
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zahra Rezaei | -- | 3102 | 2022-11-03 20:11:49 | | | |
| 2 | Amina Yu | -4 word(s) | 3098 | 2022-11-04 03:07:42 | | |
Video Upload Options
One of the most concerning issues with conventional drug delivery platforms is the elicitation of an immune response upon implantation. Different natural and artificial platforms have been used for various biomedical applications ranging from drug and metabolite delivery, gene delivery, and wound healing/regenerative applications. However, most of these platforms suffer due to a compromise on immunogenicity and their respective biomedical applications. Although hydrogels from biomaterials of different origins have shown great promise in various biomedical applications, their immunogenicity, however small, is still a matter of concern, thus preventing their widespread clinical adoption. Hydrogels have been proposed as an excellent platform for various applications in drug delivery and regenerative medicine. Hydrogels are soft, tridimensional crosslinked networks of polymers with a high-water content, similar to the percentage found in human tissue.
1. Types of Hydrogels Used for Immunomodulation
1.1. Natural Hydrogels
1.1.1. Hyaluronate/Hyaluronic Acid (HA)
1.1.2. Gelatin
1.1.3. Alginate
1.2. Synthetic Hydrogels
1.3. Hybrid Hydrogels
2. Immunomodulation Strategies
2.1. Systematic Strategies
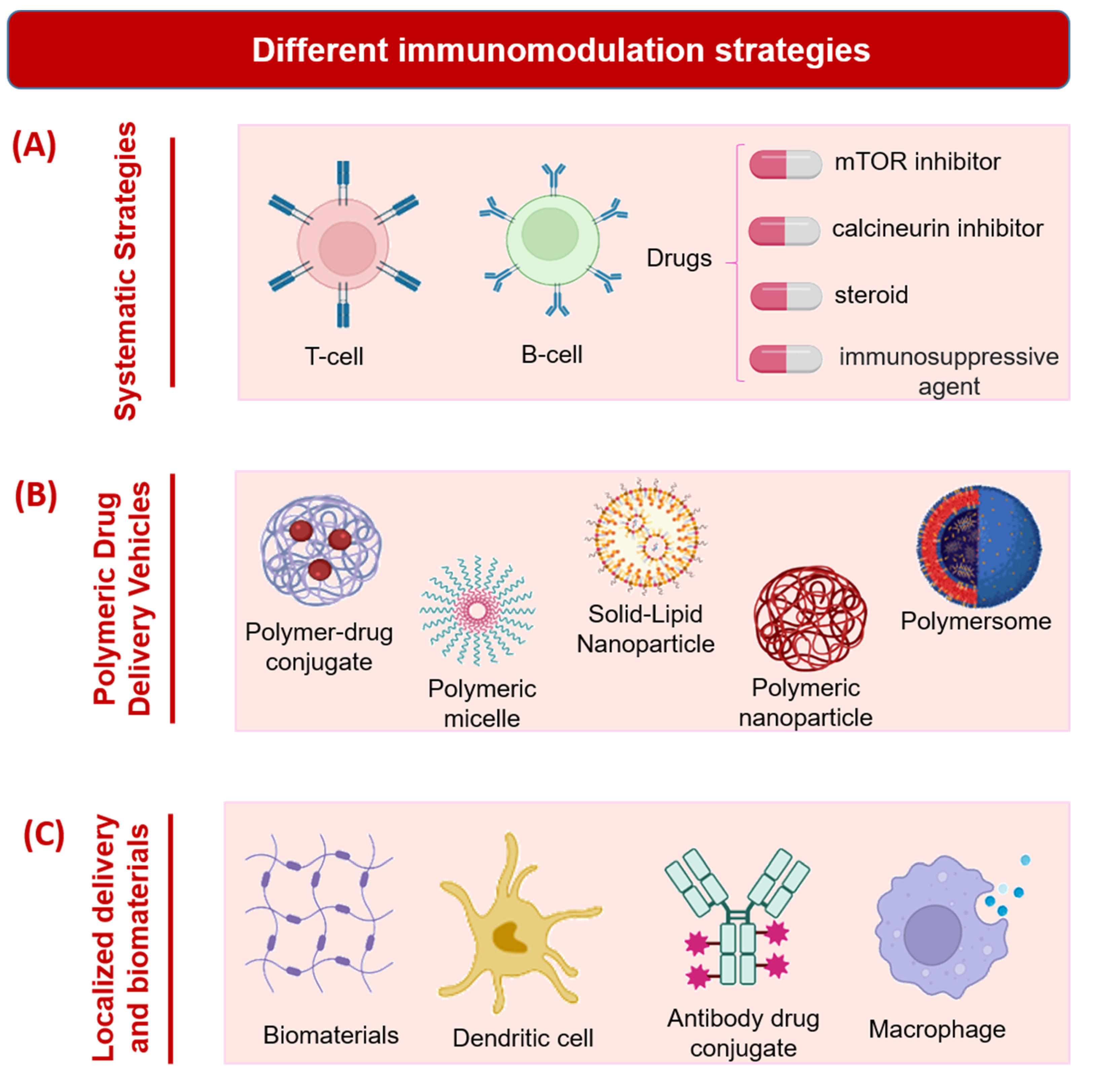
2.2. Polymeric Drug Delivery Vehicles
2.3. Localized Delivery and Biomaterials
3. Immunomodulating Molecules
3.1. Antigen Models
3.2. Immunomodulating Molecules in Cancer
3.3. Immunomodulating Molecules in Autoimmune Diseases
3.4. Microbial Molecules as Immunomodulating Agents
References
- Knopf-Marques, H.; Pravda, M.; Wolfova, L.; Velebny, V.; Schaaf, P.; Vrana, N.E.; Lavalle, P. Hyaluronic acid and its derivatives in coating and delivery systems: Applications in tissue engineering, regenerative medicine and immunomodulation. Adv. Healthc. Mater. 2016, 5, 2841–2855.
- Ferrini, A.; Stevens, M.M.; Sattler, S.; Rosenthal, N. Toward regeneration of the heart: Bioengineering strategies for immunomodulation. Front. Cardiovasc. Med. 2019, 6, 26.
- Nishiguchi, A.; Taguchi, T. Sustained-immunostimulatory nanocellulose scaffold to enhance vaccine efficacy. J. Biomed. Mater. Res. Part A 2020, 108, 1159–1170.
- Dai, M.; Sui, B.; Hua, Y.; Zhang, Y.; Bao, B.; Lin, Q.; Liu, X.; Zhu, L.; Sun, J. A well defect-suitable and high-strength biomimetic squid type II gelatin hydrogel promoted in situ costal cartilage regeneration via dynamic immunomodulation and direct induction manners. Biomaterials 2020, 240, 119841.
- Huang, L.; Zhang, J.; Hu, J.; Zhao, T.; Gu, Z. Biomimetic gelatin methacrylate/nano fish bone hybrid hydrogel for bone regeneration via osteoimmunomodulation. ACS Biomater. Sci. Eng. 2020, 6, 3270–3274.
- Reyshari, A.; Mohammadiazarm, H.; Mohammadian, T.; Torfi Mozanzadeh, M. Effects of sodium diformate on growth performance, gut microflora, digestive enzymes and innate immunological parameters of Asian sea bass (Lates calcarifer) juveniles. Aquac. Nutr. 2019, 25, 1135–1144.
- Alhaj, O.A.; Faye, B.; Agrawal, R.P. Handbook of Research on Health and Environmental Benefits of Camel Products; IGI Global: Hershey, PE, USA, 2019.
- Barthes, J.; Lagarrigue, P.; Riabov, V.; Lutzweiler, G.; Kirsch, J.; Muller, C.; Courtial, E.-J.; Marquette, C.; Projetti, F.; Kzhyskowska, J. Biofunctionalization of 3D-printed silicone implants with immunomodulatory hydrogels for controlling the innate immune response: An in vivo model of tracheal defect repair. Biomaterials 2021, 268, 120549.
- Uehara, M.; Li, X.; Sheikhi, A.; Zandi, N.; Walker, B.; Saleh, B.; Banouni, N.; Jiang, L.; Ordikhani, F.; Dai, L. Anti-IL-6 eluting immunomodulatory biomaterials prolong skin allograft survival. Sci. Rep. 2019, 9, 6535.
- Wei, W.; Zhang, Q.; Zhou, W.; Liu, Z.; Wang, Y.; Alakpa, E.V.; Ouyang, H.; Liu, H. Immunomodulatory application of engineered hydrogels in regenerative medicine. Appl. Mater. Today 2019, 14, 126–136.
- Bloise, N.; Rountree, I.; Polucha, C.; Montagna, G.; Visai, L.; Coulombe, K.L.; Munarin, F. Engineering immunomodulatory biomaterials for regenerating the infarcted myocardium. Front. Bioeng. Biotechnol. 2020, 8, 292.
- Yang, F.; Shi, K.; Jia, Y.; Hao, Y.; Peng, J.; Yuan, L.; Chen, Y.; Pan, M.; Qian, Z. A biodegradable thermosensitive hydrogel vaccine for cancer immunotherapy. Appl. Mater. Today 2020, 19, 100608.
- García, J.R.; Quirós, M.; Han, W.M.; O’Leary, M.N.; Cox, G.N.; Nusrat, A.; García, A.J. IFN-γ-tethered hydrogels enhance mesenchymal stem cell-based immunomodulation and promote tissue repair. Biomaterials 2019, 220, 119403.
- Koh, R.H.; Jin, Y.; Kim, J.; Hwang, N.S. Inflammation-modulating hydrogels for osteoarthritis cartilage tissue engineering. Cells 2020, 9, 419.
- Ji, X.; Yuan, X.; Ma, L.; Bi, B.; Zhu, H.; Lei, Z.; Liu, W.; Pu, H.; Jiang, J.; Jiang, X. Mesenchymal stem cell-loaded thermosensitive hydroxypropyl chitin hydrogel combined with a three-dimensional-printed poly (ε-caprolactone)/nano-hydroxyapatite scaffold to repair bone defects via osteogenesis, angiogenesis and immunomodulation. Theranostics 2020, 10, 725.
- Stojanovic, I.; Dimitrijevic, M.; Vives-Pi, M.; Jose Mansilla, M.; Pujol-Autonell, I.; Rodríguez-Fernandez, S.; Palova-Jelínkova, L.; Funda, D.P.; Gruden-Movsesijan, A.; Sofronic-Milosavljevic, L. Cell-based tolerogenic therapy, experience from animal models of multiple sclerosis, type 1 diabetes and rheumatoid arthritis. Curr. Pharm. Des. 2017, 23, 2623–2643.
- Dumont, C.M.; Park, J.; Shea, L.D. Controlled release strategies for modulating immune responses to promote tissue regeneration. J. Control. Release 2015, 219, 155–166.
- Srinivas, T.R.; Meier-Kriesche, H.-U. Minimizing immunosuppression, an alternative approach to reducing side effects: Objectives and interim result. Clin. J. Am. Soc. Nephrol. 2008, 3, S101–S116.
- Krzystyniak, A.; Gołąb, K.; Witkowski, P.; Trzonkowski, P. Islet cell transplant and the incorporation of Tregs. Curr. Opin. Organ Transplant. 2014, 19, 610.
- Obermajer, N.; Popp, F.C.; Soeder, Y.; Haarer, J.; Geissler, E.K.; Schlitt, H.J.; Dahlke, M.H. Conversion of Th17 into IL-17Aneg regulatory T cells: A novel mechanism in prolonged allograft survival promoted by mesenchymal stem cell–supported minimized immunosuppressive therapy. J. Immunol. 2014, 193, 4988–4999.
- Getts, D.R.; Martin, A.J.; McCarthy, D.P.; Terry, R.L.; Hunter, Z.N.; Yap, W.T.; Getts, M.T.; Pleiss, M.; Luo, X.; King, N.J. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat. Biotechnol. 2012, 30, 1217–1224.
- Dellacherie, M.O.; Seo, B.R.; Mooney, D.J. Macroscale biomaterials strategies for local immunomodulation. Nat. Rev. Mater. 2019, 4, 379–397.
- Howe, M.L.; Barres, B.A. A novel role for microglia in minimizing excitotoxicity. BMC Biol. 2012, 10, 7.
- Liu, Y.; Tian, F.; Shan, J.; Gao, J.; Li, B.; Lv, J.; Zhou, X.; Cai, X.; Wen, H.; Ma, X. Kupffer cells: Important participant of hepatic alveolar echinococcosis. Front. Cell. Infect. Microbiol. 2020, 10, 8.
- Clayton, K.; Vallejo, A.F.; Davies, J.; Sirvent, S.; Polak, M.E. Langerhans cells—Programmed by the epidermis. Front. Immunol. 2017, 8, 1676.
- Graham, J.G.; Zhang, X.; Goodman, A.; Pothoven, K.; Houlihan, J.; Wang, S.; Gower, R.M.; Luo, X.; Shea, L.D. PLG scaffold delivered antigen-specific regulatory T cells induce systemic tolerance in autoimmune diabetes. Tissue Eng. Part A 2013, 19, 1465–1475.
- Sundblad, V.; Morosi, L.G.; Geffner, J.R.; Rabinovich, G.A. Galectin-1: A jack-of-all-trades in the resolution of acute and chronic inflammation. J. Immunol. 2017, 199, 3721–3730.
- Drukker, M. Immunogenicity of embryonic stem cells and their progeny. Methods Enzymol. 2006, 420, 391–409.
- Drukker, M.; Katz, G.; Urbach, A.; Schuldiner, M.; Markel, G.; Itskovitz-Eldor, J.; Reubinoff, B.; Mandelboim, O.; Benvenisty, N. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2002, 99, 9864–9869.
- Sadtler, K.; Singh, A.; Wolf, M.T.; Wang, X.; Pardoll, D.M.; Elisseeff, J.H. Design, clinical translation and immunological response of biomaterials in regenerative medicine. Nat. Rev. Mater. 2016, 1, 16040.
- Andorko, J.I.; Jewell, C.M. Designing biomaterials with immunomodulatory properties for tissue engineering and regenerative medicine. Bioeng. Transl. Med. 2017, 2, 139–155.
- Chen, Y.; Shu, Z.; Qian, K.; Wang, J.; Zhu, H. Harnessing the properties of biomaterial to enhance the immunomodulation of mesenchymal stem cells. Tissue Eng. Part B Rev. 2019, 25, 492–499.
- Van Parijs, L.; Abbas, A.K. Role of Fas-mediated cell death in the regulation of immune responses. Curr. Opin. Immunol. 1996, 8, 355–361.
- Hume, P.S.; Anseth, K.S. Inducing local T cell apoptosis with anti-Fas-functionalized polymeric coatings fabricated via surface-initiated photopolymerizations. Biomaterials 2010, 31, 3166–3174.
- Brodbeck, W.G.; Patel, J.; Voskerician, G.; Christenson, E.; Shive, M.S.; Nakayama, Y.; Matsuda, T.; Ziats, N.P.; Anderson, J.M. Biomaterial adherent macrophage apoptosis is increased by hydrophilic and anionic substrates in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 10287–10292.
- Jones, J.A.; Chang, D.T.; Meyerson, H.; Colton, E.; Kwon, I.K.; Matsuda, T.; Anderson, J.M. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. 2007, 83, 585–596.
- Veiseh, O.; Doloff, J.C.; Ma, M.; Vegas, A.J.; Tam, H.H.; Bader, A.R.; Li, J.; Langan, E.; Wyckoff, J.; Loo, W.S. Size-and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater. 2015, 14, 643–651.
- Rowley, A.T.; Nagalla, R.R.; Wang, S.W.; Liu, W.F. Extracellular matrix-based strategies for immunomodulatory biomaterials engineering. Adv. Healthc. Mater. 2019, 8, 1801578.
- Heydari, P.; Kharaziha, M.; Varshosaz, J.; Javanmard, S.H. Current knowledge of immunomodulation strategies for chronic skin wound repair. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 265–288.
- Tomlin, H.; Piccinini, A.M. A complex interplay between the extracellular matrix and the innate immune response to microbial pathogens. Immunology 2018, 155, 186–201.
- Northrup, L.; Christopher, M.A.; Sullivan, B.P.; Berkland, C. Combining antigen and immunomodulators: Emerging trends in antigen-specific immunotherapy for autoimmunity. Adv. Drug Deliv. Rev. 2016, 98, 86–98.
- Ko, S.-Y.; Ko, H.-J.; Chang, W.-S.; Park, S.-H.; Kweon, M.-N.; Kang, C.-Y. α-Galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J. Immunol. 2005, 175, 3309–3317.
- He, H.; Fei, Z.; Guo, T.; Hou, Y.; Li, D.; Wang, K.; Ren, F.; Fan, K.; Zhou, D.; Xie, C. Bioadhesive injectable hydrogel with phenolic carbon quantum dot supported Pd single atom nanozymes as a localized immunomodulation niche for cancer catalytic immunotherapy. Biomaterials 2022, 280, 121272.
- Page, D.B.; Postow, M.A.; Callahan, M.K.; Allison, J.P.; Wolchok, J.D. Immune modulation in cancer with antibodies. Annu. Rev. Med. 2014, 65, 185–202.
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11.
- Savoia, P.; Astrua, C.; Fava, P. Ipilimumab (Anti-Ctla-4 Mab) in the treatment of metastatic melanoma: Effectiveness and toxicity management. Hum. Vaccines Immunother. 2016, 12, 1092–1101.
- Sundar, R.; Cho, B.-C.; Brahmer, J.R.; Soo, R.A. Nivolumab in NSCLC: Latest evidence and clinical potential. Ther. Adv. Med. Oncol. 2015, 7, 85–96.
- Wang, M.; Wang, J.; Wang, R.; Jiao, S.; Wang, S.; Zhang, J.; Zhang, M. Identification of a monoclonal antibody that targets PD-1 in a manner requiring PD-1 Asn58 glycosylation. Commun. Biol. 2019, 2, 392.
- Floudas, C.S.; Brar, G.; Mabry-Hrones, D.; Duffy, A.G.; Wood, B.; Levy, E.; Krishnasamy, V.; Fioravanti, S.; Bonilla, C.M.; Walker, M. A pilot study of the PD-1 targeting agent AMP-224 used with low-dose cyclophosphamide and stereotactic body radiation therapy in patients with metastatic colorectal cancer. Clin. Color. Cancer 2019, 18, e349–e360.
- Tan, S.; Liu, K.; Chai, Y.; Zhang, C.W.-H.; Gao, S.; Gao, G.F.; Qi, J. Distinct PD-L1 binding characteristics of therapeutic monoclonal antibody durvalumab. Protein Cell 2018, 9, 135–139.
- Vey, N.; Karlin, L.; Sadot-Lebouvier, S.; Broussais, F.; Berton-Rigaud, D.; Rey, J.; Charbonnier, A.; Marie, D.; André, P.; Paturel, C. A phase 1 study of lirilumab (antibody against killer immunoglobulin-like receptor antibody KIR2D.; IPH2102) in patients with solid tumors and hematologic malignancies. Oncotarget 2018, 9, 17675.
- Szymczak, F.; Colli, M.L.; Mamula, M.; Evans-Molina, C.; Eizirik, D.L. Gene expression signatures of target tissues in type 1 diabetes, lupus erythematosus, multiple sclerosis, and rheumatoid arthritis. Sci. Adv. 2021, 7, eabd7600.
- Ščigalková, I.; Bystroňová, J.; Kovářová, L.; Pravda, M.; Velebný, V.; Riabov, V.; Klüter, H.; Kzhyshkowska, J.; Vrana, N.E. The effect of healing phenotype-inducing cytokine formulations within soft hydrogels on encapsulated monocytes and incoming immune cells. RSC Adv. 2019, 9, 21396–21404.
- Mei, J.; Zhou, J.; Kong, L.; Dai, Y.; Zhang, X.; Song, W.; Zhu, C. An injectable photo-cross-linking silk hydrogel system augments diabetic wound healing in orthopaedic surgery through spatiotemporal immunomodulation. J. Nanobiotechnol. 2022, 20, 232.
- Ludvigsson, J. The role of immunomodulation therapy in autoimmune diabetes. J. Diabetes Sci. Technol. 2009, 3, 320–330.
- Herold, K.C.; Bundy, B.N.; Long, S.A.; Bluestone, J.A.; DiMeglio, L.A.; Dufort, M.J.; Gitelman, S.E.; Gottlieb, P.A.; Krischer, J.P.; Linsley, P.S. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N. Engl. J. Med. 2019, 381, 603–613.
- Sullivan, K.M.; Goldmuntz, E.A.; Keyes-Elstein, L.; McSweeney, P.A.; Pinckney, A.; Welch, B.; Mayes, M.D.; Nash, R.A.; Crofford, L.J.; Eggleston, B. Myeloablative autologous stem-cell transplantation for severe scleroderma. N. Engl. J. Med. 2018, 378, 35–47.
- Ludvigsson, J. Autoantigen treatment in type 1 diabetes: Unsolved questions on how to select autoantigen and administration route. Int. J. Mol. Sci. 2020, 21, 1598.
- Elias, D.; Avron, A.; Tamir, M.; Raz, I. DiaPep277® Preserves Endogenous Insulin Production by Immunomodulation in Type 1 Diabetes. Ann. N. Y. Acad. Sci. 2006, 1079, 340–344.
- Wherrett, D.K.; Bundy, B.; Becker, D.J.; DiMeglio, L.A.; Gitelman, S.E.; Goland, R.; Gottlieb, P.A.; Greenbaum, C.J.; Herold, K.C.; Marks, J.B. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: A randomised double-blind trial. Lancet 2011, 378, 319–327.
- Nicholas, D.; Odumosu, O.; Langridge, W.H. Autoantigen based vaccines for type 1 diabetes. Discov. Med. 2011, 11, 293.
- Jo, S.; Fang, S. Therapeutic Strategies for Diabetes: Immune Modulation in Pancreatic β Cells. Front. Endocrinol. 2021, 1019, 716692.
- Chen, Z.; Radjabzadeh, D.; Chen, L.; Kurilshikov, A.; Kavousi, M.; Ahmadizar, F.; Ikram, M.A.; Uitterlinden, A.G.; Zhernakova, A.; Fu, J. Association of Insulin Resistance and Type 2 Diabetes with Gut Microbial Diversity: A Microbiome-Wide Analysis From Population Studies. JAMA Netw. Open 2021, 4, e2118811.
- Häselbarth, L.; Ouwens, D.M.; Teichweyde, N.; Hochrath, K.; Merches, K.; Esser, C. The small chain fatty acid butyrate antagonizes the TCR-stimulation-induced metabolic shift in murine epidermal gamma delta T cells. EXCLI J. 2020, 19, 334.
- Gribova, V.; Petit, L.; Kocgozlu, L.; Seguin, C.; Fournel, S.; Kichler, A.; Vrana, N.E.; Lavalle, P. Polyarginine as a Simultaneous Antimicrobial, Immunomodulatory, and miRNA Delivery Agent within Polyanionic Hydrogel. Macromol. Biosci. 2022, 22, 2200043.
- Fallon, P.G.; Alcami, A. Pathogen-derived immunomodulatory molecules: Future immunotherapeutics? Trends Immunol. 2006, 27, 470–476.
- Adisakwattana, P.; Saunders, S.P.; Nel, H.J.; Fallon, P.G. Helminth-derived immunomodulatory molecules. Pathog.-Deriv. Immunomodulatory Mol. 2009, 666, 95–107.
- Mullick, J.; Kadam, A.; Sahu, A. Herpes and pox viral complement control proteins:‘the mask of self’. Trends Immunol. 2003, 24, 500–507.
- Viswanathan, K.; Liu, L.; Vaziri, S.; Dai, E.; Richardson, J.; Togonu-Bickersteth, B.; Vatsya, P.; Christov, A.; Lucas, A.R. Myxoma viral serpin, Serp-1, a unique interceptor of coagulation and innate immune pathways. Thromb. Haemost. 2006, 95, 499–510.
- McCoy, S.L.; Kurtz, S.E.; MacArthur, C.J.; Trune, D.R.; Hefeneider, S.H. Identification of a peptide derived from vaccinia virus A52R protein that inhibits cytokine secretion in response to TLR-dependent signaling and reduces in vivo bacterial-induced inflammation. J. Immunol. 2005, 174, 3006–3014.




