Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vivek Singh | -- | 5205 | 2022-11-04 05:51:46 | | | |
| 2 | Peter Tang | Meta information modification | 5205 | 2022-11-04 06:44:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ghosh, A.; Singh, V.K.; Basu, S.; Pati, F.; Singh, V. Molecular Therapeutics for Corneal Scar Treatment. Encyclopedia. Available online: https://encyclopedia.pub/entry/32849 (accessed on 07 February 2026).
Ghosh A, Singh VK, Basu S, Pati F, Singh V. Molecular Therapeutics for Corneal Scar Treatment. Encyclopedia. Available at: https://encyclopedia.pub/entry/32849. Accessed February 07, 2026.
Ghosh, Anwesha, Vijay K. Singh, Sayan Basu, Falguni Pati, Vivek Singh. "Molecular Therapeutics for Corneal Scar Treatment" Encyclopedia, https://encyclopedia.pub/entry/32849 (accessed February 07, 2026).
Ghosh, A., Singh, V.K., Basu, S., Pati, F., & Singh, V. (2022, November 04). Molecular Therapeutics for Corneal Scar Treatment. In Encyclopedia. https://encyclopedia.pub/entry/32849
Ghosh, Anwesha, et al. "Molecular Therapeutics for Corneal Scar Treatment." Encyclopedia. Web. 04 November, 2022.
Copy Citation
The process of corneal wound healing is complex and induces scar formation. Corneal scarring is a leading cause of blindness worldwide. The fibrotic healing of a major ocular wound disrupts the highly organized fibrillar collagen arrangement of the corneal stroma, rendering it opaque. The process of regaining this organized extracellular matrix (ECM) arrangement of the stromal layer to restore corneal transparency is complicated. The surface retention capacity of ocular drugs is poor, and there is a large gap between suitable corneal donors and clinical requirements.
corneal scar
molecular therapeutics
exosome
si/microRNAs
recombinant viral vectors
bioactive molecules
nanotechnology
1. Introduction
Unregulated tissue fibrosis is generally associated with the development of chronic diseases. Ocular insults to the cornea, such as alkali burns, injuries, and inflammation, trigger the complex process of wound healing to maintain its structural integrity, functional integrity, and transparency [1]. This is mainly characterized by the migration and proliferation of corneal epithelial cells, followed by the differentiation of stromal keratocytes into myofibroblasts, the excess deposition of extracellular matrix (ECM) components, an increase in alpha-smooth muscle actin (α-SMA) expression, and ECM remodeling, resulting in corneal opacity [2][3]. The accumulation of these events leads to a scarred cornea (Figure 1). Corneal scarring is the fourth most common cause of blindness, affecting approximately five million people globally each year [4]. A scarred cornea is classified according to its degree of opacity. Nebular opacity is caused by superficial scars on the ocular surface. As the severity of corneal scarring increases, the degree of opacity progresses from nebular to macular and leucoma [5]. Superficial scars heal over a period of three months, restoring vision; however, deep scars generally worsen, leading to blindness [6].
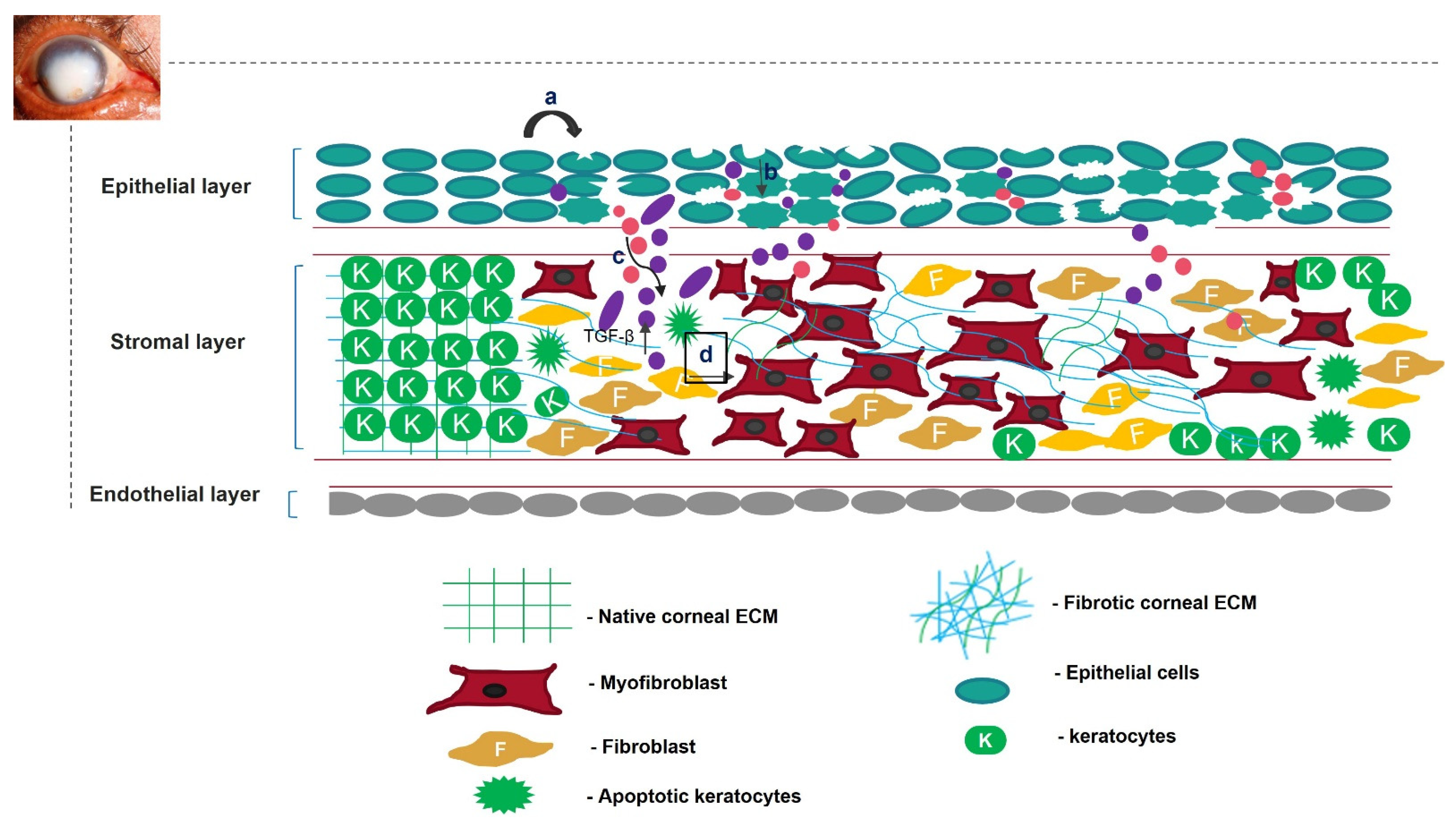
Figure 1. Overview of corneal wound-healing process and scar formation. After an ocular wound, specifically epithelial wounding, (a) the epithelial cells undergo apoptosis, (b) and epithelial cell markers are lost because of corneal epithelial injury, triggering their migration and proliferation. A deep injury disrupts Bowman’s membrane, allowing the invasion of neutrophils (pink) and macrophages (purple) into the stromal layer and releasing TGF-β. This increases the concentration of TGF-β in the stromal layer (c). Therefore, the keratocytes undergo apoptosis, differentiate into myofibroblasts, and disrupt the arrangement of the corneal ECM (d).
Finding an appropriate therapeutic technique to heal a scarred cornea efficiently has proven difficult. Corneal transplantation is an unsuitable therapy for treating scarring because of the lack of appropriate corneal donors [7]. The use of the amniotic membrane as an ocular bandage because of its anti-angiogenic and antifibrotic properties is a popular treatment method. However, its use is limited because of ethical problems related to tissue donation, potential risks of the transmission of diseases, inter-amnion variability, and reproducibility [8]. Topical ocular drops are a simple way of treating a scarred cornea. However, their low bioavailability and rapid drainage from the ocular surface limit their popularity [9].
2. Scarring of Cornea
The cornea is a structurally and functionally integrated avascular organ with three cellular layers interrupted by two acellular layers [10]. It is resistant to minor wounds; however, major ocular trauma can compromise its transparency [11]. A significant abrasion might disrupt the basement and Bowman’s membranes, leading to interactions between the epithelial and stromal layers of the cornea. This interaction initiates the complex wound-healing process [12][13]. Various growth factors, such as fibroblast growth factor (FGF), epidermal growth factor (EGF), and IGF-1, cross the disrupted Bowman’s membrane from the tear film to reach the stromal layer of the cornea during the initial wound-healing process [14][15]. In the epithelial layer, wounded epithelial cells undergo apoptosis, migration, and proliferation. The concentration of interleukin-1 (IL-1) increases in the epithelial layer upon injury. Therefore, IL-1 crosses the disrupted Bowman’s membrane into the stromal layer and becomes a major factor in killing keratocytes [16].
In the stromal layer, the corneal injury increases the expression of keratocyte membrane-bound αVβ/αβ integrins [17] and various metalloproteases, such as matrix metalloprotease 2 (MMP2) or MMP9 [18]. This cumulatively activates TGF-β from latency [19][20]. Neutrophils, monocytes, and macrophages infiltrate the wounded area during the healing process. They either become incorporated into fibrous DNA-like structures known as neutrophil extracellular traps of ECM or secrete TGF-β, increasing the concentration of TGF-β in the stromal layer [21][22][23][24]. Therefore, TGF-β takes over the control of the corneal wound-healing process. Quiescent keratocytes transdifferentiate into myofibroblasts, enlarged spindle-shaped cells characterized by the neo-expression of α-SMA in the presence of TGF-β. Incorporating α-SMA into the corneal ECM generates a contractile force that disrupts the orthogonal arrangement of its collagen fibrils, altering its normal curvature [25][26].
The active form of TGF-β first binds to the keratocyte plasma-membrane-bound TGF-β type I/II receptor (TBRI/II) and switches on the downstream SMAD-dependent signaling cascade [27]. This increases the expression of various fibrotic genes, such as MMPs and connective tissue growth factor (CTGF) [28]. In addition to TGF-β, other neuropeptides, such as calcitonin gene-related peptide, vasoactive intestinal peptide, and substance P (SP), are released from the axonal nerve endings of corneal epithelial layers. These neuropeptides cross the disrupted Bowman’s membrane to reach the stromal layer of the cornea. Substance P binds to the neurokinin 1 receptor (NK1R) of keratocytes and activates three different signaling pathways [29]. The SP/NK1R complex activates the RhoA/ROCK pathway to phosphorylate LIM kinase (LIMK). This inactivates cofilin (an actin-cleaving enzyme) [30], preventing actin degradation and promoting actin polymerization. Actin mostly remains bound to myocardin-related transcription factors A/B (MRTFA/B); however, the increased actin polymerization allows myocardin-related transcription factor A/B (MRTFA/B) to bind to serum response factor, activating profibrotic genes, such as MMPs [31][32]. The SP/NK-IR complex activates phospholipase C-beta and adenylyl cyclase, increasing the intracellular calcium level and cyclic adenosine monophosphate (cAMP) concentrations. This switches on protein kinase A and activates the extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) pathway, increasing the proliferation of keratocytes and fibroblast cells [33][34]. The increased Ca2+ ions bind to calmodulin and cohere with the Kca3.1 ion channel of keratocytes to hyperpolarize the cells. This causes the cells to skip the G1 phase of the cell cycle, promoting fibrotic cell proliferation [35]. The increase in cellular stress increases the intracellular reactive oxygen species (ROS) levels, which share a positive feedback loop with the secretion of various growth factors, such as TGF-β, proinflammatory cytokines, etc. [36] Interleukin 10 (IL-10), a proinflammatory cytokine, binds to its cognate receptor and activates the nuclear factor kappa B (NF-κβ) pathway, increasing stress and other inflammatory genes’ expression [37]. TGF-β also increases the expression of bromodomain-containing protein 4 (BRD4), increasing the expression of kelch-like ECH-associated protein 1 (keap1). Keap1 might bind to the antioxidant gene nuclear factor erythroid 2–related factor 2 (Nrf-2) and undergo proteasomal degradation [38][39]. Cellular stress also increases the expression of the ubiquitin-specific peptidase-10 (USP10) gene. The USP10 gene stabilizes p53, increasing apoptosis of wounded keratocytes. However, it also deubiquitinates integrins, contributing to the activation of TGF-β and fibrosis [40]. The apoptotic cells also release TGF-β and other inflammatory cytokines, completing the loop of fibrosis [41][42] (Figure 2).
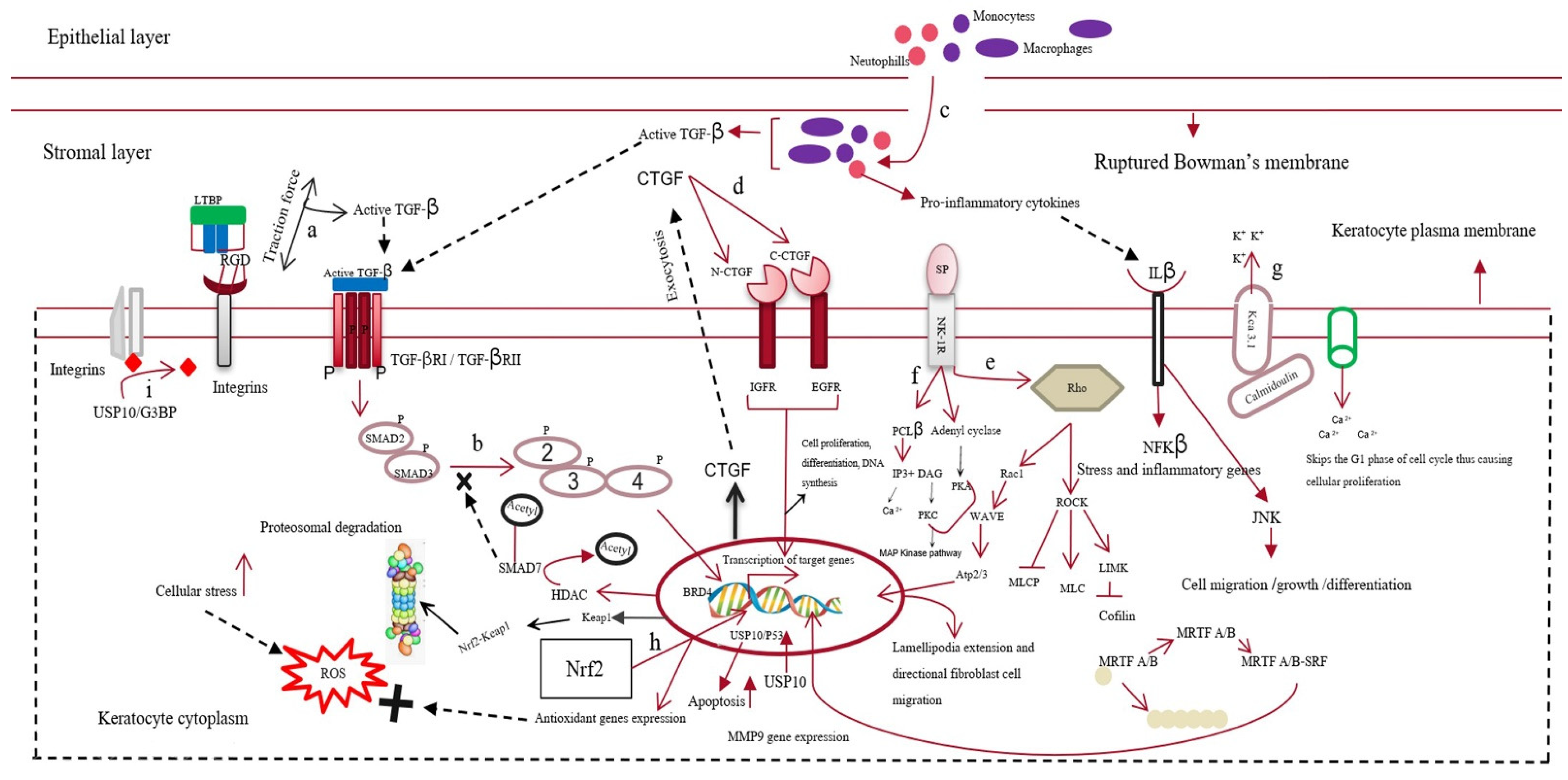
Figure 2. Mechanism of corneal scarring. This figure represents the keratocytes of the stromal layer, the epithelial layer, the disrupted Bowman’s membrane, and the stromal layer of the human cornea. The mechanism of corneal scarring is depicted in a single keratocyte cell for simplicity; (a) activation of transforming growth factor-β (TGF-β) from its latent state; (b) SMAD-dependent TGF-β signaling pathway; (c) infiltration of neutrophils and macrophages into the stromal layer and their secretion of active TGF-β; (d) CTGF (connective tissue growth factor) signaling pathway; (e) substance-P-mediated activation of the Rho/ROCK signaling cascade, leading to actin polymerization and MMP gene expression; (f) increase in intracellular Ca level by Substance-P-mediated PCL-β activation; (g) Kca3.1 ion-channel-mediated hyperpolarization of the keratocyte plasma membrane and subsequent influx of Ca ions; (h) Nrf2 (nuclear factor erythroid 2–related factor 2)-mediated antioxidant gene production; however, BRD4 (bromodomain-containing protein 4) increases keap1 expression, leading to the formation of the kelch-like ECH-associated protein 1 (keap1)/Nrf2 complex and their subsequent degradation; (i) deubiquitylation of integrins by the USP10/G3BP complex.
The complex signaling cascade signifies the corneal wound healing and scarring process. The corneal scarring process continues even after the wound has healed, making the condition worse. Keratocytes return to their quiescent state after wound healing, and the immune cells disappear. However, the disrupted Bowman’s membrane does not regenerate because of its low regenerative capacity. This causes extracellular vesicles and persisting fibroblast cells to continuously invade the stromal layer, accumulating TGF-β and repeating corneal ECM remodeling. Therefore, a scarred cornea is an immediate outcome of the multiplexed corneal wound-healing process [43].
Good vision requires a healthy corneal stromal ECM. The wound-healing process replaces the clear transparent stromal tissues with fibrotic tissues containing a disorganized collagen fibril arrangement and different proteoglycan content. There is an increase in the synthesis of chondroitin sulfate, hyaluronan, and biglycan and a decrease in the synthesis of keratin sulfate and lumican, which are required for maintaining corneal transparency [44][45]. This disordered, newly remodeled corneal ECM also lacks normal curvature and possesses low mechanical strength and elasticity. It refracts the incident light in all directions; therefore, the light is not focused on the retina, leading to vision impairment [46]. Externally stimulating the body’s response in attenuating the signaling cascade of corneal fibrosis might be a better approach to recovering a scarless cornea.
3. Regeneration of Scarless Cornea
Restoring useful vision is the primary concern of patients with corneal scarring. This can be achieved by preventing the excessive migration of corneal ECM components during wound closure. TGF-β, the growth factor responsible for disrupting the corneal ECM, must remain inactive during the wound-healing process.
3.1. Exosomes as A Therapeutic Tool
Exosomes are membrane-bound vesicles released by cells into their extracellular space. They have emerged as a novel therapeutic approach over cell-based therapy for treating corneal scarring [47]. Most stem-cell-based therapies face several challenges, such as immunogenicity, the high cost of production, ethical concerns, stability, and survival. However, exosomes have less stringent safety and regulatory requirements than other stem-cell-based therapies. These microvesicles act as biological vehicles that deliver parent cell cargo to the target cells. The exosomes are internalized by the target cells by either receptor-mediated endocytosis or simple membrane fusion. Upon reaching the cytoplasm of the target cell, the exosomes release their contents, activating various signaling cascades [48]. Exosomes can be isolated from stem cells using simple techniques, such as ultracentrifugation, ultrafiltration, size exclusion chromatography, and immunity capture. These vesicles can deliver antifibrotic proteins and miRNAs from stem cells to the ocular surface to modulate the therapeutic signaling cascade of the scarred cornea.
An increase in the concentration of chemokines and cytokines at the injury site is the hallmark of wound healing. Interleukin 8 (IL-8), a proinflammatory cytokine, binds to glycosaminoglycans (GAGs). In this GAG-bound form, IL-8 interacts with the C-X-C motif chemokine receptor 1 (CXCR1) and CXCR2 of neutrophils that infiltrate at the site of injury, leading to oligomerization and a haptotactic gradient. This haptotactic gradient directs the migration of neutrophils to the wounded area and seeds the process of corneal fibrosis [49]. Corneal stromal stem cells (CSSCs) express tumor necrosis factor (TNF)-stimulated gene 6 (TSG-6), a hyaluronan-binding protein, during inflammation [50]. This 35 kDa protein interacts with IL-8 via its link module domain and disrupts the binding of IL-8 and GAGs, preventing neutrophil migration [51][52]. Shojaati et al. [53] isolated extracellular vesicles (EVs) from CSSCs, mixed them with fibrin gel, and administered the EV–fibrin gel to the surface of an eye with corneal debridement. After 2 weeks of debridement, the EV-treated cornea showed minor corneal scarring compared to CSSC-treated and untreated controls. EVs were more effective in preventing neutrophil infiltration, reducing fibrotic marker expression, and restoring corneal morphology.
Cultured CSSCs treated with exosomes isolated from adipose-derived stem cells (ADSCs) showed optimal proliferation, reduced apoptosis, increased aldehyde dehydrogenase 1 (ALDH1), decreased MMP1, MMP3, and MMP9 expression, and increased collagen I, II, III, IV, and V expression compared to untreated CSC [54]. Therefore, ADSC-derived exosomes might revive the plasticity of transformed corneal keratocytes or stromal cells by increasing keratocyte marker expression and ECM production. The therapeutic effects of ADSCs and ADSC-derived exosomes could be attributed to microRNA-19a, which binds the 3′UTR of homeodomain-containing serine/threonine kinase 2 (HIPK2). MicroRNA-19a post-transcriptionally suppresses homeodomain-interacting protein kinase 2 (HIPK2), preventing the activation of the Jun N-terminal kinase (JNK) pathway and fibrosis [55]. Corneal keratocytes, when co-cultured with ADSC-derived exosomes, reduce HIPK2, phosphorylated SMAD3, and p53 expression, preventing the transformation of keratocytes into myofibroblasts and apoptosis of the nearby injured cells. This condition was reversed by the lentivirus-mediated overexpression of HIPK2 in keratocytes, confirming the role of miRNA-19a in corneal scar healing [56].
Exosome-based therapy is still in its nascency and requires preclinical research to be used for treating scars. Exosomes isolated from corneal stromal stem cells effectively prevent corneal scarring and require more preclinical safety and toxicity studies. Similarly, ADSC-derived exosomes reduce apoptosis and restore keratocyte marker expression in corneal stromal cells. Further characterization of these experiments is required to check their preclinical efficacy and gain better molecular insight to understand their therapeutic benefits. Exosomes from HCEs can hasten the corneal wound-healing process and prevent corneal scarring (Figure 3).The parent cells could be engineered to overexpress specific antifibrotic proteins. Exosomes consisting of this protein can be used in combination with tissue-derived microparticles to increase the effectiveness of exosome-based therapy for a scarred cornea.
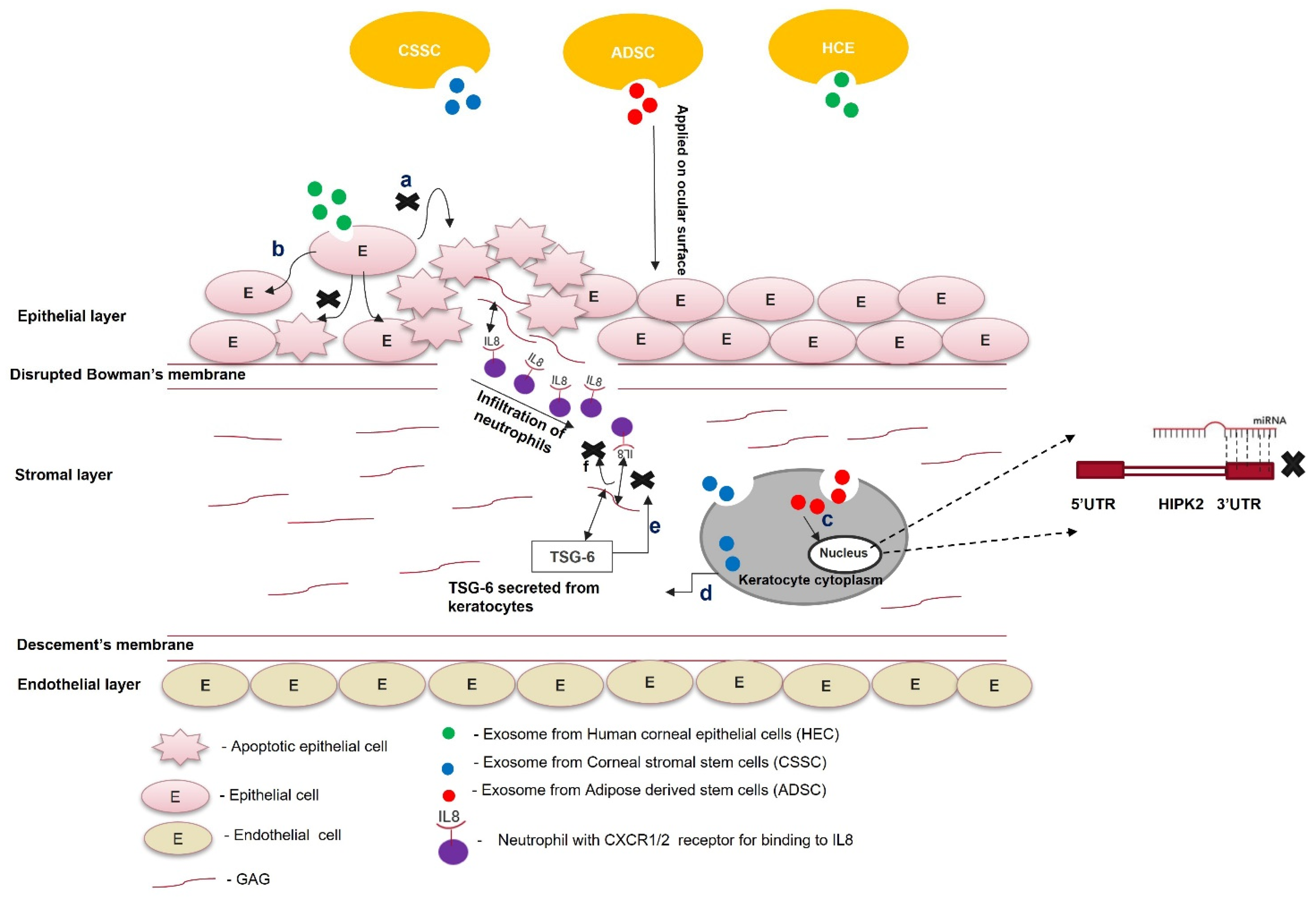
Figure 3. Role of exosomes in the treatment of scars. Exosomes from different cell types (ADSCs, CSSCs, and HECs) are loaded within the fibrin gel or encapsulated in the hydrogel and applied on the ocular surface; (a) exosomes from HECs taken up by the epithelial cells prevent paraptosis (apoptosis caused by hypoxic conditions); (b) epithelial cells treated with exosomes do not undergo apoptosis; (c) exosomes from ADSCs contain miR-19a, which post-transcriptionally silences HIPK2 and attenuates the JNK pathway; (d) exosomes from CSSCs contain TSG-6 protein, and once they are taken up by keratocytes, the TSG-6 proteins are secreted within them; (e) TSG-6 protein binds to GAG via its LINK domain and prevents the binding of IL8 to the GAG of corneal stroma; (f) the unbound form of IL-8 cannot be presented to the CXC1/2 receptor of neutrophils. It attenuates the infiltration of neutrophils to the wounded area of the cornea.
3.2. Targeted Gene Silencing for the Generation of A Scarless Cornea
The idea of directly targeting the upregulated genes of the corneal scarring signaling cascade to heal a scarred cornea has attracted researchers over the past few years. Semaphorin 3A (SEMA-3A), ubiquitin-specific protease-10 (USP 10), and calmodulin/calcium-activated K+ channel 3.1 (Kca3.1) are upregulated during the corneal wound-healing process. The increased expression of these genes contributes to corneal fibrosis. Therefore, targeting these genes using siRNA can mitigate the scarred corneal environment. The complete knockout of a targeted gene is undesirable because genetically modifying a mammalian cell is difficult, time-consuming, and expensive; moreover, knocking out a gene could have lethal consequences. Therefore, the in vivo delivery of small oligonucleotide sequences, designed to target certain mRNAs, using a suitable delivery vehicle to silence a target gene might be an effective way to prevent corneal fibrosis.
3.3. Protein Overexpression in Preventing Corneal Scarring
The cornea is an ideal tissue for gene therapy, as the ocular surface can be an excellent platform for the topical application of various viral/non-viral gene delivery vectors. The overexpression of specific genes in corneal epithelial or stromal cells that block the TGF-β signaling cascade can prevent scar formation.
3.4. MicroRNA Therapies in Regenerating Cornea without Scars
MicroRNAs are 21-nucleotide-long, non-coding RNAs that can post-transcriptionally silence the expression of their target genes. These microRNAs form an RNA-induced silencing complex (RISC) with Argonaute proteins. These proteins guide them towards the target mRNA, where they pair with the target mRNA and silence its expression [57].
MicroRNAs in the cornea affect various cellular processes, such as cell migration, differentiation, proliferation, and metabolism. Any dysregulation in miRNA levels during corneal injury can lead to corneal neovascularization and scar formation. An J et al. [58] observed a sharp decrease in the expression of miR-204 in a murine corneal wound model. MicroRNA-204 is abundant in the cornea. It inhibits corneal cell proliferation by G1 phase arrest. Therefore, low miR-204 levels during corneal wounds account for the excessive proliferation of transformed epithelial cells, myofibroblasts, ECM remodeling, and fibrosis during quick wound healing. Wang Y et al. [59] compared lens epithelial cells from healthy donors and patients with posterior capsular opacification (PCO). They showed increased α-SMA and vimentin and decreased E-cadherin in the diseased cells, indicating opacification. An in vitro PCO model was developed and transfected with miR-204. The lens epithelium cells showed increased E-cadherin and decreased α-SMA compared to non-transfected controls and miR-204-5p-inhibitor-transfected cells. MicroRNA-204 prevents TGF-β-mediated EMT and fibrosis by targeting SMAD4, disrupting the SMAD2/3-SMAD4 complex. It also decreases the expression of the Hey/HMGA transcription factors, which induce EMT through CDH1 repression and SNAIL activation [60][61]. A subconjunctival injection of a recombinant adeno-associated vector (rAAV-miR-204) prevented neovascularization in murine alkali-burned corneas, indicating the anti-angiogenic role of miR-204 [62].
The downregulation of miR-145 and the upregulation of miR-204 and miR-133b can prevent TGF-β-mediated corneal fibrosis. MicroRNA-based gene targeting is a promising therapeutic approach to prevent and heal corneal scarring (Figure 4).

Figure 4. MicroRNA therapy for corneal scarring. Upregulation of miR-204 and miR-133b and downregulation of miR-145 help in healing a scarred cornea via modulating Krüppel-like factor 4 (KLF4), Smad2/3, and CTGF.
3.5. Bioactive Molecules as HDAC Inhibitors in the Regeneration of Scarless Cornea
The wound-healing process of an injured cornea involves three overlapping phases: the inflammatory phase, the proliferative or fibrotic phase, and the final remodeling phase [63]. These events change the native morphology of the corneal stromal ECM, rendering it opaque. During the initial inflammatory phase of the healing process, corneal cells exhibit high levels of proinflammatory cytokines. During the initial phase of wound healing, the infiltrated neutrophils, monocytes, or activated macrophages secrete TGF-β, increasing its local concentration around the wounded area. TGF-β guides the transition of the wound from the inflammatory phase to the fibrotic phase by decreasing the local proinflammatory cytokine level. The timely termination of the initial inflammatory phase is required for the cells to enter the cell proliferation and ECM synthesis phase, healing the wound [64]. A lesser-known pathway that TGF-β uses for this transition is by modulating histone acetylation. TGF-β recruits histone deacetylases (HDACs), removing the acetyl group from the lysine residues of histones H3 and H4 of anti-inflammatory genes. Histone acetylation is associated with anti-inflammatory gene activation; therefore, HDAC is recruited in the presence of TGF-β, deactivating various anti-inflammatory genes and enabling the transition to the fibrotic phase [65][66][67][68][69]. Trichostatin A (TSA), a deacetylase inhibitor, prevents the deacetylation of histone H3 or H4 of anti-inflammatory genes. TSA prevented the TGF-β-mediated transformation of corneal fibroblasts to myofibroblasts in vitro and also reduced the corneal opacity in a rabbit model of corneal haze [70][71].
A possible mechanism of action for HDAC inhibitors is increasing the local concentration of TGF-β during wounding, leading to HDAC recruitment, SMAD7 deacetylation, SMAD7 ubiquitinylation, and proteasomal degradation. This paves the way for TGF-β-mediated corneal scarring. Similarly, suberoylanilide hydroxamic acid (SAHA), an HDAC inhibitor, reduced α-SMA and MMP9 expression in equine corneal fibroblasts treated with TGF-β. This shows that SAHA can prevent TGF-β-mediated corneal fibrosis [72]. Epigenetic modulators, such as TSA and SAHA, might be useful bioactive molecules for treating corneal scars.
3.6. Guided Wound Healing to Prevent Scarring
Corneal wound healing is accompanied by the secretion of various growth factors. During wound healing, these growth factors interact with their cognate receptors present on the surface of the cell to regulate the synthesis of collagen, proteoglycan, and other ECM components’ deposition. Various growth factors, such as IGF-1, platelet-derived growth factor (PDGF), TGF-β, and fibroblast growth factor 1 (FGF-1), either reach the wound site from the tear film or are secreted by the surrounding wounded or apoptotic epithelial cells. Etheredge et al. [73] reported that FGF-1 stimulates keratocyte proliferation; however, it inhibits the synthesis of type 1 procollagen and keratan sulfate. Type 1 procollagen is an essential component of the stromal ECM, and keratan sulfate directs collagen assembly to maintain corneal transparency. FGF-1 induces the formation of hypercellular keratocytes with densely packed cells in the sparse matrix. IGF-1 turns these hypercellular keratocytes into collagenous keratocytes that secrete ECM components, similar to native keratocytes. Therefore, the IGF-1/IGF-receptor signaling pathway prevents the transformation of keratocytes into myofibroblasts and ECM remodeling (Figure 5). However, this is not a predominant signaling pathway during wound healing because the local concentration of TGF-β in the wound microenvironment is high. Therefore, the TGF-β/TGF-βRI pathway takes charge, and TGF-β binds to its receptor on keratocytes to synthesize biglycan, fibronectin containing extra domain A, and hyaluronan. This proteoglycan disrupts the spacing between collagen fibrils, reducing the corneal transparency. It also guides the differentiation of keratocytes into myofibroblasts, characterized by the neo-expression of α-SMA. α-SMA incorporates actin into collagen fibrils and exerts a contractile force on the ECM, disrupting the normal corneal architecture.
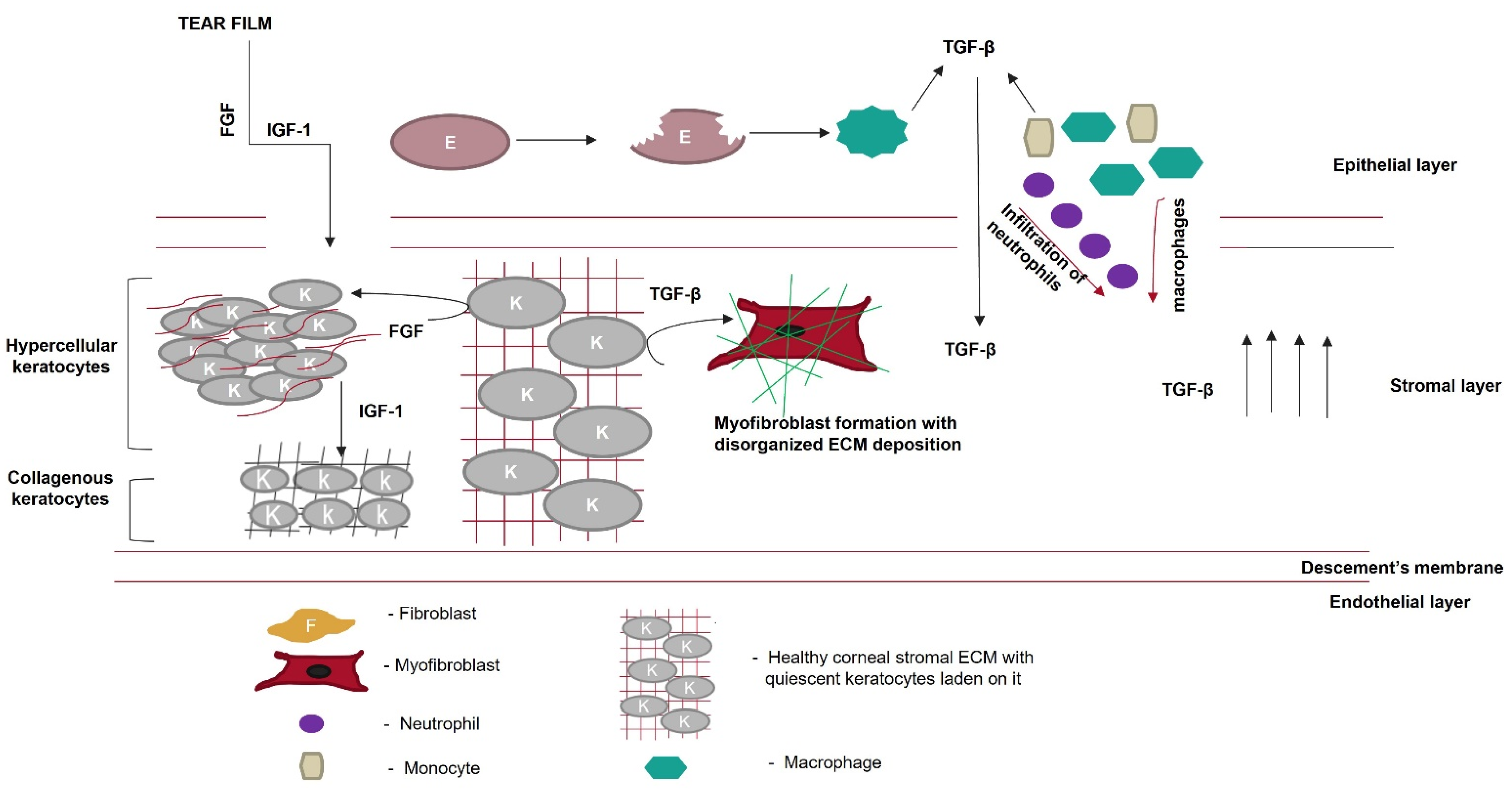
Figure 5. Role of growth factors in scar prevention. Various growth factors, such as fibroblast growth factor (FGF) or insulin-like growth factor (IGF-1), are secreted from the tear film. These growth factors cross the disrupted Bowman’s membrane to reach the corneal stromal layer. FGF converts keratocytes to hypercellular keratocytes. IGF-1 converts hypercellular keratocytes to collagenous keratocytes, which secrete ECM components similarly to the native cornea. Meanwhile, in the epithelial layer, after wounding, epithelial cells undergo apoptosis, and the apoptotic epithelial cells release TGF-β. Neutrophils, macrophages, and monocytes, which migrate from the epithelial layer to the stromal layer, secrete TGF-β, thereby increasing its local concentration. Keratocytes in the presence of TGF-β are converted to myofibroblasts, which disrupt the highly organized fibrillar arrangement of the corneal ECM.
TGF-β heals corneal wounds and is a hallmark of the process of scarring. In contrast, IGF-1 guides the process of wound healing and circumvents scarring. So far, IGF-1 has been studied as an external growth factor, either alone or together with a bioactive compound, such as SAHA or substance P. Bioengineering keratocytes to overexpress IGF-1 can increase its local concentration and allow it to control the process of wound healing.
3.7. Clinical Therapy for Scar Prevention
A gold-standard technique to prevent corneal scarring is still under research. Various biomolecules, such as decorin, TPCA-1, glucosamine, acetylcholine, and chitosan, have the potential to heal a scarred cornea via their respective biological pathways. Chen et al. [74] reported the significance of glucosamine in corneal scar treatment. This amino sugar finds clinical application in osteoarthritis management [75]. However, it significantly enhances intraocular pressure [76]. Park et al. [77] noted that glucosamine attenuated renal fibrosis by downregulating SMAD2 phosphorylation; therefore, a study of glucosamine’s effects on corneal fibrosis can provide new insights into its clinical applications. Chen’s group observed an increase in the expression, stability, and nuclear localization of KLF4 and a decrease in fibrotic gene expression in HCFs treated with glucosamine and TGF-β. The mechanism underlying the glucosamine-mediated increase in KLF4 expression is still under research. However, a study by Wang DF et al. [78] reported that glucosamine increases the expression of the phosphorylated form of PTEN (a phosphatase), leading to an increase in the phosphorylated form of KLF4. This increases the P300 histone acetylase activity, mediating H3 histone acetylation [79][80].
Glucosamine can form O-linked N-acetylglucosamine via glucosamine transferase activity and the post-translational modification of the proteasome complex to prevent KLF4 degradation [81]. Moreover, KLF4 interacts with TGF-β control elements and prevents a proinflammatory environment [82]. Therefore, glucosamine might find clinical applications in healing corneal scarring (Figure 6).
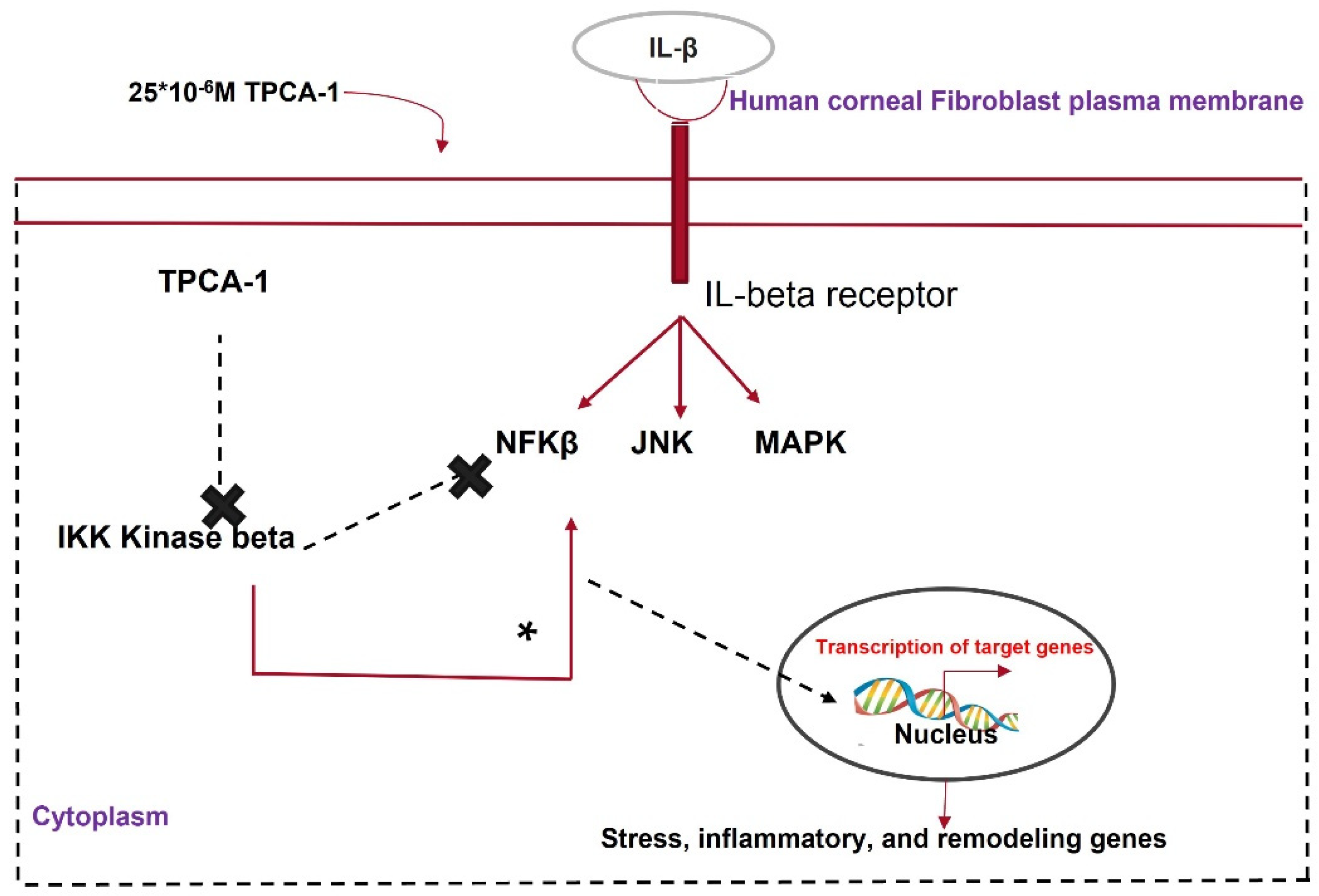
Figure 6. Mechanism of action of TPCA1 in attenuating corneal fibrosis.
Chitosan is an effective therapeutic tool for wound healing because of its antifibrotic and anti-angiogenic properties and its ability to promote rapid re-epithelization for wound closure [83][84]. Fischak et al. [85] formulated a topical eye drop for corneal epithelial wound dressing by functionalizing chitosan with N-acetylcysteine to improve its mucoadhesive properties. They observed faster wound closure in a rabbit corneal wound model. Similarly, thiolated chitosan nanoparticles (TCS-NPs) prevented myofibroblast formation in IL-6-treated HCFs [86]. The TCS-NPs reduced fibronectin or collagen I expression and VEGF expression by 99.9%. Chitosan downregulated TGF-β expression by promoting a more stable interaction between miRNA-29b and the CDS region of TGF-β, attenuating TGF-β signaling [87]. However, the expression of miRNA-29b was not upregulated after TCS-NP treatment. The cellular pathway underlying the chitosan-mediated attenuation of TGF-β expression remains unclear; however, functionalized chitosan can be a novel therapeutic approach to prevent corneal scarring.
Lycium barbarum polysaccharide (LBP), derived from the ancient herb Lycium Barbarum, is a bioactive molecule clinically used in kidney, eye, and liver fibrosis [88]. Du et al. [89] observed that LBP prevented UV-B-induced apoptosis of corneal epithelial cells (CECs) by preventing the phosphorylation and activation of the JNK pathway. In a proof-of-concept experiment to test LBP’s efficacy in attenuating corneal fibrosis, TGF-β stimulated HCF cells subjected to varying concentrations of LBP showed a dose-dependent decrease in α-SMA expression and a reduction in profibrotic collagen [90].
Fibrosis is generally accompanied by the production of reactive oxygen or nitrogen species (ROS/RNS) [91]. ROS are produced because of the TGF-β-mediated activation of NADPH-oxidase 4 via the SMAD3 signaling pathway [92]. ROS directly contribute to fibrosis by inducing TGF-β expression [93]. They also indirectly contribute to fibrosis by activating various growth factors, chemokines, cytokines, and other inflammatory molecules [94]. Increased ROS levels have been observed in skin specimens of patients with systemic sclerosis or skin fibrosis [95]. This increase is associated with EMT and myofibroblast accumulation [96]. Therefore, attenuating ROS production might prevent scarring events. Nuclear factor erythroid 2–related factor 2 (Nrf2), a leucine zipper transcription factor, decreases ROS production by increasing the expression of antioxidant genes, such as superoxide dismutase and NADPH quinone oxidoreductase, by binding to the antioxidant response element. Nrf2 is subjected to proteasomal degradation in the presence of kelch-like ECH-associated protein 1 (keap1), which is activated by BRD4 expression triggered by TGF-β. JQ1, an inhibitor of BRD4, reduces the expression of α-SMA and collagen I. It increased Nrf2 nuclear localization in HCF cells stimulated with TGF-β in vitro. It also increased corneal scarring in an injured mouse model by reducing fibrotic marker expression, supporting the antifibrotic role of JQ1 [97]. A similar antifibrotic effect was observed when immortalized HCFs pretreated with TGF-β were transfected with siRNA targeting BRD4, indicating that BRD4 inhibition prevents TGF-β-mediated corneal fibrosis.
IL-β, a proinflammatory cytokine, crosses the disrupted Bowman’s membrane and binds to receptors on keratocytes upon injury, promoting apoptosis or angiogenesis in the cornea [98]. IL-1β activates the NF-κβ pathway, which causes inflammation and fibrosis. The NF-κβ pathway is activated by the phosphorylation of either the IKKα or IKKβ subunit of its key modulator, IKK. TPCA1 inhibits the phosphorylation of the IKKβ subunit and deactivates the NF-κβ pathway. Zhang et al. [99] showed that TPCA1 preserved the keratocyte phenotype in HCFs pretreated with IL-β via nuclear accumulation of phosphorylated NF-κβ. Corneal keratocytes stimulated with IL-1β showed the reduced expression of keratocyte markers such as KERA, LUM, and ALDH3A1 and elevated levels of phosphorylated NF-κβ [100]. IL-1β treatment elevated MMP2, MMP3, and MMP9 expression in corneal fibroblasts. This was reversed by TPCA-1 application to IL-1β-stimulated fibroblasts [101]. MMPs have multiple roles in wound healing, such as ECM remodeling, TGF-β cleavage, and the activation of EMT [102]. TPCA-1 can prevent corneal scarring by inhibiting IKK and MMP (Figure 6).
Acetylcholine is a neurotransmitter that is released by cholinergic receptors. CECs show a very high expression of acetylcholine [103]. Acetylcholine interacts with its nicotine receptor, causing the influx of Na+ or Ca2+ ions and the efflux of K+ ions. This modulates various signaling pathways through protein kinases and phosphatases [104]. Acetylcholine can also act via a murine receptor (mAchR) to activate multiple downstream signaling pathways by activating protein kinase C. mAchR (M1, M3) and nAchR are present in CECs, and acetylcholine can promote re-epithelization in an injured rat cornea [105]. In contrast, keratocytes produce a meager amount of acetylcholine. Acetylcholine promotes keratocyte proliferation by activating the mAchR present on keratocytes. Sloniecka et al. [106] showed reduced fibrotic gene expression in primary human keratocytes cultured in the presence of acetylcholine with concentrations of 10−7 M and 10−8 M. The low dose of acetylcholine had a pronounced antifibrotic effect, indicating that it can be developed as topical eye drops for safe and antifibrotic wound healing.
Decorin, a prime proteoglycan of the corneal ECM, can interact with all three isoforms of TGF-β. It can directly sequester the active form of the growth factor and attenuate its function [107]. The overexpression of decorin using a mammalian expression vector prevents myofibroblast transformation in TGF-β-stimulated HCFs [108]. Decorin binds to its cognate receptor and increases the intracellular calcium level. This increase in calcium activates calmodulin, which phosphorylates CAM Kinase II and inhibits the TGF-β-mediated activation of fibrotic genes via SMAD 2 phosphorylation [109]. The administration of decorin, using fluid gel with a better drug retention capacity, promoted rapid re-epithelization in ex vivo injured rat corneas [110]. Therefore, the ocular administration of decorin using a delivery vehicle with increased retention time exerts a better therapeutic response in regaining a scarless cornea [111].
3.8. Nanomedicine in Corneal Scarring Treatment
Nanomedicine is an emerging field in medical science with the potential to revolutionize corneal fibrosis therapy (Figure 7). Nanomedicine utilizes materials with nanometer dimensions to target living cells at the genetic and molecular levels. It ensures efficient and sustained drug release, increasing the bioavailability of drugs. This is especially good for topical eye drops, which have a bioavailability of only 5% [112].

Figure 7. Nanotechnology as a therapeutic tool for reviving scarred corneas. Keratocytes transform into fibroblasts and then into myofibroblasts in the presence of TGF-β. This disrupts the highly organized fibrillar arrangement of the corneal stromal ECM. Nanoparticles loaded with the gene of interest or specific drugs, nanofiber scaffolds loaded with drugs, dendrimers, nanomicelles, and nanopolymers are a few nanomedical approaches for treating a scarred cornea.
A polymeric acid nanofiber scaffold covalently attached to antisense oligonucleotides targeting diabetes-linked genes was conjugated to a monoclonal antibody for cell targeting. A trileucine repeat for endosomal rupture (caused by low pH) and an Alexa Fluor for cell tracking showed the release of the antisense oligonucleotides in the cytoplasm of the target cell. The oligonucleotides were released because of covalent bond cleavage by cellular glutathione, resulting in the binding and suppression of diabetes-linked genes [113]. Ma et al. [114] prepared a poly-lactic co-glycolic acid (PLGA) scaffold that served as an ideal platform for corneal keratocyte growth and differentiation. This scaffold repaired corneal stromal defects. Polylactic acid nanofiber scaffolds can be loaded with drugs such as cyclosporine A to accelerate corneal stromal wound healing [115].
Dendrimers are branched three-dimensional nanostructures. They can be coupled with polypropylene and crosslinked with collagen. They have high mechanical strength and can serve as an optimal tissue-engineering scaffold for corneal cells [116]. Nanodevice soft lenses can be loaded with suitable drugs for sustained release over time and can be placed on the ocular surface. Pullulan and poly-caprolactone can be used to prepare nanospheres that can be loaded with antimicrobial drugs, such as ciprofloxacin. These nanospheres can be coated on the surface of the lens to prevent Staphylococcus aureus and Pseudomonas aeruginosa infections in the eye [117]. Nanosponges are highly branched colloidal nanostructures that can be synthesized by crosslinking β-cyclodextrin and di-phenyl carbonate. When loaded with dexamethasone, these nanosponges increase the drugs’ permeability on the ocular surface [118].
Although nanomedicine has proved to be an effective tool for corneal wound treatment, high production costs, tissue accumulation, and in vivo stability are the major limitations of their therapeutic application [119]. Nanomedicine is a promising approach because of its drug release kinetics over conventional drug delivery methods. Therefore, it should be explored further for treating corneal scars.
References
- Song, P.; Wang, S.; Zhang, P.; Sui, W.; Zhang, Y.; Liu, T.; Gao, H. The Superficial Stromal Scar Formation Mechanism in Keratoconus: A Study Using Laser Scanning In Vivo Confocal Microscopy. Biomed Res. Int. 2016, 2016, 7092938.
- Wilson, S.E.; Netto, M.; Ambrósio, R. Corneal Cells: Chatty in Development, Homeostasis, Wound Healing, and Disease. Am. J. Ophthalmol. 2003, 136, 530–536.
- West-Mays, J.A.; Dwivedi, D.J. The Keratocyte: Corneal Stromal Cell with Variable Repair Phenotypes. Int. J. Biochem. Cell Biol. 2006, 38, 1625–1631.
- Whitcher, J.P.; Srinivasan, M.; Upadhyay, M.P. Corneal Blindness: A Global Perspective. Bull. World Health Organ. 2001, 79, 214–221.
- Agrawal, P.K. The pathology of cornea (a histopathological study). Indian J. Ophthalmol. 1983, 31, 662.
- McClintic, S.M.; Srinivasan, M.; Mascarenhas, J.; Greninger, D.A.; Acharya, N.R.; Lietman, T.M.; Keenan, J.D. Improvement in Corneal Scarring Following Bacterial Keratitis. Eye 2013, 27, 443–446.
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173.
- Tseng, S.C.G.; Prabhasawat, P.; Lee, S.-H. Amniotic Membrane Transplantation for Conjunctival Surface Reconstruction. Am. J. Ophthalmol. 1997, 124, 765–774.
- Salminen, L. Review: Systemic Absorption of Topically Applied Ocular Drugs in Humans. J. Ocul. Pharmacol. Ther. 1990, 6, 243–249.
- Eghrari, A.O.; Riazuddin, S.A.; Gottsch, J.D. Overview of the Cornea: Structure, Function, and Development. In Progress in Molecular Biology and Translational Science; Academic Press: Waltham, MA, USA, 2015; Volume 134, pp. 7–23.
- Mobaraki, M.; Abbasi, R.; Omidian Vandchali, S.; Ghaffari, M.; Moztarzadeh, F.; Mozafari, M. Corneal Repair and Regeneration: Current Concepts and Future Directions. Front. Bioeng. Biotechnol. 2019, 7, 135.
- Wilson, S.E. Bowman’s Layer in the Cornea– Structure and Function and Regeneration. Exp. Eye Res. 2020, 195, 108033.
- Ljubimov, A.V.; Saghizadeh, M. Progress in Corneal Wound Healing. Prog. Retin. Eye Res. 2015, 49, 17–45.
- Imanishi, J.; Kamiyama, K.; Iguchi, I.; Kita, M.; Sotozono, C.; Kinoshita, S. Growth Factors: Importance in Wound Healing and Maintenance of Transparency of the Cornea. Prog. Retin. Eye Res. 2000, 19, 113–129.
- Haber, M.; Cao, Z.; Panjwani, N.; Bedenice, D.; Li, W.W.; Provost, P.J. Effects of Growth Factors (EGF, PDGF-BB and TGF-Beta1) on Cultured Equine Epithelial Cells and Keratocytes: Implications for Wound Healing. Vet. Ophthalmol. 2003, 6, 211–217.
- Hong, J.W.; Liu, J.J.; Lee, J.S.; Mohan, R.R.; Mohan, R.R.; Woods, D.J.; He, Y.G.; Wilson, S.E. Proinflammatory chemokine induction in keratocytes and inflammatory cell infiltration into the cornea. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2795–2803.
- Stepp, M.A. Corneal Integrins and Their Functions. Exp. Eye Res. 2006, 83, 3–15.
- Imai, K.; Hiramatsu, A.; Fukushima, D.; Pierschbacher, M.D.; Okada, Y. Degradation of Decorin by Matrix Metalloproteinases: Identification of the Cleavage Sites, Kinetic Analyses and Transforming Growth Factor-Β1 Release. Biochem. J. 1997, 322, 809–814.
- Tandon, A.; Tovey, J.C.K.; Sharma, A.; Gupta, R.; Mohan, R.R. Role of Transforming Growth Factor Beta in Corneal Function, Biology and Pathology. Curr. Mol. Med. 2010, 10, 565–578.
- Blanco-Mezquita, J.T.; Hutcheon, A.E.K.; Stepp, M.A.; Zieske, J.D. AVβ6 Integrin Promotes Corneal Wound Healing. Investig. Opthalmol. Vis. Sci. 2011, 52, 8505–8513.
- Martin, P.; Leibovich, S.J. Inflammatory Cells during Wound Repair: The Good, the Bad and the Ugly. Trends Cell Biol. 2005, 15, 599–607.
- Marrazzo, G.; Bellner, L.; Halilovic, A.; Li Volti, G.; Drago, F.; Dunn, M.W.; Schwartzman, M.L. The Role of Neutrophils in Corneal Wound Healing in HO-2 Null Mice. PLoS ONE 2011, 6, e21180.
- Prame Kumar, K.; Nicholls, A.J.; Wong, C.H.Y. Partners in Crime: Neutrophils and Monocytes/Macrophages in Inflammation and Disease. Cell Tissue Res. 2018, 371, 551–565.
- Chrysanthopoulou, A.; Mitroulis, I.; Apostolidou, E.; Arelaki, S.; Mikroulis, D.; Konstantinidis, T.; Sivridis, E.; Koffa, M.; Giatromanolaki, A.; Boumpas, D.T.; et al. Neutrophil Extracellular Traps Promote Differentiation and Function of Fibroblasts. J. Pathol. 2014, 233, 294–307.
- Phan, S.H. Biology of Fibroblasts and Myofibroblasts. Proc. Am. Thorac. Soc. 2008, 5, 334–337.
- Wilson, S.E. Corneal Myofibroblast Biology and Pathobiology: Generation, Persistence, and Transparency. Exp. Eye Res. 2012, 99, 78–88.
- Huang, T.; David, L.; Mendoza, V.; Yang, Y.; Villarreal, M.; De, K.; Sun, L.; Fang, X.; López-Casillas, F.; Wrana, J.L.; et al. TGF-β Signalling Is Mediated by Two Autonomously Functioning TβRI:TβRII Pairs. EMBO J. 2011, 30, 1263–1276.
- Walton, K.L.; Johnson, K.E.; Harrison, C.A. Targeting TGF-β Mediated SMAD Signaling for the Prevention of Fibrosis. Front. Pharmacol. 2017, 8, 461.
- Słoniecka, M.; Danielson, P. Substance P Induces Fibrotic Changes through Activation of the RhoA/ROCK Pathway in an in Vitro Human Corneal Fibrosis Model. J. Mol. Med. 2019, 97, 1477–1489.
- Prudnikova, T.Y.; Rawat, S.J.; Chernoff, J. Molecular Pathways: Targeting the Kinase Effectors of RHO-Family GTPases. Clin. Cancer Res. 2015, 21, 24–29.
- Yu-Wai-Man, C.; Treisman, R.; Bailly, M.; Khaw, P.T. The Role of the MRTF-A/SRF Pathway in Ocular Fibrosis. Investig. Opthalmol. Vis. Sci. 2014, 55, 4560–4567.
- Harvey, S.A.K.; Anderson, S.C.; SundarRaj, N. Downstream Effects of ROCK Signaling in Cultured Human Corneal Stromal Cells: Microarray Analysis of Gene Expression. Investig. Opthalmol. Vis. Sci. 2004, 45, 2168–2176.
- Snijdelaar, D.G.; Dirksen, R.; Slappendel, R.; Crul, B.J.P. Substance P. Eur. J. Pain 2000, 4, 121–135.
- Suvas, S. Role of Substance P Neuropeptide in Inflammation, Wound Healing, and Tissue Homeostasis. J. Immunol. 2017, 199, 1543–1552.
- Grgic, I.; Kiss, E.; Kaistha, B.P.; Busch, C.; Kloss, M.; Sautter, J.; Muller, A.; Kaistha, A.; Schmidt, C.; Raman, G.; et al. Renal Fibrosis Is Attenuated by Targeted Disruption of KCa3.1 Potassium Channels. Proc. Natl. Acad. Sci. USA 2009, 106, 14518–14523.
- Krstić, J.; Trivanović, D.; Mojsilović, S.; Santibanez, J.F. Transforming Growth Factor-Beta and Oxidative Stress Interplay: Implications in Tumorigenesis and Cancer Progression. Oxid. Med. Cell. Longev. 2015, 2015, 654594.
- Chen, L.; Mongan, M.; Meng, Q.; Wang, Q.; Kao, W.; Xia, Y. Corneal Wound Healing Requires IKB Kinase β Signaling in Keratocytes. PLoS ONE 2016, 11, e0151869.
- Stratton, M.S.; Haldar, S.M.; McKinsey, T.A. BRD4 Inhibition for the Treatment of Pathological Organ Fibrosis. F1000Research 2017, 6, 1015.
- Liu, X.-F.; Zhou, D.-D.; Xie, T.; Malik, T.H.; Lu, C.-B.; Li, H.-J.; Wang, F.; Shu, C.; Liu, C.; Lu, C.-W.; et al. Nrf2, a Potential Therapeutic Target against Oxidative Stress in Corneal Diseases. Oxid. Med. Cell. Longev. 2017, 2017, 2326178.
- Yuan, J.; Luo, K.; Zhang, L.; Cheville, J.C.; Lou, Z. USP10 Regulates P53 Localization and Stability by Deubiquitinating P53. Cell 2010, 140, 384–396.
- Steen, E.H.; Wang, X.; Balaji, S.; Butte, M.J.; Bollyky, P.L.; Keswani, S.G. The Role of the Anti-Inflammatory Cytokine Interleukin-10 in Tissue Fibrosis. Adv. Wound Care 2020, 9, 184–198.
- Ramaesh, T.; Ramaesh, K.; Leask, R.; Springbett, A.; Riley, S.C.; Dhillon, B.; West, J.D. Increased Apoptosis and Abnormal Wound-Healing Responses in the Heterozygous Pax6+/− Mouse Cornea. Investig. Opthalmol. Vis. Sci. 2006, 47, 1911–2171.
- Zheng, K.; Huang, H.; Peng, K.; Cai, J.; Jhanji, V.; Chen, H. Change of Optical Intensity during Healing Process of Corneal Wound on Anterior Segment Optical Coherence Tomography. Sci. Rep. 2016, 6, 32352.
- Ghatak, S.; Maytin, E.V.; Mack, J.A.; Hascall, V.C.; Atanelishvili, I.; Moreno Rodriguez, R.; Markwald, R.R.; Misra, S. Roles of Proteoglycans and Glycosaminoglycans in Wound Healing and Fibrosis. Int. J. Cell Biol. 2015, 2015, 1–20.
- Shu, D.Y.; Lovicu, F.J. Myofibroblast Transdifferentiation: The Dark Force in Ocular Wound Healing and Fibrosis. Prog. Retin. Eye Res. 2017, 60, 44–65.
- Cintron, C.; Covington, H.I.; Kublin, C.L. Morphologic Analyses of Proteoglycans in Rabbit Corneal Scars. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1789–1798.
- Raposo, G.; Stoorvogel, W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013, 200, 373–383.
- Turturici, G.; Tinnirello, R.; Sconzo, G.; Geraci, F. Extracellular Membrane Vesicles as a Mechanism of Cell-to-Cell Communication: Advantages and Disadvantages. Am. J. Physiol. Physiol. 2014, 306, C621–C633.
- Das, C.K.; Jena, B.C.; Banerjee, I.; Das, S.; Parekh, A.; Bhutia, S.K.; Mandal, M. Exosome as a Novel Shuttle for Delivery of Therapeutics across Biological Barriers. Mol. Pharm. 2019, 16, 24–40.
- Kuschert, G.S.V.; Hoogewerf, A.J.; Proudfoot, A.E.I.; Chung, C.; Cooke, R.M.; Hubbard, R.E.; Wells, T.N.C.; Sanderson, P.N. Identification of a Glycosaminoglycan Binding Surface on Human Interleukin-8. Biochemistry 1998, 37, 11193–11201.
- Hertsenberg, A.J.; Shojaati, G.; Funderburgh, M.L.; Mann, M.M.; Du, Y.; Funderburgh, J.L. Corneal Stromal Stem Cells Reduce Corneal Scarring by Mediating Neutrophil Infiltration after Wounding. PLoS ONE 2017, 12, e0171712.
- Dyer, D.P.; Thomson, J.M.; Hermant, A.; Jowitt, T.A.; Handel, T.M.; Proudfoot, A.E.I.; Day, A.J.; Milner, C.M. TSG-6 Inhibits Neutrophil Migration via Direct Interaction with the Chemokine CXCL8. J. Immunol. 2014, 192, 2177–2185.
- Shojaati, G.; Khandaker, I.; Funderburgh, M.L.; Mann, M.M.; Basu, R.; Stolz, D.B.; Geary, M.L.; Dos Santos, A.; Deng, S.X.; Funderburgh, J.L. Mesenchymal Stem Cells Reduce Corneal Fibrosis and Inflammation via Extracellular Vesicle-Mediated Delivery of MiRNA. Stem Cells Transl. Med. 2019, 8, 1192–1201.
- Shen, T.; Zheng, Q.-Q.; Shen, J.; Li, Q.-S.; Song, X.-H.; Luo, H.-B.; Hong, C.-Y.; Yao, K. Effects of Adipose-Derived Mesenchymal Stem Cell Exosomes on Corneal Stromal Fibroblast Viability and Extracellular Matrix Synthesis. Chin. Med. J. (Engl). 2018, 131, 704–712.
- Hofmann, T.G.; Stollberg, N.; Schmitz, M.L.; Will, H. HIPK2 Regulates Transforming Growth Factor-Beta-Induced c-Jun NH(2)-Terminal Kinase Activation and Apoptosis in Human Hepatoma Cells. Cancer Res. 2003, 63, 8271–8277.
- Shen, T.; Zheng, Q.; Luo, H.; Li, X.; Chen, Z.; Song, Z.; Zhou, G.; Hong, C. Exosomal MiR-19a from Adipose-Derived Stem Cells Suppresses Differentiation of Corneal Keratocytes into Myofibroblasts. Aging (Albany NY) 2020, 12, 4093–4110.
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. (Lausanne) 2018, 9, 402.
- An, J.; Chen, X.; Chen, W.; Liang, R.; Reinach, P.S.; Yan, D.; Tu, L. MicroRNA Expression Profile and the Role of MiR-204 in Corneal Wound Healing. Investig. Opthalmol. Vis. Sci. 2015, 56, 3673–3683.
- Wang, Y.; Li, W.; Zang, X.; Chen, N.; Liu, T.; Tsonis, P.A.; Huang, Y. MicroRNA-204-5p Regulates Epithelial-to-Mesenchymal Transition during Human Posterior Capsule Opacification by Targeting SMAD4. Investig. Opthalmol. Vis. Sci. 2013, 54, 323–332.
- Li, Y.; Zhao, Z.; Xu, C.; Zhou, Z.; Zhu, Z.; You, T. HMGA2 Induces Transcription Factor Slug Expression to Promote Epithelial-to-Mesenchymal Transition and Contributes to Colon Cancer Progression. Cancer Lett. 2014, 355, 130–140.
- Medici, D.; Hay, E.D.; Olsen, B.R. Snail and Slug Promote Epithelial-Mesenchymal Transition through β-Catenin–T-Cell Factor-4-Dependent Expression of Transforming Growth Factor-Β3. Mol. Biol. Cell 2008, 19, 4875–4887.
- Lu, Y.; Tai, P.W.L.; Ai, J.; Gessler, D.J.; Su, Q.; Yao, X.; Zheng, Q.; Zamore, P.D.; Xu, X.; Gao, G. Transcriptome Profiling of Neovascularized Corneas Reveals MiR-204 as a Multi-Target Biotherapy Deliverable by RAAVs. Mol. Ther. Nucleic Acids 2018, 10, 349–360.
- Ashby, B.D.; Garrett, Q.; Willcox, M.D.P. Corneal Injuries and Wound Healing—Review of Processes and Therapies. Austin J. Clin. Ophthalmol. 2014, 1, 1017.
- Landén, N.X.; Li, D.; Ståhle, M. Transition from Inflammation to Proliferation: A Critical Step during Wound Healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885.
- Zhou, Q.; Wang, Y.; Yang, L.; Wang, Y.; Chen, P.; Wang, Y.; Dong, X.; Xie, L. Histone Deacetylase Inhibitors Blocked Activation and Caused Senescence of Corneal Stromal cells. Mol. Vis. 2008, 14, 2556–2565.
- Sun, G.; Reddy, M.A.; Yuan, H.; Lanting, L.; Kato, M.; Natarajan, R. Epigenetic Histone Methylation Modulates Fibrotic Gene Expression. J. Am. Soc. Nephrol. 2010, 21, 2069–2080.
- Lu, C.; Sidoli, S.; Kulej, K.; Ross, K.; Wu, C.H.; Garcia, B.A. Coordination between TGF-β Cellular Signaling and Epigenetic Regulation during Epithelial to Mesenchymal Transition. Epigenetics Chromatin 2019, 12, 11.
- Tang, J.; Yan, H.; Zhuang, S. Histone Deacetylases as Targets for Treatment of Multiple Diseases. Clin. Sci. 2013, 124, 651–662.
- Zhou, Q.; Yang, L.; Wang, Y.; Qu, M.; Chen, P.; Wang, Y.; Xie, L.; Zhao, J.; Wang, Y. TGFβ Mediated Transition of Corneal Fibroblasts from a Proinflammatory State to a Profibrotic State through Modulation of Histone Acetylation. J. Cell. Physiol. 2010, 224, 135–143.
- Xiao, W.; Chen, X.; Liu, X.; Luo, L.; Ye, S.; Liu, Y. Trichostatin A, a Histone Deacetylase Inhibitor, Suppresses Proliferation and Epithelial–Mesenchymal Transition in Retinal Pigment Epithelium Cells. J. Cell. Mol. Med. 2014, 18, 646–655.
- Sharma, A.; Mehan, M.M.; Sinha, S.; Cowden, J.W.; Mohan, R.R. Trichostatin A Inhibits Corneal Haze In Vitro and In Vivo. Investig. Opthalmol. Vis. Sci. 2009, 50, 2695–2701.
- Donnelly, K.S.; Giuliano, E.A.; Sharma, A.; Mohan, R.R. Suberoylanilide Hydroxamic Acid (Vorinostat): Its Role on Equine Corneal Fibrosis and Matrix Metalloproteinase Activity. Vet. Ophthalmol. 2014, 17, 61–68.
- Etheredge, L.; Kane, B.P.; Hassell, J.R. The Effect of Growth Factor Signaling on Keratocytes In Vitro and Its Relationship to the Phases of Stromal Wound Repair. Investig. Opthalmol. Vis. Sci. 2009, 50, 3128–3136.
- Chen, Y.-J.; Huang, Y.-S.; Chen, J.-T.; Chen, Y.-H.; Tai, M.-C.; Chen, C.-L.; Liang, C.-M. Protective Effects of Glucosamine on Oxidative-Stress and Ischemia/Reperfusion-Induced Retinal Injury. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1506–1516.
- Pham, T.; Cornea, A.; Jenkins, A.; Blick, K.E.; Scofield, R.H. Oral Glucosamine in Doses Used to Treat Osteoarthritis Worsens Insulin Resistance. Am. J. Med. Sci. 2007, 333, 333–339.
- Esfandiari, H.; Loewen, N.A. Effect of Glucosamine on Intraocular Pressure. In Handbook of Nutrition, Diet, and the Eye, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019.
- Park, J.; Lee, S.-Y.; Ooshima, A.; Yang, K.-M.; Kang, J.M.; Kim, Y.-W.; Kim, S.-J. Glucosamine Hydrochloride Exerts a Protective Effect against Unilateral Ureteral Obstruction-Induced Renal Fibrosis by Attenuating TGF-β Signaling. J. Mol. Med. 2013, 91, 1273–1284.
- Wang, D.-F.; Yang, H.-J.; GU, J.-Q.; Cao, Y.-L.; Meng, X.; Wang, X.-L.; Lin, Y.-C.; Gao, M. Suppression of Phosphatase and Tensin Homolog Protects Insulin-Resistant Cells from Apoptosis. Mol. Med. Rep. 2015, 12, 2695–2700.
- Li, H.; Han, M.; Bernier, M.; Zheng, B.; Sun, S.; Su, M.; Zhang, R.; Fu, J.; Wen, J. Krüppel-like Factor 4 Promotes Differentiation by Transforming Growth Factor-β Receptor-Mediated Smad and P38 MAPK Signaling in Vascular Smooth Muscle Cells. J. Biol. Chem. 2010, 285, 17846–17856.
- He, M.; Zheng, B.; Zhang, Y.; Zhang, X.-H.; Wang, C.; Yang, Z.; Sun, Y.; Wu, X.-L.; Wen, J.-K. KLF4 Mediates the Link between TGF-Β1-Induced Gene Transcription and H3 Acetylation in Vascular Smooth Muscle Cells. FASEB J. 2015, 29, 4059–4070.
- Zhang, F.; Su, K.; Yang, X.; Bowe, D.B.; Paterson, A.J.; Kudlow, J.E. O-GlcNAc Modification Is an Endogenous Inhibitor of the Proteasome. Cell 2003, 115, 715–725.
- Swamynathan, S.; Buela, K.-A.; Kinchington, P.; Lathrop, K.L.; Misawa, H.; Hendricks, R.L.; Swamynathan, S.K. Klf4 Regulates the Expression of Slurp1, Which Functions as an Immunomodulatory Peptide in the Mouse Cornea. Investig. Opthalmol. Vis. Sci. 2012, 53, 8433–8446.
- Azad, A.K.; Sermsintham, N.; Chandrkrachang, S.; Stevens, W.F. Chitosan Membrane as a Wound-Healing Dressing: Characterization and Clinical Application. J. Biomed. Mater. Res. 2004, 69, 216–222.
- Alsarra, I.A. Chitosan Topical Gel Formulation in the Management of Burn Wounds. Int. J. Biol. Macromol. 2009, 45, 16–21.
- Fischak, C.; Klaus, R.; Werkmeister, R.M.; Hohenadl, C.; Prinz, M.; Schmetterer, L.; Garhöfer, G. Effect of Topically Administered Chitosan N Acetylcysteine on Corneal Wound Healing in a Rabbit Model. J. Ophthalmol. 2017, 2017, 5192924.
- Zahir-Jouzdani, F.; Mahbod, M.; Soleimani, M.; Vakhshiteh, F.; Arefian, E.; Shahosseini, S.; Dinarvand, R.; Atyabi, F. Chitosan and Thiolated Chitosan: Novel Therapeutic Approach for Preventing Corneal Haze after Chemical Injuries. Carbohydr. Polym. 2018, 179, 42–49.
- Chen, Q.; Lu, H.; Yang, H. Chitosan Inhibits Fibroblasts Growth in Achilles Tendon via TGF-Β1/Smad3 Pathway by MiR-29b. Int. J. Clin. Exp. Pathol. 2014, 7, 8462–8470.
- Gan, F.; Liu, Q.; Liu, Y.; Huang, D.; Pan, C.; Song, S.; Huang, K. Lycium Barbarum Polysaccharides Improve CCl4-Induced Liver Fibrosis, Inflammatory Response and TLRs/NF-KB Signaling Pathway Expression in Wistar Rats. Life Sci. 2018, 192, 205–212.
- Du, S.; Han, B.; Li, K.; Zhang, X.; Sha, X.; Gao, L. Lycium Barbarum Polysaccharides Protect Rat Corneal Epithelial Cells against Ultraviolet B-Induced Apoptosis by Attenuating the Mitochondrial Pathway and Inhibiting JNK Phosphorylation. Biomed Res. Int. 2017, 2017, 1–10.
- Kwok, S.S.; Wong, F.S.-Y.; Shih, K.C.; Chan, Y.-K.; Bu, Y.; Chan, T.C.-Y.; Ng, A.L.-K.; Lo, A.C.-Y.; Tong, L.; Yam, G.H.-F.; et al. Lycium Barbarum Polysaccharide Suppresses Expression of Fibrotic Proteins in Primary Human Corneal Fibroblasts. J. Clin. Med. 2020, 9, 3572.
- Richter, K.; Konzack, A.; Pihlajaniemi, T.; Heljasvaara, R.; Kietzmann, T. Redox-Fibrosis: Impact of TGFβ1 on ROS Generators, Mediators and Functional Consequences. Redox Biol. 2015, 6, 344–352.
- Cucoranu, I.; Clempus, R.; Dikalova, A.; Phelan, P.J.; Ariyan, S.; Dikalov, S.; Sorescu, D. NAD(P)H Oxidase 4 Mediates Transforming Growth Factor-Β1–Induced Differentiation of Cardiac Fibroblasts Into Myofibroblasts. Circ. Res. 2005, 97, 900–907.
- Liu, R.-M.; Desai, L.P. Reciprocal Regulation of TGF-β and Reactive Oxygen Species: A Perverse Cycle for Fibrosis. Redox Biol. 2015, 6, 565–577.
- Barnes, J.L.; Gorin, Y. Myofibroblast Differentiation during Fibrosis: Role of NAD(P)H Oxidases. Kidney Int. 2011, 79, 944–956.
- Bourji, K.; Meyer, A.; Chatelus, E.; Pincemail, J.; Pigatto, E.; Defraigne, J.-O.; Singh, F.; Charlier, C.; Geny, B.; Gottenberg, J.-E.; et al. High Reactive Oxygen Species in Fibrotic and Nonfibrotic Skin of Patients with Diffuse Cutaneous Systemic Sclerosis. Free Radic. Biol. Med. 2015, 87, 282–289.
- Serafini, M.M.; Catanzaro, M.; Fagiani, F.; Simoni, E.; Caporaso, R.; Dacrema, M.; Romanoni, I.; Govoni, S.; Racchi, M.; Daglia, M.; et al. Modulation of Keap1/Nrf2/ARE Signaling Pathway by Curcuma- and Garlic-Derived Hybrids. Front. Pharmacol. 2020, 10, 1597.
- Qu, M.; Zhang, X.; Hu, X.; Dong, M.; Pan, X.; Bian, J.; Zhou, Q. BRD4 Inhibitor JQ1 Inhibits and Reverses Mechanical Injury-Induced Corneal Scarring. Cell Death Discov. 2018, 4, 64.
- Balser, C.; Wolf, A.; Herb, M.; Langmann, T. Co-Inhibition of PGF and VEGF Blocks Their Expression in Mononuclear Phagocytes and Limits Neovascularization and Leakage in the Murine Retina. J. Neuroinflamm. 2019, 16, 1–12.
- Zhang, W.; Chen, J.; Qu, M.; Backman, L.J.; Zhang, A.; Liu, H.; Zhang, X.; Zhou, Q.; Danielson, P. Sustained Release of TPCA-1 from Silk Fibroin Hydrogels Preserves Keratocyte Phenotype and Promotes Corneal Regeneration by Inhibiting Interleukin-1 β Signaling. Adv. Healthc. Mater. 2020, 9, e2000591.
- Kondo, Y.; Fukuda, K.; Adachi, T.; Nishida, T. Inhibition by a Selective IκB Kinase-2 Inhibitor of Interleukin-1–Induced Collagen Degradation by Corneal Fibroblasts in Three-Dimensional Culture. Investig. Opthalmol. Vis. Sci. 2008, 49, 4850–4857.
- Mulholland, B.; Tuft, S.J.; Khaw, P.T. Matrix Metalloproteinase Distribution during Early Corneal Wound Healing. Eye 2005, 19, 584–588.
- Pardo, A.; Cabrera, S.; Maldonado, M.; Selman, M. Role of Matrix Metalloproteinases in the Pathogenesis of Idiopathic Pulmonary Fibrosis. Respir. Res. 2016, 17, 23.
- Stevenson, R.W.; Wilson, W.S. Drug-Induced Depletion of Acetylcholine in the Rabbit Corneal Epithelium. Biochem. Pharmacol. 1974, 23, 3449–3457.
- Chernyavsky, A.I.; Galitovskiy, V.; Shchepotin, I.B.; Jester, J.V.; Grando, S.A. The Acetylcholine Signaling Network of Corneal Epithelium and Its Role in Regulation of Random and Directional Migration of Corneal Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6921–6933.
- Öztürk, F.; Kurt, E.; Inan, Ü.Ü.; Emiroglu, L.; Ilker, S.S. The Effects of Acetylcholine and Propolis Extract on Corneal Epithelial Wound Healing in Rats. Cornea 1999, 18, 466–471.
- Słoniecka, M.; Backman, L.J.; Danielson, P. Acetylcholine Enhances Keratocyte Proliferation through Muscarinic Receptor Activation. Int. Immunopharmacol. 2015, 29, 57–62.
- Gubbiotti, M.A.; Vallet, S.D.; Ricard-Blum, S.; Iozzo, R.V. Decorin Interacting Network: A Comprehensive Analysis of Decorin-Binding Partners and Their Versatile Functions. Matrix Biol. 2016, 55, 7–21.
- Mohan, R.R.; Gupta, R.; Mehan, M.K.; Cowden, J.W.; Sinha, S. Decorin Transfection Suppresses Profibrogenic Genes and Myofibroblast Formation in Human Corneal Fibroblasts. Exp. Eye Res. 2010, 91, 238–245.
- ABDEL-WAHAB, N.; WICKS, S.J.; MASON, R.M.; CHANTRY, A. Decorin Suppresses Transforming Growth Factor-β-Induced Expression of Plasminogen Activator Inhibitor-1 in Human Mesangial Cells through a Mechanism That Involves Ca2+-Dependent Phosphorylation of Smad2 at Serine-240. Biochem. J. 2002, 362, 362–643.
- Chouhan, G.; Moakes, R.J.A.; Esmaeili, M.; Hill, L.J.; deCogan, F.; Hardwicke, J.; Rauz, S.; Logan, A.; Grover, L.M. A Self-Healing Hydrogel Eye Drop for the Sustained Delivery of Decorin to Prevent Corneal Scarring. Biomaterials 2019, 210, 41–50.
- Hill, L.J.; Moakes, R.J.A.; Vareechon, C.; Butt, G.; Ng, A.; Brock, K.; Chouhan, G.; Vincent, R.C.; Abbondante, S.; Williams, R.; et al. Sustained release of decorin to the surface of the eye enables scarless corneal regeneration. npj Regen. Med. 2018, 3, 23.
- Chaurasia, S.; Lim, R.; Lakshminarayanan, R.; Mohan, R. Nanomedicine Approaches for Corneal Diseases. J. Funct. Biomater. 2015, 6, 277–298.
- Kramerov, A.A.; Shah, R.; Ding, H.; Holler, E.; Turjman, S.; Rabinowitz, Y.S.; Ghiam, S.; Maguen, E.; Svendsen, C.N.; Saghizadeh, M.; et al. Novel Nanopolymer RNA Therapeutics Normalize Human Diabetic Corneal Wound Healing and Epithelial Stem Cells. Nanomed. Nanotechnol. Biol. Med. 2021, 32, 102332.
- Ma, X.-Y.; Bao, H.-J.; Cui, L.; Zou, J. The Graft of Autologous Adipose-Derived Stem Cells in the Corneal Stromal after Mechanic Damage. PLoS ONE 2013, 8, e76103.
- Cejkova, J.; Cejka, C.; Trosan, P.; Zajicova, A.; Sykova, E.; Holan, V. Treatment of Alkali-Injured Cornea by Cyclosporine A-Loaded Electrospun Nanofibers—An Alternative Mode of Therapy. Exp. Eye Res. 2016, 147, 128–137.
- Duan, X.; Sheardown, H. Dendrimer Crosslinked Collagen as a Corneal Tissue Engineering Scaffold: Mechanical Properties and Corneal Epithelial Cell Interactions. Biomaterials 2006, 27, 4608–4617.
- Ibrahim Al-Mashahedah, A.M.; Kanwar, R.K.; Kanwar, J.R. Utility of Nanomedicine Targeting Scar-Forming Myofibroblasts to Attenuate Corneal Scarring and Haze. Nanomedicine 2019, 14, 1049–1072.
- Swaminathan, S.; Vavia, P.R.; Trotta, F.; Cavalli, R. Nanosponges Encapsulating Dexamethasone for Ocular Delivery: Formulation Design, Physicochemical Characterization, Safety and Corneal Permeability Assessment. J. Biomed. Nanotechnol. 2013, 9, 998–1007.
- Weng, Y.; Liu, J.; Jin, S.; Guo, W.; Liang, X.; Hu, Z. Nanotechnology-Based Strategies for Treatment of Ocular Disease. Acta Pharm. Sin. B 2017, 7, 281–291.
More
Information
Subjects:
Ophthalmology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
04 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




