1. Types of Hydrogels Used for Immunomodulation
1.1. Natural Hydrogels
1.1.1. Hyaluronate/Hyaluronic Acid (HA)
Hyaluronate is a glycosaminoglycan (GAG), a key component of extracellular matrix (ECM) which is present in the majority of tissues but mainly in connective, epithelial, and nerve tissues
[1](Figure 2A) [54]. HA can modulate cell migration, inflammatory response, and angiogenesis
[1][2][9,54].
1.1.2. Gelatin
Gelatin can be obtained from numerous animal sources such as swine
[3][58], squid
[4][8], fish
[5][57], beef
[6][59], and camel
[7][60]. Gelatin has been conjugated with a wide range of immunomodulating molecules
(Figure 2B). One such example includes a gelatin-based hydrogel with IL-10 and PG-E2 that has been used as an immunomodulatory coating for silicone 3D tracheal implants to prevent granuloma formation. This formulation demonstrated an upregulation of M2 macrophages and low levels of proinflammatory cytokines, thereby controlling the inflammatory response
[8][55]. Furthermore, gelatin methacryloyl (GelMA) when combined with IL-6 resulted in double survival of skin allografts compared with IL-6 administered alone
[9][61].
1.1.3. Alginate
Alginate is an alga-derived anionic natural polymer. In the presence of Ca
2+ ions and mature dendritic cells, alginate hydrogels have been demonstrated to attract immune cells to the site of injection, leading to a greater immune response against infection
[10]. Moreover, alginate gel formulations with calcium carbonate (CaCO
3) have displayed faster-wound healing by macrophage polarization to the M2 phenotype when applied to skin flap ischemic wounds
[11] (Figure 2C) [56].
1.2. Synthetic Hydrogels
P
olyethylene glycol (PEG
) is a synthetic polymer that is used as a constituent in healthcare-related products. It has also been used for hydrogel synthesis and applications. Several formulations with PEG have demonstrated biocompatibility and effectiveness for cancer immunotherapy when combined with growth factors, cancer antigens, and antigen-presenting cells
[10][12][10,62]. Additionally, PEG with TGB-ß1 and IL-10 has also been effective for immunosuppression by decreasing dendritic cell maturation
[10]. Furthermore, PEG-based hydrogels loaded with human mesenchymal stem cells (hMSC) and IFN-γ have also shown accelerated colonic mucosal wound healing in immunocompetent and immunocompromised mice
[13][63]. PEG and other types of synthetic hydrogels such as polyvinyl alcohol (PVA) and polylactic co-glycolic acid (PLGA) have been useful for cartilage tissue engineering as well
[14] (Figure 2D) [24].
1.3. Hybrid Hydrogels
To improve mechanical properties as well as biocompatibility, natural and synthetic hydrogels can be conjugated together. For example, it has been demonstrated that gelatin derived from squid cartilage when combined with methacrylated HA (HAMA) and crosslinked with PEG has improved mechanical properties. Furthermore, this hybrid hydrogel is capable of tissue restoration as well as inducing an immunomodulatory response to neutrophils and anti-inflammatory macrophages, resulting in cartilage formation
[4][8]. Also, an injectable hydrogel composed of PEG and vascular endothelial growth factor (VEGF) showed cardioprotective properties and improved vascularization in a rat model
[2][9]. Additionally, hydroxyapatite combined with an hMSC hydrogel and a 3D-printed model for bone regeneration has demonstrated enhanced osteogenesis, macrophage regeneration, and angiogenic properties
[15][64]. These are only a few examples of the diversity that can be achieved by combining natural and synthetic polymers in order to obtain better hydrogel properties
(Figure 2F).
In the next sections, thwe focus is on discuss immunomodulating strategies and different molecules that might be potential new candidates to explore when designing immunomodulating hydrogels (IMHs). The IMHs. We will briefly discuss strategies that can be used on a systemic level or localized together with candidates for immunomodulation to be used for specific diseases will be discussed.
2. Immunomodulation Strategies
2.1. Systematic Strategies
Tolerogenic therapy and systematic immune suppression following allogeneic tissue or cell transplantation are often needed to avert pernicious autoimmune events
[16][65]. These same issues also need to be considered when using IMHs for biomedical applications. T cell activation, effector cell differentiation, immunological priming, and effector T cell trafficking have all been documented to occur with the clinical introduction of scaffolds, devices, implants, and any materials which are recognized as foreign by the immune system
[17][66]. Defective T cells also produce rigorous, unwanted autoimmune cycles leading to antigen-specific inflammation, which resemble phenomena occurring in the spinal cord and other traumatic injuries. Indeed, T cells are often the key players or targets in adaptive immune system remodeling and their modulation can be achieved by alterations that are made to the innate immune system
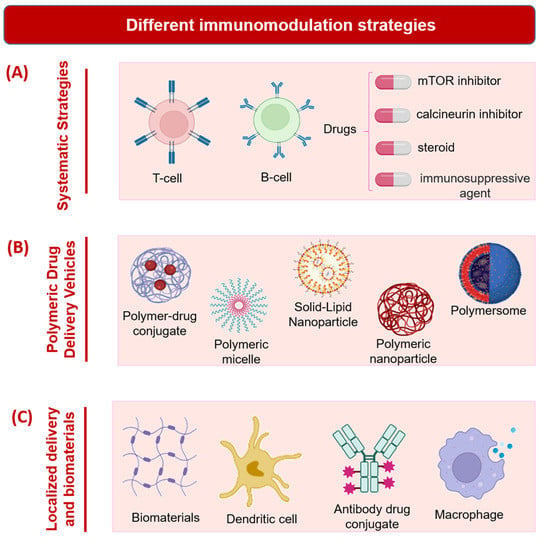
[17][66]. Currently, blocking antibodies or immunosuppressants of different drug classes including, but not limited to, sirolimus (mammalian target of rapamycin, or mTOR inhibitor), tacrolimus (calcineurin inhibitor), and prednisone (steroid) are used to dampen auto- or alloimmune responses (
Figure 13A)
[18][67]. However, these often produce a significant decline in anti-infectivity and leukocyte-mediated tissue regeneration at the site of an injury. In one study, administrating targeting molecules or substances systemically has been shown to aid in immunomodulation and antigen tolerance
[17][66]. For instance, by the intravenous route, Tregs have been utilized to reduce critical immunity caused by isoantigens arising from islet graft transplantations
[19][68]. This occurs due to their counteractivity to effector T cells that are antigen-specific. Mesenchymal stem cells, combined with mycophenolate mofetil (MMF), have also been delivered into the veins of mice after transplantation surgeries, and their function is to increase the number of T helper 17 cells, which MMF interacts with to produce effector T cells
[20][69]. In fact, systematically rather than locally administered MSCs lead to the higher presentation of Tregs throughout the body and reduce the potential for inflammation following allograft implantation
[17][66].
Figure 13. Different immunomodulation strategies. (A) Systematic strategies. (B) Polymeric drug delivery vehicles. (C) Localized delivery and biomaterials. Schematics prepared using Biorender.
2.2. Polymeric Drug Delivery Vehicles
In another strategy for the modulation of the immune response, polymeric nanoparticles have been systemically introduced in many instances to emulate apoptotic cell fragments (
Figure 13B). In one study, non-biodegradable, carboxylated and myelin integral membrane protein-conjugated polystyrene (PS) particles (500 nm) were fabricated and intravenously administered into mice with autoimmune encephalomyelitis
[21][70]. With the (139–151) protein epitope, the polymer nanoparticles prevented or slowed progression of the brain and spinal cord inflammation associated with this condition. Moreover, studies investigating PS nanospheres, showed that those with a larger size (500 nm
−1 µm) and with an anionic charge on their membranes were more likely to interact with macrophage receptors with collagenase structure (MARCO), which determines the degree of diminished immunogenicity
[21][70]. Other strategies attempted for treating autoimmune encephalomyelitis have used poly (lactide coglycolide) (PLG) nanoparticles as carriers of different substances, such as antigen-specific peptides, and immunosuppressant drugs, which can transiently inhibit the activation of T helper 1 and 17 cells, monocytes, and CD4+ and CD8+ T cells, among others
[21][70].
2.3. Localized Delivery and Biomaterials
Systemic strategies have been shown to be effective, although potential antagonistic responses, including intolerances in the gastrointestinal tract, secondary immune side effects, or the evolution of antibiotic-resistant microbes could occur
[17][66]. The local delivery of proteins, genes, cells, or biomaterials could help to avoid these potential side effects and still enable immunomodulation in the absence of circulating factors. Moreover, there are advantages of using local over systemic approaches, especially when targeting molecules or antibodies that are not incorporated into the formulation being administered, of improved tissue specificity and functional modulation
[22][71]. In one well-characterized phenomenon that occurs in the central nervous system, microglia and astrocytes co-operate to reduce inflammation caused by injury and eliminate excitotoxicity
[23][72]. The local delivery of immunomodulators could promote direct interaction with these cells and any associated inflammatory cells or molecules. Indeed, design considerations often implicate the interacting immune cell populations that will become affected by a locally administered immunomodulating agent. Often, these populations are somewhat distinct in their functions or responses across tissue types, despite falling into the same categories that encompass the traditional immune cells, such as the macrophages, lymphocytes, and neutrophils (
Figure 13C). For example, the liver and epidermis, respectively, contain specialized Kupffer cells or hepatic macrophages and Langerhans cells, which have a macrophagic cell lineage as well as dendritic cell properties, that are situated in the outer skin layers
[24][25][73,74]. As elaborated above in the discussion on systemic cell delivery, stem cells, especially MSCs, are excellent for re-configuring immune system functions (e.g., by altering macrophage polarization states, changing immune cell phenotypes, or reducing inflammation). Their localized transplantation can be utilized to achieve good clinical outcomes in terms of tissue regeneration and immunomodulation. This is accomplished by direct interactions between native and transplanted cells or by the transmission of cell signaling proteins or molecules, such as cytokines and hormones, that broadcast messages between interacting cell types. Tregs have also been transplanted singularly or together with PLG biomaterials in order to inhibit effector T cell and dendritic cell responses and to prevent implant rejection by the immune system
[26][75]. They function by secreting proteins including the cytokines IL-10, galectin-1, and TGF-β, or by interacting with the host cells through several immune-regulating protein-mediated pathways
[27][76]. An interesting study investigating locally transplanted Tregs demonstrated that systemic protection (in distal areas) could only be achieved via regional administration of these cells
[26][75]. Moreover, when immune aversion, rather than modulation, is desired in tissue engineering applications, embryonic stem or other fetal cells which are deficient in major histocompatibility complexes I and II can be transplanted
[28][29][77,78].
This leads to a discussion on biomaterials and tissue growth scaffolds such as IMHs as modifiable substrates that can be used locally to prevent autoimmunity from occurring or lessen the extent or severity of an autoimmune response. Regenerative medicine utilizes biomaterials and hydrogels in order to internally support the growth, proliferation, and differentiation of autogenous or allogeneic cells, including stem cells; to provide material or a base for host tissue complexation and integration, and to restore functional performance in damaged organs or tissues
[30][79]. Properties of biomaterials, including hydrogels, therefore must be evaluated in order to determine the optimal structure, porosity, surface features, chemical composition, and potential for functionalization with cells, nanoparticles, proteins, genes, or other molecules
[31][32][80,81]. These properties influence the eventual performance, including the immunomodulation, of hydrogels and other biomaterials in vivo. An example of this would be the prevention of lymphocyte adhesion onto biomaterials by attaching the Fas ligand to them, which can bind the Fas receptors on lymphocytes and deactivate them
[33][34][82,83]. In alternate approaches, structural porosity, as well as fiber alignments and diameters in electrospun scaffolds, have been optimized to minimize fibrous capsule formation and improve host/implant assimilation
[17][66]. These properties have been determined to influence the release of cytokines and other biomolecules by host immune cells.
The hydrophobicity of a material can also modulate the behavior of immune cells, as more hydrophilic surfaces tend to recruit few monocytes and macrophages overall
[35][84]. Foreign-body giant cells, which are conglomerates of macrophages, are also less likely to become activated when hydrophilic materials are implanted
[36][37][85,86].
3. Immunomodulating Molecules
3.1. Antigen Models
The properties of the extracellular matrix (ECM) constitute one of the most important parameters in the regulation of an immune response. ECM constitutes different factors such as laminin, collagen, fibronectin, and proteoglycan, which affect cell function and immunogenicity. Furthermore, ECM contains bioactive agents such as antigens, cytokines, and other immunomodulatory molecules. The interaction between ECM components and antigen molecules has been shown to positively affect tissue regeneration, healing, and therapeutic applications
[38][39][40][87,88,89]. Proof of concept studies in immunomodulation have utilized antigen molecules in order to assess strategies in vaccination efficacy, though many of their effects are only partially translated to human studies
[41][90]. These model antigens include ovalbumin from chicken eggs, bovine serum albumin, and dinitrophenol, which are classically recognized as allergenic proteins or small molecule antigens, respectively. In turn, experimental molecules such as α-galactosylceramide, which is a natural killer T cell ligand that functions as an adjuvant, can also be co-administered with the model antigens to increase the potency of the complete formulation. Besides producing a potent immune response by nasal spray application in BALB/c and C57BL/6 mice, this adjuvant and antigen complex has been proven effective in combatting EG7 lymphoblasts cells
[42][91].
3.2. Immunomodulating Molecules in Cancer
Immunotherapy has become a promising technique to treat cancer by using localized immune niches such as hydrogels, which can act as three-dimensional biomedical cargo carriers and can regulate the tumor microenvironment for better therapeutic output
[43][38]. In immunomodulation, proteins, and small molecules such as antibodies and immune checkpoint inhibitor drugs, which are often composed of antibodies as their active ingredients, are used. For example, in 2011, the immunotherapeutic ipilimumab, a monoclonal antibody and checkpoint inhibitor, was approved by the FDA for use in melanoma and other cancer patients
[44][92]. These checkpoint inhibitors primarily govern the native T cell processes after their administration
[45][93]. For instance, the co-inhibitory molecule cytotoxic T lymphocyte antigen 4, or CTLA-4, which is found in high amounts in regulatory T cells, is blocked by ipilimumab or tremelimumab from binding B7 protein, thus allowing the elimination of tumor cells by T cells
[44][46][92,94].
Advanced checkpoint inhibitors have also been produced to target apoptotic cell death proteins and ligands, including PD-1 (programmed cell death protein 1), a receptor of the B7 family, and the associated ligands PD-L1 and PD-L2 (programmed death ligands 1 and 2), especially in cancer immunotherapy
[44][92]. Nivolumab, a G4 immunoglobulin, has been shown to be safe and effective as a PD-1 inhibitor, and other molecules that are undergoing evaluation for their anti-PD1 activity include pembrolizumab and pidilizumab humanized monoclonal antibodies, as well as AMP-224 protein
[47][48][49][95,96,97]. Unlike ipilimumab, nivolumab was not dose-dependent in terms of the efficacy return following administration, although both drugs demonstrated anti-cancer responses only after prolonged treatment, during which time there was a stable progression of the disease state before amelioration occurred
[44][92]. Atezolizumab and BMS-936559 are, similarly, antibody treatments that target the PD-L1 ligand in cancer cells, and in other instances, checkpoint inhibitors such as lirilumab are being investigated for their ability to bind alternate T and natural killer cell receptors, other than CTLA-4 or PD-1
[50][51][98,99]. Many of these molecule drugs can be used in consortium with chemotherapeutics in designed IMHs to achieve a potent anti-cancerous effect.
3.3. Immunomodulating Molecules in Autoimmune Diseases
There are more than 80 different autoimmune diseases, with the most common ones being type-1 diabetes, rheumatoid arthritis, multiple sclerosis, and systemic lupus erythematosus
[52][100]. Complementary strategies have been applied in the immunomodulation of these and other autoimmune diseases. In the case of implantable biomaterials, a common problem with implications for their effectiveness is an immune response from the host
[8][55]. Many factors such as physicochemical and biocompatible properties, and the location of an implanted material can determine the severity of the immune response
[53][101]. To boost diabetic-wound healing, a photocurable methacryloxylated silk fibroin hydrogel (Sil-MA) system co-encapsulated with metformin-loaded mesoporous silica microspheres (MET@ MSNs) and silver nanoparticles (Ag NPs) was developed. Sil-MA-based hydrogel showed a sustained and controlled release of the Ag nanoparticles. Moreover, this hydrogel platform inhibited the formation of neutrophil extracellular traps, which induces pro-inflammatory factors. Due to its ability to modify the immune microenvironment, this IMH system promoted fibroblast migration and endothelial cell angiogenesis
[54][102].
Other strategies and approaches have also been developed and applied in the immunomodulation of autoimmune diseases. In type I diabetes, the preservation of beta cells is a primary objective for which drugs and therapies have been developed
[55][103]. One approach utilizes anti-CD3 antibodies in order to slow beta cell deterioration since the CD3 receptor is involved in a rigorous autoimmune response in diabetic patients
[56][104]. This tactic delayed the progression of type I diabetes by just one year, perhaps, alternate strategies including an anti-CD3 booster are needed for the long-term. Moreover, cytokine release syndrome, low blood platelet counts, and Epstein-Barr viral reactivation were serious side effects associated with the CD3 antibody treatment approach
[55][103].
To overcome these challenges, other approaches undergoing empirical evaluation include the nonmyeloablative transplantation of autologous stem cells, autoantigen treatment, and vaccination with glutamic acid decarboxylase
[55][103]. Autologous stem cell transplantation is risky and can lead to high rates of treatment-related mortality, as discerned from previous applications to other autoimmune conditions, hence other methods may be preferred
[55][56][57][103,104,105]. For instance, in the autoantigen approach, one direct influencer of beta cell functions is insulin, which has been subcutaneously administered to pre-diabetic patients as a preventative measure
[58][106]. However, the preventative success of insulin injections was not substantiated by clinical trials, despite the efficacious use of insulin in treating type I diabetes. Other indirect autoantigens are heat shock proteins, or their synthetic peptide counterparts such as DiaPep277, which has been used to preserve insulin control and secretion by the beta cells without producing adverse side effects in adult patients with type I diabetes
[59][107]. Glutamic acid decarboxylase, a metabolic rate-determining enzyme for the process of glutamate to gamma-aminobutyric acid conversion, has also been shown, like autoantigens, to cause a Th1 to Th2 transition of T cells
[60][108]. A vaccine, Diamyd, containing this enzyme and the aluminum hydroxide adjuvant has demonstrated the potential to prevent diabetes in numerous clinical trials in the United States and Europe
[61][109].
3.4. Microbial Molecules as Immunomodulating Agents
Microbiota-derived molecules have also been used in immunomodulation, and they will be briefly described here
[62][110]. Increasingly, type I and type II diabetes are linked to intrinsic changes in gut microbiota, which may release smaller quantities of short-chain fatty acids, such as butyrate
[62][63][110,111]. Diminished butyrate conversion from lactate has been correlated with reduced regulatory T cell generation and a weakened suppression of the autoimmune system
[64][112].
One recent study has described hyaluronic acid (HA) hydrogels as having triple biological activities of being antimicrobial, immunomodulatory, and capable of acting as a miRNA delivery agent. MiRNA with antibacterial and anti-inflammatory properties was loaded into these hydrogels. Furthermore, the HA hydrogel was fabricated with polyarginine, which reduced the inflammatory response of lipopolysaccharide-stimulated macrophages
[65][113].
In other instances, immunomodulatory molecules derived from pathogenic or non-pathogenic microbes have been applied in acquiescing inflammatory conditions
[66][67][114,115]. For instance, a protein of the vaccinia virus has been demonstrated to interrupt the complement system cascade that occurs following the implantation of a foreign body, and also to avert immune system-induced damage to the central nervous system
[66][68][114,116]. Another example is of a protease inhibitor released by the myxoma virus, stress-associated endoplasmic reticulum protein 1, which is anti-inflammatory in patients with acute coronary syndrome
[66][69][114,117]. Numerous cytokines, chemokine homologs, chemokine binding proteins, and cell signaling molecules released by viruses, bacteria, and fungal species have also shown efficacy in blocking infections, suppressing inflammation, and inhibiting chemokines, for example, the A52R protein, which is also a product of the vaccinia virus, reduces inflammation by inhibiting the generation of NF-kappaB proteins through toll-like receptor mechanisms
[70][118].