Mediterranean diet (Med-D) represents one of the most famous and well received diet food habits, particularly in the western countries
[1]. Med-D originates from the eating patterns that people around Mediterranean basin have been following since the ancient years, along with the adoption of food products that were cultivated later in the area (i.e., potatoes)
[2][3]. Med-D promotes the consumption of all food types but focuses on vegetables, fruits, the nearly exclusive use of olive oil in food preparation, routine consumption of marine food, low fat white meat (chicken, turkey) along with cereals, grains, nuts, wine and lesser consumption of high-fat meat products
[3]. UNESCO states regarding Med-D:
“The Mediterranean diet is characterized by a nutritional model that has remained constant over time and space, consisting mainly of olive oil, cereals, fresh or dried fruit and vegetables, a moderate amount of fish, dairy and meat, and many condiments and spices, all accompanied by wine or infusions, always respecting beliefs of each community” [4]. Since the first observations in 1960s
[5], Med-D has shown undisputable, longitudinal, and high-quality evidence to support its health benefits against specific diseases for people that remain adherent to Med-D dietary patterns
[6][7][8][9]. A reduced risk of overall mortality related with metabolic syndrome, cardiovascular diseases, coronary heart disease, myocardial infarction, cancer incidence, diabetes, neurodegenerative diseases, kidney disease and arthritis are mostly identified regarding clinical data availability
[10][11][12][13][14][15][16][17][18][19][20][21]. Moreover, for Med-D food products we can recognize specific active constituents (i.e., oleuropein, resveratrol, retinoids, flavonoids, terpenes, catechins, ω-3-fatty acids etc.) with pharmacological effects (i.e., anti-oxidant and anti-inflammatory) that are related with the health benefits from adherence in Med-D
[22][23][24][25][26][27][28][29][30][31]. In this respect, the question that arises is whether there could be cases in which pharmacologically active compounds that are present in Med-D foods could potentially interfere with the pharmacological action of drugs and lead in clinically significant DFIs.
2. Mediterranean Food Products and Potential DFIs
2.1. Med-D Food Products
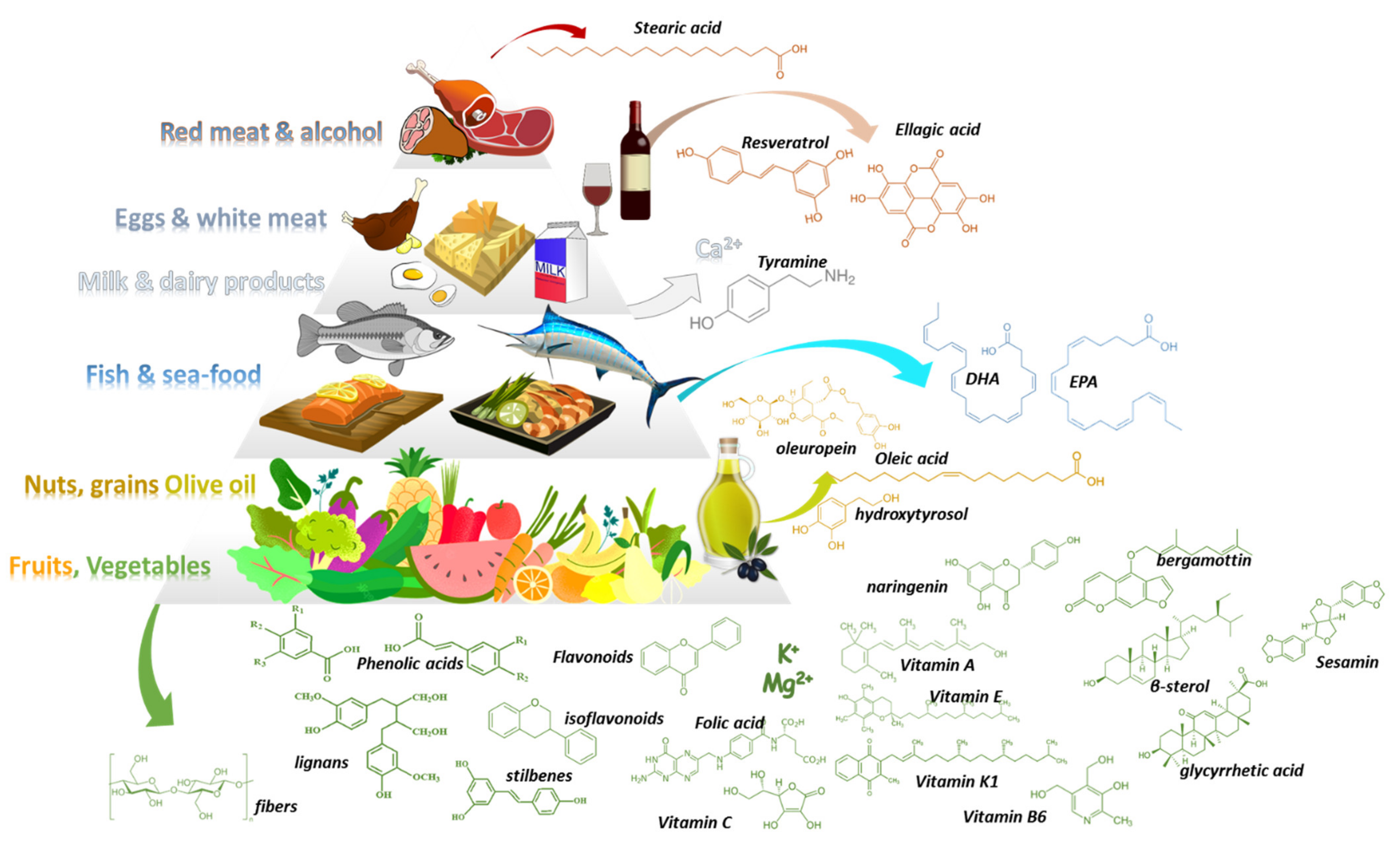
Med-D allows the intake of all types of foods following a general food guidance pyramid (
Figure 1)
[2][3]. In its base (level 1) are vegetables, fruits, nuts, and cereals that should be consumed in greater amounts and at daily frequency. Above them (level-2), sea food proteins and omega-3-fatty acids (n-3 FAs) are suggested to be consumed biweekly. Animal food products (level3) such as dairy, cheese, and eggs can be consumed in moderate portions during the week. Red meat (level 4) should be consumed in less proportions during the week along with saturated fat products and sweets. Regarding alcohol, a moderate consumption of wine and other fermented beverages is recommended (one to two glasses with meals). The pyramid is complete considering daily activities and physical exercise.
Table 1,
Table 2 and
Table 3 summarize available data regarding potential DFIs for food products of level1 in the Med-D pyramid.
Figure 1. The Mediterranean diet pyramid along with examples of characteristic constituents that can be found in Med-D’s food products.
Table 1. DFIs, pharmacological mechanism, significance, and level of evidence for vegetables and herbs that are contained in the base of Med-D pyramid (PK: pharmacokinetic, PD: Pharmacodynamic, DFI: drug-food interaction).
Table 2. DFIs, pharmacological mechanism, significance, and level of evidence for fruits that are contained in the base of Med-D pyramid (PK: pharmacokinetic, PD: Pharmacodynamic, DFI: drug-food interaction).
Table 3. DFIs, pharmacological mechanism, significance, and level of evidence for nuts, and cereals that are contained in the base of Med-D pyramid.
2.2. Drug-Med Diet Interactions
2.2.1. Vegetables, Herbals, Olive Oil, Cereals, and Nuts
The Med-D has its basis in foods of plant-origin with a wide variety of vegetables along with other herbals. It contains an extensive list of domestic and imported vegetables that reached the area through historical trading routes. The most common vegetables include artichokes, arugula, asparagus, beetroots, broccoli, brussel sprouts, cabbage, carrots, celery, collard greens, cucumbers, dandelion greens, eggplant, fennel, garlic leeks, lettuce, mushrooms, mustard greens, onions (all types), peas, peppers, potatoes, pumpkin, radishes, spinach, turnips, zucchini. Case reports and clinical data suggest that potential PK-DFIs can result from consumption of artichoke, broccoli, brussel sprouts, cabbage, cauliflower, and tomatoes
[46][47][48]. A pharmacological mechanism can be attributed to the isothiocyanate content (i.e., broccoli, cauliflower etc.) and their capability to modulate drugs’ CYP-mediated metabolism or transport (ABC-transporters)
[47]. Especially for CYP1A2, it has been shown through clinical trial that brassica vegetables can induce CYP1A2 metabolic activity modulating caffeine’s pharmacokinetics
[49]. In addition, celery and other apiaceous vegetables (i.e., carrot, celery, dill, cilantro, parsnip, parsley etc.) can decrease cytochrome CYP1A2 activity as has been shown through several studies
[47][49]. Garlic components have shown inhibitory action for CYPs 2C, 2D, and 3A-mediated metabolism in vitro but in a later clinical pharmacokinetic study, long-term use of garlic caplets led to a significant decline in the plasma concentrations of saquinavir which is metabolized from CYP3A4
[50][51]. Tomato juice was shown in vitro to contain mechanism-based and competitive inhibitor(s) of CYP3A4
[52][53]. Cabbage and onion juices have also shown potential inhibiting activities on CYP3A4 in vitro
[54]. Basil demonstrated in vitro potential reversible and time-dependent inhibition of CYP2B6 and CYP3A4 as well as esterase-mediated metabolism of rifampicin, but the concentrations were higher than the ones used in daily food consumption
[55]. The significance of these potential PK-DFIs is currently unresolved and the level of evidence for most of the cases is low. The frequent consumption of these foods may contribute in an observed inter-individual variability within the treatment goals. A recent systematic review and meta-analysis of twenty-three dietary intervention trials in humans analyzed the effect of cruciferous vegetable-enriched diets on drug metabolism. The meta-analyses showed a significant effect on CYP1A2 and glutathione S-transferase-alpha (GSTa)
[48]. Thus, healthcare advice is needed in case patients habitually consume excessive amounts of vegetables such as broccoli, brussel sprouts, cabbage, cauliflower, radish, and watercress and are under treatment with CYP1A2 substrates (i.e., clozapine, olanzapine, fluvoxamine, haloperidol, melatonin, ramelteon, tizanidine, and theophylline).
Regarding PD-DFIs, arugula, asparagus, bell peppers, broccoli, celery, collard greens, kale, onions and leeks, spinach, and chard due to their content of vitamin-K could modulate the INR for people treated with coumadin analogues such as warfarin or acenocoumarol. However, despite this, DFI represents a clinically significant case; it is estimated to be of moderate importance and has a low level of evidence with studies suggesting that a balanced consumption of vegetables does not interfere with INR in a clinically significant way
[56][57]. Concerning other potential PD-DFIs, anise and aniseed’s essential oil (used to enhance the flavor of Greek Ouzo and mastic) in vivo enhanced the effects of CNS drugs (codeine, diazepam, midazolam, pentobarbital, imipramine and fluoxetine) in mice suggesting potential synergism and a clinically significant PD-DFI if it is used in extensive doses
[58]. Garlic has shown promising data as an antidiabetic agent; thus it may enhance the pharmacologic effect of antidiabetic medicines
[59]. Turnips have demonstrated synergism with antidiabetic drugs towards hypoglycemia while yams have a good quality of evidence for synergy with estrogens and thus special precautions should be made for patients under estrogen therapy
[60][61]. Naturally occurring levodopa and carbidopa have been quantified in fava beans in fair amounts, thus patients with Parkinson’s under treatment should be aware of possible synergism with co-administered medications
[62].
Concerning olive oil, its protective role against inflammation-related chronic non-communicable diseases (cardiovascular, diabetes, cancer etc.) have been described thoroughly
[63]. Proposed mechanisms involve the action of bioactive constituents of olive oil on interleukin-6 (IL-6) and platelet activating factor (PAF) inflammation pathways. In particular, PAF, which is a class of lipid chemical mediators with messenger functions, has gained research attention as a potential drug target due to its involvement in inflammatory diseases such as allergies, asthma, atherosclerosis, diabetes etc.
[64]. Moreover, it is a point of focus as a contributing biological mechanism for anti-inflammatory action of several food products with protective roles against inflammation
[31]. Several bioactive phytochemical compounds such as terpenes and constituents in olive oil with PAF action have been related to protective mechanisms against atherosclerosis
[65][66]. In addition, as of today there is no contribution of olive oil or its constituents in potential DFIs. On the other hand, for some terpenes with PAF action, i.e., cedrol, inhibiting properties against human P450 have been described in vitro and further studies are needed to clarify potential DFIs
[67]. Regarding potential PD-DFIs of PAF modulators with anti-platelet medications, there are not any reports suggesting potential contribution in DFIs.
2.2.2. Fruits and Fruit Juices
The Mediterranean fruits are among the most famous and widely consumed food products globally. Apples, apricots, avocados, cherries, clementines, figs, grapefruits, grapes, melons, nectarines, oranges, peaches, pears, pomegranates, strawberries, tangerines are the most common ones that are consumed by people following the Med-D style. Regarding fruit juices, the fermentable but unfermented product obtained from the edible part of the fruit and preserved fresh, they sometimes contain (due to the extraction process) different constituents or quantities from the original fruit.
The most notorious DFI regarding fruits and/or fruit juice with medications is that of grapefruit and its juice (GFJ)
[41][68][69][70]. GFJ constituents (i.e., furanocoumarins etc.) can inhibit CYP activity (mainly CYP3A) through mechanism-based inhibition as well as transporter proteins in the intestine and liver (i.e., P-gp)
[68][70][71][72][73]. This can elevate drugs’ bioavailability which, along with reduction in drugs’ intrinsic clearance, can result in increased concentrations and potential side effects. Typical examples are the concomitant use of GFJ with: (i) Ca
2+ channel antagonists that result in low blood pressure; (ii) HMG-CoA reductase inhibitors that may lead to rhabdomyolysis and renal impairment; and (iii) adverse pulmonary effects caused by amiodarone co-administration
[41][68][74][75].
Grapefruit as a plant belongs to the plant family of Rutaceae within the genus of Citrus fruits such as lemons, oranges, limes, tangerines fruits that are widely consumed. As a result, the frequent consumption of these products alerted the previous years the scientific community to the need to examine if DFIs could be further observed
[76][77][78]. Until today, the most often described mechanism for potential DFIs are related with modulation of the activity of OATP transporters and CYPs metabolic activities
[79][80][81]. Citrus fruits such as orange, lemon, pomelo, and lime have been assessed for potential DFIs and compared with GFJ. Pomelo juice increased the bioavailability of cyclosporine in an open-label crossover PK study probably due to inhibition of CYP3A4 and P-gp
[82]. Orange juice reduced the bioavailability of alendronate and aliskiren
[83][84], whereas Seville orange juice interacts with felodipine in a similar way to GFJ
[85]. Lime juice has been shown in vivo to increase (similarly to GFJ) the bioavailability and systemic concentrations of carbamazepine with a clinically significant risk for liver and kidney toxicity
[86]. Although tangerine (fruit and juice) showed some effect in vitro on CYP3A4-mediated metabolism of midazolam, this was of no clinical significance
[87]. Narirutin found in Citrus fruits has also been observed in vivo to inhibit OATP1A2 and OATP2B1
[88].
Regarding apple juice, there is evidence from in vitro, in vivo and clinical studies of inhibiting activity on OATPs and modulation of the PK profile of drugs such as fexofenadine, montelukast and aliskiren
[80][81][84][89]. It also contributed to a DFI with atenolol in a dose-response relationship but with limited effect on the PD-profile of the drug
[90]. Cranberry juice and its constituent avicularin inhibited uptake transporters OATP1A2 and AOATP2B1 in vitro
[91]. In addition, in vitro data indicated inhibition of CYP-mediated metabolism (CYP2C9 and CYP3A4) similar to ketoconazole and fluconazole. The effect is mainly attributed to anthocyanins content but these compounds show poor bioavailability, thus in vitro data were not repeated in vivo or through clinical studies
[92][93][94]. Pomegranate juice has demonstrated in vitro/in vivo inhibiting action against CYP2C9 and CYP3A4 but with no clinical impact based on the available clinical data
[95].
Drug interactions with fruits, fruit juices or pulps can also be related with PD-DFIs, and especially for fruits that contain considerable amounts of potassium (K
+). Bananas, apricots, and oranges are some typical examples of high-K
+ fruits and in theory their over consumption can be implicated in potential PD-DFIs with ARBs and diuretics
[96][97]. Although this effect is based in theoretical statements, an in vivo study with palm fruits and lisinopril demonstrated elevated serum K
+ levels
[98]. The risk of potential hyperkaliemia is clinically significant, especially in cases of kidney diseases. Although observational studies suggest that adherence to Med-D improves survival for CKD patients, there is a lack of conclusive clinical data regarding DFIs and hyperkaliemia, hence vigilance should be advised from healthcare providers
[99][100].
2.2.3. Fish and Sea Food
Fish and sea food (clams, cockles, crabs, groupers, lobsters, mackerel, mussels, octopuses, oysters, salmons, sardines, sea basses, shrimps, squids, sea breams, tunas, etc.) are the main sources of protein and fat within the Med-D diet. They contain high amounts of essential amino acids along with n-3 fatty acids (FA) (i.e., eicosapentaenoic acid and docosahexaenoic acid) especially the pelagic fishes (sardines, anchovies, mackerels etc.)
[101]. Apart of the nutritional value in Med-D, marine n-3 FAs are known to have positive effects on human health such as a protecting role in cardiovascular diseases, inflammation, diabetes, neurocognitive disorders etc.
[102]. Regarding DFIs, omega-3 FAs seem to reduce coagulation factors (i.e., fibrinogen and prothrombin), thus in theory can potentiate the effects of anticoagulants
[103]. As of today there have been some case reports of interactions between warfarin co-administration with fish-oil supplements, but the results were not repeated in a retrospective study of a larger cohort of patients with atrial fibrillation and deep vein thrombosis
[104][105][106]. Considering also that DS usually have a higher content of n-3 FAs than the consumed food, the potential DFI is of minor importance and negligible for patients that remain adherent in their treatment plan.
2.2.4. Milk Dairy Products, White and Red Meat
Milk and dairy products (yoghurt, cheese) are part of the traditional domestic livestock practices around the Mediterranean basin and are part of the historical heritage of the dietary habits for the region. In medicine, milk and dairy products are an old case of potential DFIs due to their content in Ca
2+ and tyramine. The presence of Ca
2+ in milk and dairy products can create unabsorbed chelate ligands with antibiotic classes of tetracyclines and quinolones resulting in reduced bioavailability (PK-DFIs)
[107]. Tyramine is a precursor of catecholamines and the inhibition of their metabolism from MAOIs can lead to increased catecholamine levels which can cause hypertension. Tyramine is known to interact with mono-amino oxidase inhibitors (MAOIs) resulting in an effect known as tyramine pressor response with high blood pressure and risk of cerebral hemorrhage which can be fatal
[42][108]. Finally, another important PK-DFI is the reduced bioavailability of ferrous from cow milk. Caseins in cow milk bind Fe
2+ by clusters of phosphoserines, keeping it soluble in GI’s alkaline pH, preventing its free form from being available for absorption, thus decreasing its bioavailability in cases of ferrous supplement co-administration
[109]. In addition, co-administration of mercaptopurine and cow milk in patients with chronic myelogenous leukemias reduces the bioavailability of the drug due to milk’s high content of xanthine oxidase, thus this co-administration should be avoided
[110][111].
Meat products within Med-D, although consumed to a limited extent, are a valuable source of nutrients for a healthy and balanced diet. Their dietary value lies in their high protein content with essential amino acids, ferrous from red meat, vitamin B12 and other vitamins of B-complex, zinc, selenium, and phosphorus. Fat content is dependent on meat species, feeding system, as well as the meat part that is used in food
[112]. As stated earlier, high-fat content may lead to raised salt and increase the solubilization of lipophilic drugs
[38].
2.2.5. Wine and Other Beverages
Wines, except for their alcohol content (~11% for whites and 15% for reds), have a rich composition of bioactive compounds such as polyphenols. Resveratrol, anthocyanins, catechins, and tannins (proanthocyanins and ellagitannins) are some of the most often found polyphenols with a higher content in red wines which also explicate the beneficial effect of from wine consumption
[113][114]. One of the most known examples is the French paradox, the epidemiological observation of low coronary heart disease death rates despite high intake of dietary cholesterol and saturated fat in southern France which is attributed in red wine consumption in those populations
[115]. Regarding DFIs, polyphenols can modulate the phase I and II metabolism as stated earlier but for wine this effect can be considered minimal compared to the effects of alcohol in the case of regular or heavy drinking.
Alcohol can enhance the effects of medications, especially in cases of chronic conditions. PK-DFIs of alcohol are related mostly with induction of CYP2E1 and to a lesser extent CYP3A3 and CYP2A1. Heavy alcohol drinking can lead to PD-DFIs of alcohol such as sedation when combined with CNS acting drugs (sedatives, antihistamines, antidepressants, antipsychotics etc.), induce gastric bleeding when combined with aspirin and relative painkillers or anticoagulants, and hypoglycemia with antidiabetic drugs
[43][116]. Regarding the vexed issue, the widespread opinion that concomitant drink of alcohol with antibiotics or other antimicrobials will cause toxicity or treatment failure, a recent review of the available evidence suggested that the data are poor and sometimes controversial
[117]. The reduced efficacy refers mostly to erythromycin and doxycycline. Disulfiram-like reaction (distress, pain, flushes, irregular heartbeat) can occurs in co-administration of metronidazole, ketoconazole, griseofulvin and cephalosporines (i.e., cefuroxime, cefotetan, ceftriaxone, cefoperazone, ceftriazone). Ambiguous data for ADRs exist for trimethoprim sulfamethoxazole. On the other hand, penicillins, fluoroquinolones, azithromycin, tetracyclines, nitrofurantoin, secnidazole, tinidazole, and fluconazole have not been causally related to ADRs
[117]. Another mechanism involved is the reduction in the immune response and epidemiological studies have shown alcohol abuse to be associated with an increased incidence of infectious diseases. But this is related mostly to cases of alcohol abuse, consumption of high content alcoholic drinks and overall, a poor quality of life regarding well-being and disease prevention. Thus, it is a good idea from the healthcare perspective, especially for heavy-drinking patients under treatment, to advise towards drinking cessation
[118][119]. On the other hand, the moderate consumption of red wine seems to be beneficial in cases of immune protection due to its polyphenol content
[118]. Thus, although the quality and quantity of data are vague, the avoidance of or reduction in consuming alcohol in low or moderate amounts (e.g., a social occasion with one glass of wine or beer) as is suggested through Med-D can be enjoyed.