Gupta, J.; Khurana, N.; Kaur, J.; Khatik, G.L. Synthesis and evaluation of new 1,2,4-oxadiazole based trans- acrylic acid derivatives as potential PPAR-alpha/gamma dual agonist. Bioorgan. Chem. 2020, 100, 103867.
- Obermoser, V.; Mauersberger, R.; Schuster, D.; Czifersky, M.; Lipova, M.; Siegl, M.; Kintscher, U.; Gust, R. Importance of 5/6-aryl substitution on the pharmacological profile of 4ʹ-((2-propyl-1H-benzoimidazol-1-yl)methyl)--2-carboxylic acid derived PPARγ agonists. Eur. J. Med. Chem. 2017, 126, 590–603.
- Shinozuka, T.; Tsukada, T.; Fujii, K.; Tokumaru, E.; Shimada, K.; Onishi, Y.; Matsui, Y.; Wakimoto, S.; Kuroha, M.; Ogata, T.; et al. Discovery of DS-6930, a potent selective PPARγ modulator. Part I: Lead Identif. Bioorgan. Med. Chem. 2018, 26, 5079–5098.
- Yamamoto, K.; Tamura, T.; Nakamura, R.; Hosoe, S.; Matsubara, M.; Nagata, K.; Kodaira, H.; Uemori, T.; Takahashi, Y.; Suzuki, M.; et al. Development of a novel class of peroxisome proliferator-activated receptor (PPAR) gamma ligands as an anticancer agent with a unique binding mode based on a non-thiazolidinedione scaffold. Bioorgan. Med. Chem. 2019, 27, 115122.
- An, S.; Kim, G.; Kim, H.J.; Ahn, S.; Kim, H.Y.; Ko, H.; Hyun, Y.E.; Nguyen, M.; Jeong, J.; Liu, Z.; et al. Discovery and Structure-Activity Relationships of Novel Template, Truncated 1′-Homologated Adenosine Derivatives as Pure Dual PPARγ/δModulators. J. Med. Chem. 2020, 63, 16012–16027.
- Li, Z.; Xu, Y.; Cai, Z.; Wang, X.; Ren, Q.; Zhou, Z.; Xie, R. Discovery of novel dual PPARα/δ agonists based on benzimidazole scaffold for the treatment of non-alcoholic fatty liver disease. Bioorgan. Chem. 2020, 99, 103803.
- Ma, L.; Lian, Y.; Tang, J.; Chen, F.; Gao, H.; Zhou, Z.; Hou, N.; Yi, W. Identification of the anti-fungal drug fenticonazole nitrate as a novel PPARγ-modulating ligand with good therapeutic index: Structure-based screening and biological validation. Pharmacol. Res. 2021, 173, 105860.
- Bhalla, J.; Bari, S.S.; Chaudhary, G.R.; Kumar, A.; Rathee, A.; Sharma, R.; Bhalla, A. Stereoselective synthesis and in-silico evaluation of C4-benzimidazolyloxyphenyl substituted trans-β-lactam derivatives as promising novel PPARγ activators. Synth. Commun. 2021, 51, 3758–3767.
- Kharbanda, C.; Alam, M.S.; Hamid, H.; Javed, K.; Bano, S.; Ali, Y.; Dhulap, A.; Alam, P.; Pasha, M.A.Q. Novel Piperine Derivatives with Antidiabetic Effect as PPAR-γ Agonists. Chem. Biol. Drug Des. 2016, 88, 354–362.
- Li, Z.; Chen, Y.; Zhou, Z.; Deng, L.; Xu, Y.; Hu, L.; Liu, B.; Zhang, L. Discovery of first-in-class thiazole-based dual FFA1/PPARδ agonists as potential anti-diabetic agents. Eur. J. Med. Chem. 2019, 164, 352–365.
- Ammazzalorso, A.; de Lellis, L.; Florio, R.; Laghezza, A.; de Filippis, B.; Fantacuzzi, M.; Giampietro, L.; Maccallini, C.; Tortorella, P.; Veschi, S.; et al. Synthesis of novel benzothiazole amides: Evaluation of PPAR activity and anti-proliferative effects in paraganglioma, pancreatic and colorectal cancer cell lines. Bioorgan. Med. Chem. Lett. 2019, 29, 2302–2306.
- Mourad, A.A.E.; Mourad, M.A.E. Enhancing insulin sensitivity by dual PPARγ partial agonist, β-catenin inhibitor: Design, synthesis of new αphthalimido-o-toluoyl2-aminothiazole hybrids. Life Sci. 2020, 259, 118270.
- Patchipala, S.B.; Pasupuleti, V.R.; Audipudi, A.V.; babu Bollikolla, H. Synthesis, in-vivo anti-diabetic & anticancer activities and molecular modeling studies of tetrahydrobenzothiazole tethered nicotinohydrazide derivatives. Arab. J. Chem. 2022, 15, 103546.
- Fu, Y.; Ma, J.; Shi, X.; Song, X.Y.; Yang, Y.; Xiao, S.; Li, J.; Gu, W.J.; Huang, Z.; Zhang, J.; et al. A novel pyrazole-containing indolizine derivative suppresses NF-κB activation and protects against TNBS-induced colitis via a PPAR-γ-dependent pathway. Biochem. Pharmacol. 2017, 135, 126–138.
- Boubia, B.; Poupardin, O.; Barth, M.; Binet, J.; Peralba, P.; Mounier, L.; Jacquier, E.; Gauthier, E.; Lepais, V.; Chatar, M.; et al. Design Synthesis, and Evaluation of a Novel Series of Indole Sulfonamide Peroxisome Proliferator-Activated Receptor (PPAR) α/γ/δ Triple Activators: Discovery of Lanifibranor, a New Antifibrotic Clinical Candidate. J. Med. Chem. 2018, 61, 2246–2265.
- El-Zahabi, M.A.; Elbendary, E.R.; Bamanie, F.H.; Radwan, M.F.; Ghareib, S.A.; Eissa, I.H. Design, synthesis, molecular modeling and anti-hyperglycemic evaluation of phthalimide-sulfonylurea hybrids as PPARγ and SUR agonists. Bioorgan. Chem. 2019, 91, 103115.
- Liu, J.; Su, X.; Li, H.; Fan, L.; Li, Y.; Tang, X.; Yan, J.; Chen, X.; Chen, F.; Liu, J.; et al. Design, synthesis, and evaluation of novel L-phenyl glycine derivatives as potential PPARγ lead compounds. Bioorgan. Med. Chem. 2018, 26, 4153–4167.
- Ding, X.; Kang, D.; Sun, L.; Zhan, P.; Liu, X. Combination of 2D and 3D-QSAR studies on DAPY and DANA derivatives as potent HIV-1 NNRTIs. J. Mol. Struct. 2022, 1249, 131603.
- Liu, K.; Zhao, X.; Qi, X.; Hou, D.L.; Li, H.b.; Gu, Y.H.; Xu, Q.L. Design, synthesis, and biological evaluation of a novel dual peroxisome proliferator-activated receptor alpha/delta agonist for the treatment of diabetic kidney disease through anti-inflammatory mechanisms. Eur. J. Med. Chem. 2021, 218, 1802–1812.
- Niu, H.; Wang, W.; Li, J.; Lei, Y.; Zhao, Y.; Yang, W.; Zhao, C.; Lin, B.; Song, S.; Wang, S. A novel structural class of coumarin-chalcone fibrates as PPARα/γ agonists with potent antioxidant activities: Design, synthesis, biological evaluation, and molecular docking studies. Eur. J. Med. Chem. 2017, 138, 212–220.
- Bermejo, A.; Collado, A.; Barrachina, I.; Marqués, P.; el Aouad, N.; Franck, X.; Garibotto, F.; Dacquet, C.; Caignard, D.H.; Suvire, F.D.; et al. Polycerasoidol, a Natural Prenylated Benzopyran with a Dual PPARα/PPARγAgonist Activity and Anti-inflammatory Effect. J. Nat. Prod. 2019, 82, 1802–1812.
- Ahn, S.; Kim, J.; An, S.; Pyo, J.J.; Jung, D.; Lee, J.; Hwang, S.Y.; Gong, J.; Shin, I.; Kim, H.P.; et al. 2-Phenyl-8-(1-phenyl allyl)-chromenone compounds have a pan-PPAR modulator pharmacophore. Bioorgan. Med. Chem. 2019, 27, 2948–2958.
- Vila, L.; Cabedo, N.; Villarroel-Vicente, C.; García, A.; Bernabeu, Á.; Hennuyer, N.; Staels, B.; Franck, X.; Figadère, B.; Sanz, M.J.; et al. Synthesis and biological studies of “Polycerasoidol” and “trans-δ-Tocotrienolic acid” derivatives as PPARα and/or PPARγ agonists. Bioorgan. Med. Chem. 2022, 53, 116532.
- Du, G.; Zhao, Y.; Feng, L.; Yang, Z.; Shi, J.; Huang, C.; Li, B.; Guo, F.; Zhu, W.; Li, Y. Design, Synthesis, and Structure-Activity Relationships of Bavachinin Analogues as Peroxisome Proliferator-Activated Receptor γ Agonists. ChemMedChem 2017, 12, 183–193.
- Yi, J.; Du, G.; Zhao, Y.; Zhang, L.; Li, B.; Zhu, W.; Huang, C.; Li, Y.; Guo, F. Bavachinin analogs as agonists of pan-peroxisome proliferator-activated receptors. Med. Chem. Res. 2018, 27, 1851–1862.
- Ahn, S.; Lee, M.; An, S.; Hyun, S.; Hwang, J.; Lee, J.; Noh, M. 2-Formyl-komarovicine promotes adiponectin production in human mesenchymal stem cells through PPARγ partial agonism. Bioorgan. Med. Chem. 2018, 26, 1069–1075.
- Dixit, V.A.; Rathi, P.C.; Bhagat, S.; Gohlke, H.; Petersen, R.K.; Kristiansen, K.; Chakraborti, A.K.; Bharatam, P.V. Design and synthesis of novel Y-shaped barbituric acid derivatives as PPARγ activators. Eur. J. Med. Chem. 2016, 108, 423–435.
- Frkic, R.L.; He, Y.; Rodriguez, B.B.; Chang, M.R.; Kuruvilla, D.; Ciesla, A.; Abell, A.D.; Kamenecka, T.M.; Griffin, P.R.; Bruning, J.B. Structure-Activity Relationship of 2,4-Dichloro-N-(3,5-dichloro-4-(quinolin-3-yloxy)phenyl)benzenesulfonamide (INT131) Analogs for PPARγ-Targeted Antidiabetics. J. Med. Chem. 2017, 60, 4584–4593.
- Gan, L.; Gan, Z.; Dan, Y.; Li, Y.; Zhang, P.; Chen, S.; Ye, Z.; Pan, T.; Wan, C.; Hu, X.; et al. Tetrazanbigen Derivatives as Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) Partial Agonists: Design, Synthesis, Structure-Activity Relationship, and Anticancer Activities. J. Med. Chem. 2021, 64, 1018–1036.
- Zhao, S.; Kanno, Y.; Li, W.; Wakatabi, H.; Sasaki, T.; Koike, K.; Nemoto, K.; Li, H. Picrasidine N Is a Subtype-Selective PPARβ/δ Agonist. J. Nat. Prod. 2016, 79, 879–885.
- Ibrahim, M.K.; Eissa, I.H.; Alesawy, M.S.; Metwaly, A.M.; Radwan, M.M.; ElSohly, M.A. Design, synthesis, molecular modeling and anti-hyperglycemic evaluation of quinazolin-4(3H)-one derivatives as potential PPARγ and SUR agonists. Bioorgan. Med. Chem. 2017, 25, 4723–4744.
- Yu, D.D.; van Citters, G.; Li, H.; Stoltz, B.M.; Forman, B.M. Discovery of novel modulators for the PPARα (peroxisome proliferator-activated receptor α): Potential therapies for nonalcoholic fatty liver disease. Bioorgan. Med. Chem. 2021, 41, 116193.
- Mandal, S.P.; Reji, A.; Bhavimani, G.; Prabitha, P.; Durai, P.; Yuvaraj, S.; Shashank, A.; Krishna, K.L.; Kumar, B.R.P. Rational Design, Synthesis and Evaluation of Novel Rodanine Derivatives for Antihyperglycemic Activity. Polycycl. Aromat. Compd. 2020, 42, 1794–1805.
- Hu, X.; Wan, C.; Gan, Z.; Liu, R.; Chen, Y.; Wang, J.; Gan, L.; Chen, Y.; Li, Y.; He, B.; et al. TNBG-5602, a novel derivative of quinoxaline, inhibits liver cancer growth via upregulating peroxisome proliferator-activated receptor γ in vitro and in vivo. J. Pharm. Pharmacol. 2019, 71, 1684–1694.
- Xie, Y.D.; Xu, Y.H.; Liu, J.P.; Wang, B.; Shi, Y.H.; Wang, W.; Wang, X.P.; Sun, M.; Xu, X.Y.; Bian, X.L. 1,3-Benzodioxole-based fibrate derivatives as potential hypolipidemic and hepatoprotective agents. Bioorgan. Med. Chem. Lett. 2021, 43, 127898.
- Li, Z.; Ren, Q.; Zhou, Z.; Cai, Z.; Wang, B.; Han, J.; Zhang, L. Discovery of the first-in-class dual PPARδ/γ partial agonist for the treatment of metabolic syndrome. Eur. J. Med. Chem. 2021, 225, 113807.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
08 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




