Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kazuhisa Maeda | -- | 1621 | 2022-11-03 18:52:43 | | | |
| 2 | Peter Tang | Meta information modification | 1621 | 2022-11-04 03:14:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Maeda, K. Tranexamic Acid in Melasma and Sun-Induced Hyperpigmentation Treatment. Encyclopedia. Available online: https://encyclopedia.pub/entry/32773 (accessed on 07 February 2026).
Maeda K. Tranexamic Acid in Melasma and Sun-Induced Hyperpigmentation Treatment. Encyclopedia. Available at: https://encyclopedia.pub/entry/32773. Accessed February 07, 2026.
Maeda, Kazuhisa. "Tranexamic Acid in Melasma and Sun-Induced Hyperpigmentation Treatment" Encyclopedia, https://encyclopedia.pub/entry/32773 (accessed February 07, 2026).
Maeda, K. (2022, November 03). Tranexamic Acid in Melasma and Sun-Induced Hyperpigmentation Treatment. In Encyclopedia. https://encyclopedia.pub/entry/32773
Maeda, Kazuhisa. "Tranexamic Acid in Melasma and Sun-Induced Hyperpigmentation Treatment." Encyclopedia. Web. 03 November, 2022.
Copy Citation
Tranexamic acid (TXA) has anti-plasmin activity and has been shown when administered orally to be effective against melasma, for which it is considered first-line pharmacotherapy. Several studies have shown that topically applied TXA is also effective against melasma and skin hyperpigmentation caused by sunburn and inflammation. The TXA concentration in the epidermis and dermis/vasculature has been estimated from its distribution in the skin after closed application, and topically applied TXA has thus been shown to act on neutrophils and mast cells in the dermis and on the vascular system.
tranexamic acid
topical application
melasma
sun-induced skin hyperpigmentation
skin-lightening agent
quasi-drug
plasmin
1. Introduction
Melanin is produced by melanocytes in the basal layer of the epidermis and is constantly supplied to the surrounding keratinocytes. The keratinocytes are regulated to maintain a constant amount of melanin, and skin color therefore should not fluctuate. However, aging, ultraviolet radiation, and hormonal imbalance disrupt the skin’s regulatory mechanisms, resulting in increased melanin and excessive melanin accumulation in keratinocytes, which manifests as pigment spots [1]. The turnover time of the human epidermis varies among individuals, regions, and ages and is approximately 1 month on the forearm of a 37-year-old individual [2]. Hyperpigmentation produced by ultraviolet light fades gradually through epidermal turnover, while melasma fades gradually after menopause when factors that stimulate melanocytes return to normal levels. Senile lentigo, which increases with age, does not fade, and its pathogenic mechanism differs from that of melasma.
To date, approximately 20 quasi-drug whitening active ingredients have been developed for use in Japan [3]. Tranexamic acid (trans-4-aminomethylcyclohexanecarboxylic acid; TXA, Figure 1) is a drug with approximately 10-fold the anti-plasmin action exhibited by ε-aminocaproic acid [4] and has been used for more than 30 years to treat bleeding tendency and abnormal bleeding potentially related to increased fibrinolysis [5]. In the field of dermatology, TXA at 750–2000 mg per day in 3–4 divided doses have been used in the treatment of skin diseases such as eczema and related conditions, urticaria, drug eruptions, and toxic reactions, and its effects on itching, swelling, erythema, and other symptoms have been established [5]. In addition to inhibiting plasmin [6], TXA has recently been shown to readily compete with tyrosinase activity [7] and can inhibit hyperpigmentation by decreasing melanin synthesis [8][9]. TXA is available as a pharmaceutical cosmetic (quasi-drug containing TXA) that is effective against freckles and other pigment spots, and women with freckles can easily use it as part of their skin-lightening skincare regimen.
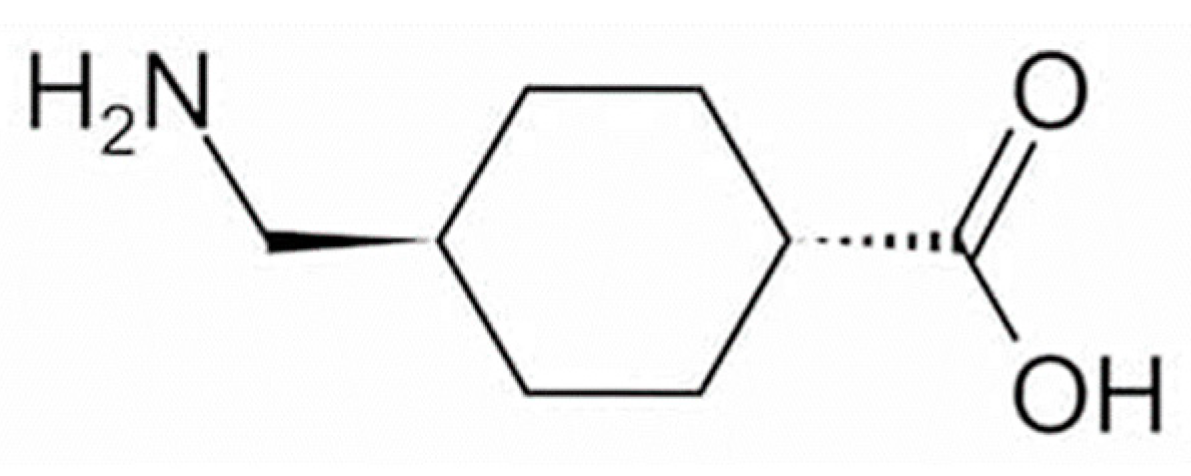
Figure 1. Structural formula of tranexamic acid (trans-4-aminomethylcyclohexanecarboxylic acid).
2. Effectiveness of TXA for Melasma
Melasma is a common hyperpigmentation disorder, especially in women of reproductive age who are pregnant or using oral contraceptives [10][11]. Increased numbers of mast cells have been observed in the skin at the site of melasma lesions [12]. Mast cells may produce vascular endothelial growth factor (VEGF), transforming growth factor-β (TGF-β), and basic fibroblast growth factor (bFGF), all of which promote vascular growth and therefore contribute to the development of melasma [13][14]. Despite the important role of mast cells and histamine in the pathogenesis of melasma, anti-histamines have failed to show a benefit in the treatment of this condition. Oral TXA (the clinical trial registration number; DH-4243) has been shown to be effective against melasma [15] and has been available in Japan as an over-the-counter treatment for melasma since 2007. Several additional studies have demonstrated the efficacy of oral TXA [16][17][18][19][20][21][22] and its usefulness as a topical agent [23][24][25][26][27], and its ability to reduce epidermal hyperpigmentation of melasma and improve dermal disorders such as vascular and mast cell counts [16] has also been documented. In addition to oral TXA, topical hydroquinone is considered an effective treatment for melasma [28] and is widely used as a 2–5% ointment despite reported skin irritation and formulation instability [29]. Laser treatment, another option, can cause aggravation of lesions. Satisfactory results have been obtained after oral TXA treatment with a combination of oral and topical medications [20][30][31][32]. The toxicity of TXA to melanocytes or adverse effects of the topical application have not been reported.
The clinical efficacy of oral TXA has been well-established by a number of research groups [33]. Higashi et al. reported that oral administration of 750–1500 mg per day of TXA resulted in a significant improvement in hyperpigmentation 2–3 months after treatment initiation and an almost complete resolution in cases where treatment was continued for six months to one year [34]. A previous study evaluated the topical application of TXA as a cream containing 1% vitamin C for twice daily use (in the morning and before bedtime) for more than six months in 10 patients with melasma. Melasma was fully resolved in one patient who had light pigmentation at the start of treatment, while the nine remaining patients showed a reduction in hyperpigmentation, indicating the efficacy of the treatment [34].
The effectiveness against melasma of the topical application of a liposomal lotion containing 2% TXA, 3% ascorbic acid phosphate magnesium salt, and 2% α-tocopherol for at least three months has also been demonstrated [35]. Although quasi-drugs containing TXA take longer to show efficacy than oral TXA, a reduction in hyperpigmentation can nevertheless be observed (Figure 2). However, oral administration of TXA may cause mild gastrointestinal disturbance and irregular menstruation [36]. Therefore, given that melasma can be aggravated by UV light and inflammation, treatment with topical TXA may be beneficial after oral TXA administration.
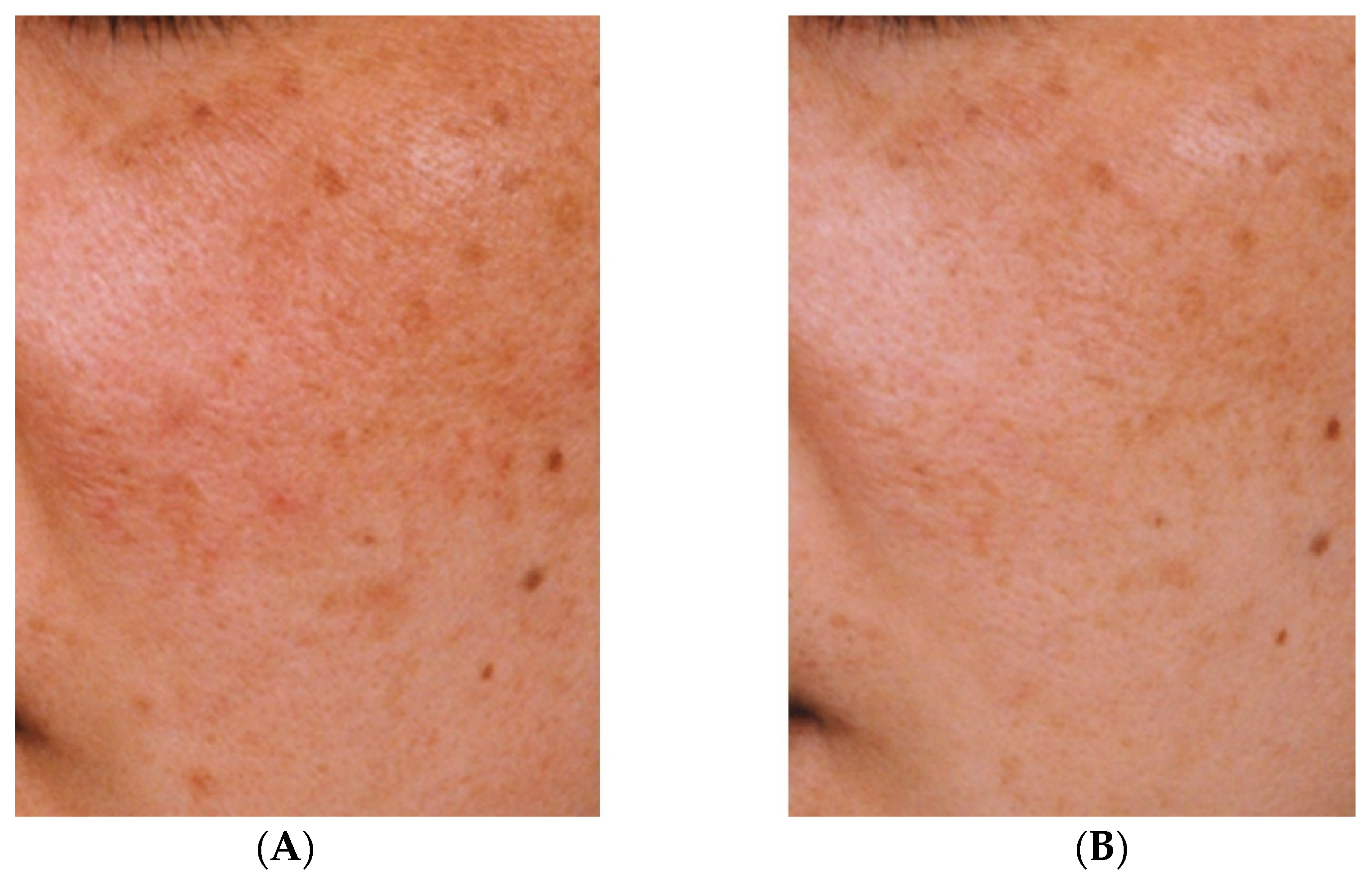
Figure 2. Improvement of hyperpigmentation after three months (B) of continuous use of an emulsion containing tranexamic acid compared with pre-treatment hyperpigmentation (A) [33].
3. Effectiveness of TXA for Sun-Induced Hyperpigmentation
The effectiveness of an emulsion containing TXA in inhibiting pigmentation caused by UV irradiation was compared with that of an emulsion containing magnesium ascorbate phosphate salt. A significant difference (p < 0.01) was observed between the two emulsions (Figure 3) [33]. No adverse effects were reported in either group. Next, 30 women (aged 33–53 years) who were concerned about spots and dullness were treated with an emulsion containing TXA morning and night, twice a day for 3 months. Skin lightening with the improvement of freckles, dark spots, and dullness was observed, along with improvements in the texture and the appearance of pores [33]. There were no side effects reported during the 3-month treatment period [33].
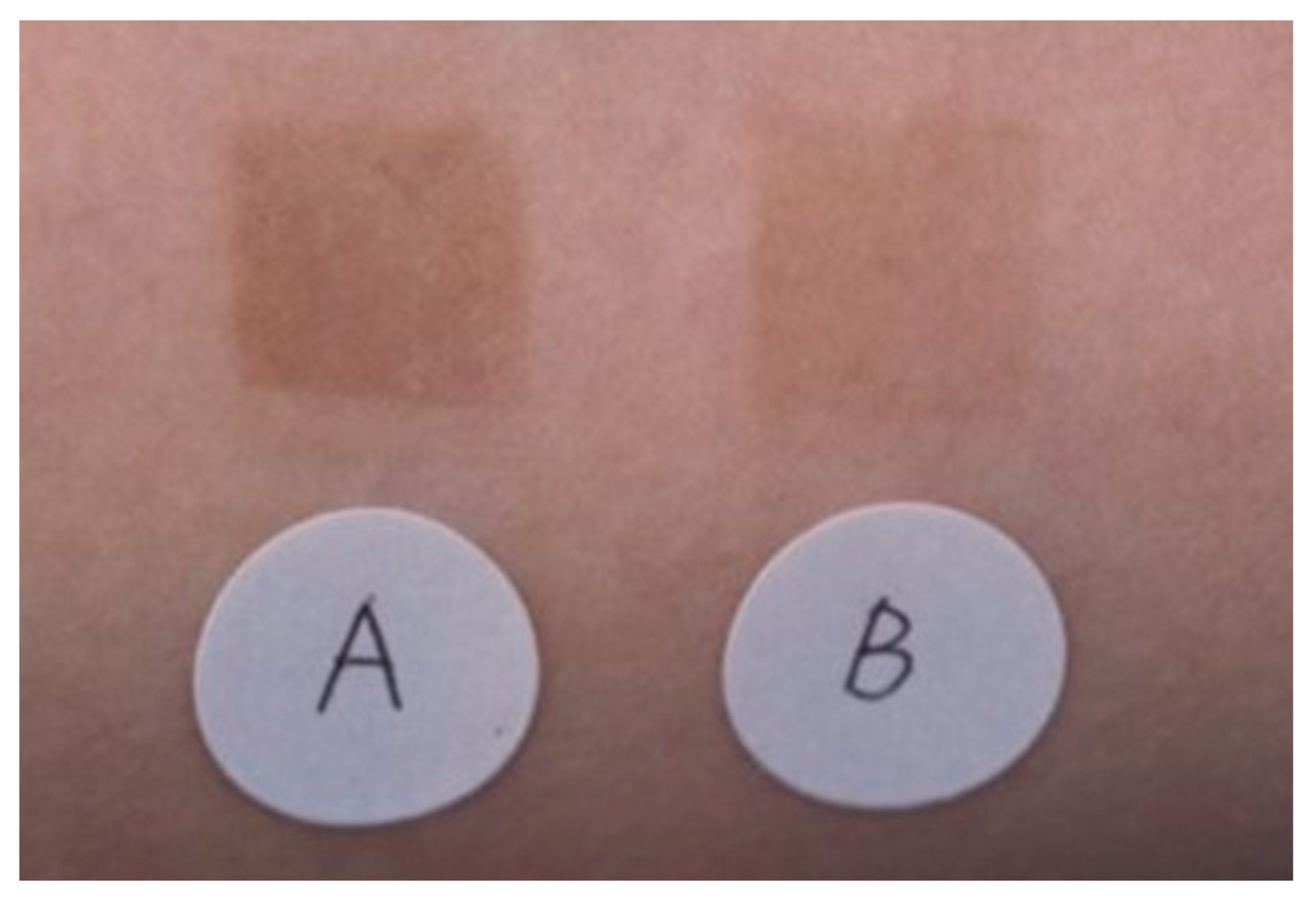
Figure 3. Superior pigmentation suppression effect of an emulsion containing 2% TXA compared to that of an emulsion containing 3% magnesium ascorbate phosphate salt (APM). A: APM application site, B: TXA application site [33].
Two forms of sun-induced skin hyperpigmentation have been established: immediate pigment darkening (immediate tanning) and delayed darkening (delayed tanning). Immediate pigment darkening is primarily caused by long-wave ultraviolet radiation (UVA) and manifests as a grayish-black color during irradiation that fades within about 30 min and may be attributable to a redox reaction of existing melanin and its intermediates [37][38]. Conversely, delayed-type darkening is primarily caused by medium-wavelength ultraviolet (UVB) radiation, leading to dark brown coloring on approximately the fourth day after sunburn and clearly visible pigmentation on about the sixth day, persisting for weeks to months [39]. This hyperpigmentation is thought to be caused by an increase in tyrosinase activity in melanosomes (premelanosomes) organelles within pigment cells in the basal layer of the epidermis, resulting in an increased synthesis of melanin [39]. Histologically, such hyperactive melanocytes have been shown to have large dendrite extensions [39].
There are two major hypotheses regarding the mechanism of melanocyte activation in sun-induced skin hyperpigmentation (delayed-type darkening). One hypothesis is that UV light directly activates melanocytes and promotes melanin synthesis, while small DNA fragments produced during the repair and removal of DNA in UV-damaged melanocytes have been reported to increase tyrosinase activity, leading to increased melanin synthesis [40]. The other hypothesis is that epidermal cells exposed to UV light release various melanocyte-activating factors (signaling molecules) that target melanocytes to increase melanin synthesis and proliferation. Candidate signaling molecules have been described in the literature and include arachidonic acid metabolites such as prostaglandin E2 (PGE2) [41][42][43][44][45][46][47], various hormones such as melanocyte-stimulating hormone (MSH) [48][49], fibroblast growth factor [50], endothelin [51], and histamine [52]. The observation that these signaling molecules regulate melanocyte activity indicates that UV-induced melanocyte activation may be controlled by a high-level regulatory mechanism.
4. Mechanism of Action of TXA
TXA exhibits hemostatic, anti-inflammatory, and anti-allergic effects through its anti-plasmin activity [5], which results from the strong binding of TXA to the lysine binding site and fibrin affinity site of plasmin and plasminogen. The production by plasmin of kinins and other active peptides responsible for increased vascular permeability, allergy, and inflammatory lesions is subsequently inhibited [5].
In addition to its inhibitory effect on fibrin degradation (anti-fibrinolytic effect) [4], TXA has been reported to inhibit arachidonic acid release [53], prostaglandin and leukotriene production [54][55], neutrophil reactive oxygen species (ROS) release [56], histamine release in mast cells [57], TGF-β1 expression in keratinocytes [58], and vascular VEGF receptor inhibition [59]. In addition, arachidonic acid metabolites such as PGE2 [44][45][46][47] and histamine [52] have been reported to promote dendrite formation, proliferation, and melanogenesis in melanocytes.
Phospholipase A2, which mediates the release of arachidonic acid; activates cultured human melanocytes to promote melanin synthesis, and its metabolites, prostaglandins, and leukotrienes; activate cultured human melanocytes; and induce skin hyperpigmentation [44][45][46][47][60][61][62]. The action of phospholipase A2 on cell membrane phospholipids releases arachidonic acid, and plasmin promotes the conversion of phospholipase A2 from its inactive precursor to the active form [63]. Therefore, TXA may inhibit the release of arachidonic acid and the production of prostaglandins and other substances via its anti-plasmin action at the cell membrane.
The anti-fibrotic effect of TXA, its inhibitory effect on the release of ROS by neutrophils, and its inhibitory effect on the release of histamine by mast cells have been observed in vitro at concentrations of 7.8 μg/mL, 50 μg/mL, and 10 μg/mL, respectively, although the distribution of TXA in guinea pig skin following closed application has been measured at <3.66 μg/g in the epidermis and <0.012 μg/g in the dermis and vascular system [33]. The concentration of TXA in the dermis during topical application is therefore presumed to be lower than the effective concentration, and it is unlikely that TXA acts on dermal neutrophils, mast cells, or on the vascular system to form thrombi.
References
- Maeda, K. Large melanosome complex is increased in keratinocytes of solar lentigo. Cosmetics 2017, 4, 49.
- Maeda, K. New method of measurement of epidermal turnover in humans. Cosmetics 2017, 4, 47.
- Maeda, K. Timeline of the development of skin-lightening active ingredients in Japan. Molecules 2022, 27, 4774.
- Abiko, Y.; Iwamoto, M. Plasminogen-plasmin system: VII. Potentiation of antifibrinolytic action of a synthetic inhibitor, tranexamic acid, by α2-macroglobulin antiplasmin. Biochim. Biophys. Acta 1970, 214, 411–418.
- Japanese Pharmacopoeia and Related Informations. The Japanese Pharmacopoeia 18th Edition, Tranexamic Acid, 1850~1851. Available online: https://jpdb.nihs.go.jp/kyokuhou/indexe.html (accessed on 15 September 2022).
- Dai, L.; Bevan, D.; Rangarajan, S.; Sørensen, B.; Mitchell, M. Stabilization of fibrin clots by activated prothrombin complex concentrate and tranexamic acid in FVIII inhibitor plasma. Haemophilia 2011, 17, e944–e948.
- Zhang, X.Y.; Yang, X.H.; Yang, H.; Yang, Y.P. Study of inhibitory effect of acidum tranexamicum on melanin synthesis. Chin. J. Dermatovenerol. Int. Tradit. West. Med. 2003, 2, 227–229.
- Kim, M.S.; Bang, S.H.; Kim, J.H.; Shin, H.J.; Choi, J.H.; Chang, S.E. Tranexamic acid diminishes laser-induced melanogenesis. Ann. Dermatol. 2015, 27, 250–256.
- Lindgren, A.L.; Austin, A.H.; Welsh, K.M. The Use of Tranexamic acid to prevent and treat post-inflammatory hyperpigmentation. J. Drugs Dermatol. 2021, 20, 344–345.
- Passeron, T. Melasma pathogenesis and influencing factors—An overview of the latest research. J. Eur. Acad. Dermatol. Venereol. 2013, 27 (Suppl. 1), 5–6.
- Lee, A.Y. Recent progress in melasma pathogenesis. Pigment Cell Melanoma Res. 2015, 28, 648–660.
- Hernández-Barrera, R.; Torres-Alvarez, B.; Castanedo-Cazares, J.P.; Oros-Ovalle, C.; Moncada, B. Solar elastosis and presence of mast cells as key features in the pathogenesis of melasma. Clin. Exp. Dermatol. 2008, 33, 305–308.
- Kim, E.H.; Kim, Y.C.; Lee, E.S.; Kang, H.Y. The vascular characteristics of melasma. J. Dermatol. Sci. 2007, 46, 111–116.
- Kim, S.J.; Park, J.Y.; Shibata, T.; Fujiwara, R.; Kang, H.Y. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin. Exp. Dermatol. 2016, 41, 480–485.
- Kawashima, M.; Kawada, A.; Takiwaki, H.; Mizuno, A.; Torii, H.; Hayashi, N.; Nogita, T.; Akiyoshi, E.; Yoshikawa, N.; Watanabe, C.; et al. Clinical efficacy of DH-4243 for Chloasma: A multi-center randomized controlled trial. Jpn. J. Clin. Dermatol. 2007, 61, 735–743. (In Japanese)
- Na, J.I.; Choi, S.Y.; Yang, S.H.; Choi, H.R.; Kang, H.Y.; Park, K.C. Effect of TXA on melasma: A clinical trial with histological evaluation. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 1035–1039.
- Lee, H.C.; Thing, T.G.; Goh, C.L. Oral Tranexamic acid (TA) in the treatment of melasma: A retrospective analysis. J. Am. Acad. Dermatol. 2016, 75, 385–392.
- Del Rosario, E.; Florez-Pollack, S.; Zapata, L., Jr.; Hernandez, K.; Tovar-Garza, A.; Rodrigues, M. Randomized, placebo-controlled, double-blind study of oral tranexamic acid in the treatment of moderate to severe melasma. J. Am. Acad. Dermatol. 2018, 78, 63–369.
- Tse, T.W.; Hui, E. Tranexamic acid: An important adjuvant in the treatment of melasma. J. Cosmet. Dermatol. 2013, 12, 57–66.
- Cho, H.H.; Choi, M.; Cho, S.; Lee, J.H. Role of oral tranexamic acid in melasma patients treated with IPL and low fluence QS Nd: YAG laser. J. Dermatolog. Treat. 2013, 24, 292–296.
- Wu, S.; Shi, H.; Wu, H.; Yan, S.; Guo, J.; Sun, Y.; Pan, L. Treatment of melasma with oral administration of tranexamic acid. Aesthetic Plast. Surg. 2012, 36, 964–970.
- Kato, H.; Araki, J.; Eto, H.; Doi, K.; Hirai, R.; Kuno, S.; Higashino, T.; Yoshimura, K. A prospective randomized controlled study of oral tranexamic acid for preventing postinflammatory hyperpigmentation after Q-switched ruby laser. Dermatol. Surg. 2011, 37, 605–610.
- Kondo, S.; Okada, Y.; Tomita, Y. Clinical study of effect of tranexamic acid emulsion on melasma and freckles. Skin Res. 2007, 6, 309–315. (In Japanese)
- Ebrahimi, B.; Naeini, F.F. Topical tranexamic acid as a promising treatment for melasma. J. Res. Med. Sci. 2014, 19, 753–757.
- Banihashemi, M.; Zabolinejad, N.; Jaafari, M.R.; Salehi, M.; Jabari, A. Comparison of therapeutic effects of liposomal tranexamic acid and conventional hydroquinone on melasma. J. Cosmet. Dermatol. 2015, 14, 174–177.
- Na Ayuthaya, P.K.; Niumphradit, N.; Manosroi, A.; Nakakes, A. Topical 5% tranexamic acid for the treatment of melasma in Asians: A double-blind randomized controlled clinical trial. J. Cosmet. Laser Ther. 2012, 14, 150–154.
- Xu, Y.; Ma, R.; Juliandri, J.; Wang, X.; Xu, B.; Wang, D.; Lu, Y.; Zhou, B.; Luo, D. Efficacy of functional microarray of microneedles combined with topical tranexamic acid for melasma: A randomized, self-controlled, split-face study. Medicine 2017, 96, e6897.
- Shihab, N.; Prihartono, J.; Tovar-Garza, A.; Agustin, T.; Legiawati, L.; Pandya, A.G. Randomised, controlled, double-blind study of combination therapy of oral tranexamic acid and topical hydroquinone in the treatment of melasma. Australas. J. Dermatol. 2020, 61, 237–242.
- Igarashi, M.; Tomita, Y.; Seiji, M. Hydroquinone therapy for chloasma. Rinsho Derma 1977, 19, 761–765. (In Japanese)
- Shin, J.U.; Park, J.; Oh, S.H.; Lee, J.H. Oral tranexamic acid enhances the efficacy of low-fluence 1064-nm Q-switched neodymium-doped yttrium aluminum garnet laser treatment for melasma in Koreans: A randomized, prospective trial. Dermatol. Surg. 2013, 39, 435–442.
- Qu, Y.; Wang, F.; Liu, J.; Xia, X. Clinical observation and dermoscopy evaluation of fractional CO2 laser combined with topical tranexamic acid in melasma treatments. J. Cosmet. Dermatol. 2021, 20, 1110–1116.
- Agamia, N.; Apalla, Z.; Salem, W.; Abdallah, W. A comparative study between oral tranexamic acid versus oral tranexamic acid and Q-switched Nd-YAG laser in melasma treatment: A clinical and dermoscopic evaluation. J. Dermatolog. Treat. 2021, 32, 819–826.
- Maeda, K. Tranexamic acid. Mon. Book Derma 2005, 98, 35–42. (In Japanese)
- Higashi, N. Treatment of melasma with oral tranexamic acid. Ski. Res. 1988, 30, 676–680. (In Japanese)
- Kita, Y.; Sugai, T. Effect of bleach agents on chloasma. Ski. Res. 1992, 34, 142–146. (In Japanese)
- Zhu, C.Y.; Li, Y.; Sun, Q.N.; Takada, A.; Kawada, A. Analysis of the effect of different doses of oral tranexamic acid on melasma: A multicentre prospective study. Eur. J. Dermatol. 2019, 29, 55–58.
- Miescher, G.; Minder, H. Untersuchungen über die durch langwelliges Ultraviolett hervorgerufene Pigmetdunkelung. Strahlentherapie 1939, 66, 6–23.
- Pathak, M.A.; Stratton, K. Free radicals in human skin before and after exposure to light. Arch. Biochem. Biophys. 1968, 123, 468–476.
- Mizuno, N. Behavior of melanocyte after single ultraviolet irradiation. Jpn. J. Clin. Dermatol. 1968, 22, 131–143. (In Japanese)
- Eller, M.S.; Yaar, S.M.; Gilchrest, B.A. DNA damage and melanogenesis. Nature 1994, 372, 413–414.
- Abdel-Malek, Z.A. Endocrine factors as effectors of integumental pigmentation. In Dermatologic Clinics; Nordlund, J.J., Ed.; W.B. Saunders: New York, NY, USA, 1988; Volume 6, pp. 175–183.
- Nordlund, J.J.; Abdel-Malek, Z.A.; Boissy, R.E.; Rheins, L.A. Pigment cell biology: An historical review. J. Invest. Dermatol. 1989, 92, 53S–60S.
- Gilchrest, B.A.; Park, H.-Y.; Eller, M.S.; Yaar, M. Mechanisms of ultraviolet light-induced pigmentation. Photochem. Photobiol. 1996, 63, 1–10.
- Tomita, Y.; Maeda, K.; Tagami, H. Mechanisms for hyperpigmentation in postinflammatory pigmentation, ulticaria pigmentosa and sunburn. Dermatologica 1989, 179, 49–53.
- Nordlund, J.J.; Collins, C.E.; Rheins, L.A. Prostaglandin E2 and D2 but not MSH stimulate the proliferation of pigment cells in the pinnal epidermis of the DBA/2 mouse. J. Invest. Dermatol. 1986, 86, 433–437.
- Tomita, Y.; Maeda, K.; Tagami, H. Melanocyte-stimulating properties of arachidonic acid metabolites: Possible role in postinflammatory pigmentation. Pigment. Cell Res. 1992, 5, 357–361.
- Maeda, K.; Naganuma, M. Melanocyte-stimulating properties of secretory phospholipase A2. Photochem. Photobiol. 1997, 65, 145–149.
- Birchall, N.; Orlow, S.J.; Kupper, T.; Pawelek, J. Interactions between ultraviolet light and interleukin-1 on MSH binding in both mouse melanoma and human sequamous carcinoma cells. Biochem. Biophys. Res. Commun. 1991, 175, 839–845.
- Schauer, E.; Trautinger, F.; Köck, A.; Schwarz, A.; Bhardwaj, R.; Simon, M.; Ansel, J.C.; Schwarz, T.; Luger, T.A. Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J. Clin. Invest. 1994, 93, 2258–2262.
- Halaban, R.; Langdon, R.; Birchall, N.; Cuono, C.; Baird, A.; Scott, G.; Moellmann, G.; McGuire, J. Basic fibroblast growth factor from human keratinocytes is a natural mitogen for melanocytes. J. Cell Biol. 1988, 107, 1611–1619.
- Imokawa, G.; Yada, Y.; Miyagishi, M. Endothelins secreted from human keratinocytes are intrinsic mitogens for human melanocytes. J. Biol. Chem. 1992, 267, 24675–24680.
- Tomita, Y.; Maeda, K.; Tagami, H. Stimulatory effect of histamine on normal human melanocytes in vitro. Tohoku J. Exp. Med. 1998, 155, 209–210.
- Chang, W.C.; Shi, G.Y.; Chow, Y.H.; Chang, L.C.; Hau, J.S.; Lin, M.T.; Jen, C.J.; Wing, L.Y.; Wu, H.L. Human plasmin induces a receptor-mediated arachidonate release coupled with G proteins in endothelial cells. Am. J. Physiol. 1993, 264, C271–C281.
- Ishihara, Y.; Kitamura, S.; Kosaka, K.; Harasawa, M. Tranexamic acid no prostaglandin gouseisogai ni kansuru kenkyu. Jpn. Pharmacol. Ther. 1978, 6, 398–402. (In Japanese)
- Weide, I.; Tippler, B.; Syrovets, T.; Simmet, T. Plasmin is a specific stimulus of the 5-lipoxygenase pathway of human peripheral monocytes. Thromb. Haemost. 1996, 76, 561–568.
- Sasaki, H.; Akamatsu, H.; Matoba, Y.; Ri, S.; Ito, A.; Asada, Y. Effects of Tranexamic Acid on Neutrophil Chemotaxis, Phagocytosis and Reactive Oxygen Species Generation in vitro. Jpn. Pharmacol. Ther. 1994, 22, 1429–1435. (In Japanese)
- Toki, N.; Takasugi, S.; Fujii, K. Basic research of histaminergic drugs and antihistaminergic drugs. Med. Consult. New Remedies 1981, 18, 1195–1202. (In Japanese)
- Xing, X.; Xu, Z.; Chen, L.; Jin, S.; Zhang, C.; Xiang, L. Tranexamic acid inhibits melanogenesis partially via stimulation of TGF-β1 expression in human epidermal keratinocytes. Exp. Dermatol. 2022, 31, 633–640.
- Zhu, J.W.; Ni, Y.J.; Tong, X.Y.; Guo, X.; Wu, X.P.; Lu, Z.F. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int. J. Med. Sci. 2020, 17, 903–911.
- Tomita, Y.; Maeda, K.; Tagami, H. Leukotrienes and thromboxane B2 stimulate normal human melanocytes in vitro: Possible inducers of postinflammatory pigmentation. Tohoku J. Exp. Med. 1988, 156, 303–304.
- Morelli, J.G.; Hake, S.S.; Murphy, R.C.; Norris, D.A. Leukotriene B4-induced human melanocyte pigmentation and leukotriene C4-induced human melanocyte growth are inhibited by different isoquinolinesulfonamides. J. Invest. Dermatol. 1992, 98, 55–58.
- Maeda, K.; Naganuma, M. Topical trans-4-aminomethylcyclohexanecarboxylic acid prevents ultraviolet radiation-induced pigmentation. J. Photochem. Photobiol. B Biol. 1998, 47, 136–141.
- Nakano, T.; Fujita, H.; Kikuchi, N.; Arita, H. Plasmin converts pro-form of group I phospholipase A2 into receptor binding, active forms. Biochem. Biophys. Res. Commun. 1994, 198, 10–15.
More
Information
Subjects:
Dermatology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
2 times
(View History)
Update Date:
04 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




