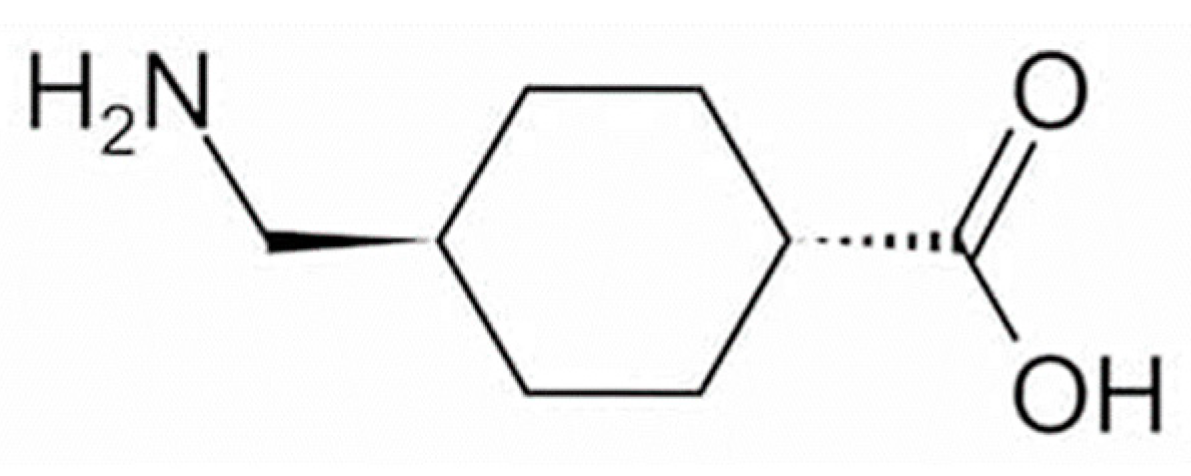
Tranexamic acid (TXA) has anti-plasmin activity and has been shown when administered orally to be effective against melasma, for which it is considered first-line pharmacotherapy. Several studies have shown that topically applied TXA is also effective against melasma and skin hyperpigmentation caused by sunburn and inflammation. The TXA concentration in the epidermis and dermis/vasculature has been estimated from its distribution in the skin after closed application, and topically applied TXA has thus been shown to act on neutrophils and mast cells in the dermis and on the vascular system.
- tranexamic acid
- topical application
- melasma
- sun-induced skin hyperpigmentation
- skin-lightening agent
- quasi-drug
- plasmin
1. Introduction
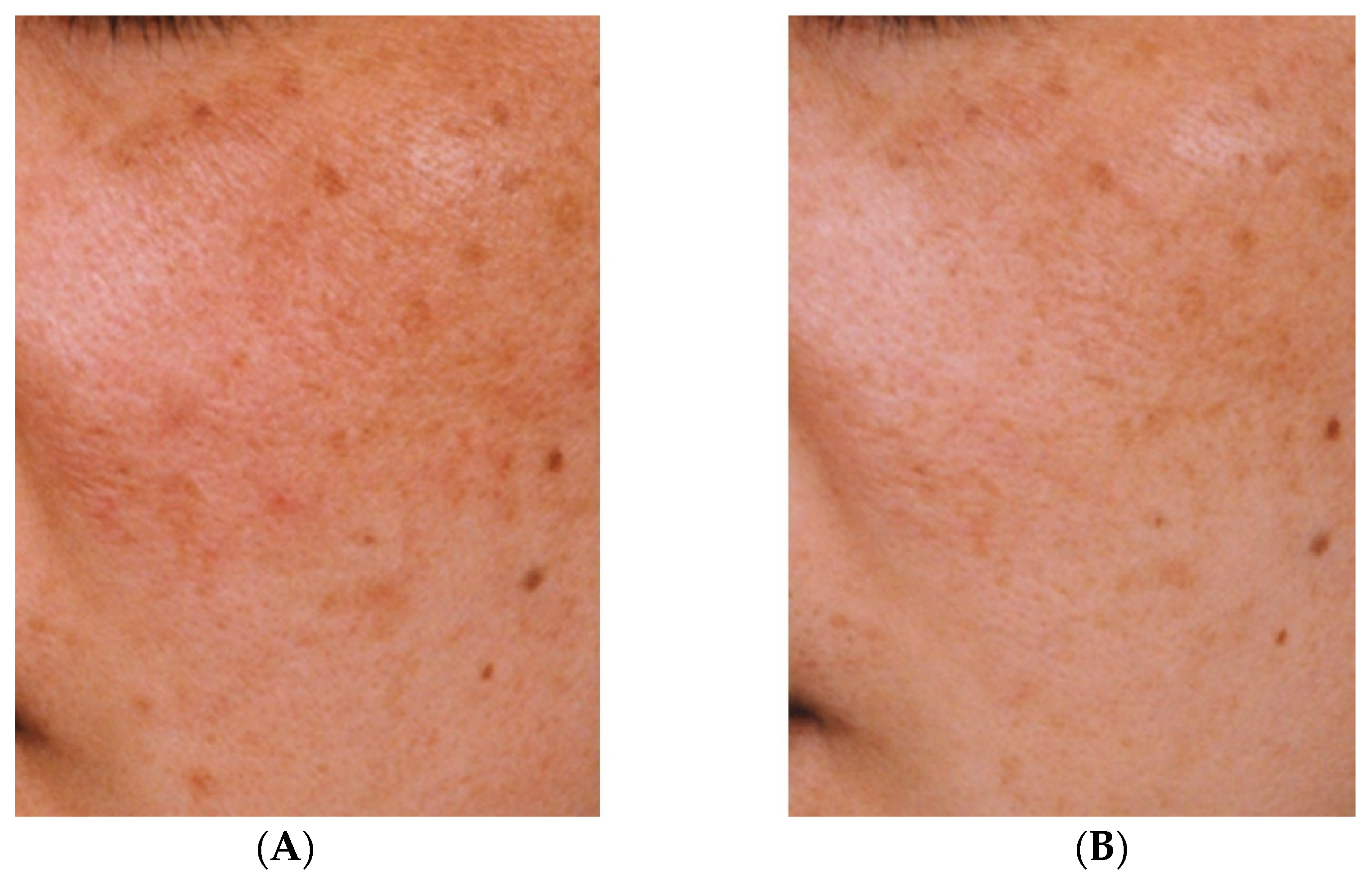
2. Effectiveness of TXA for Melasma
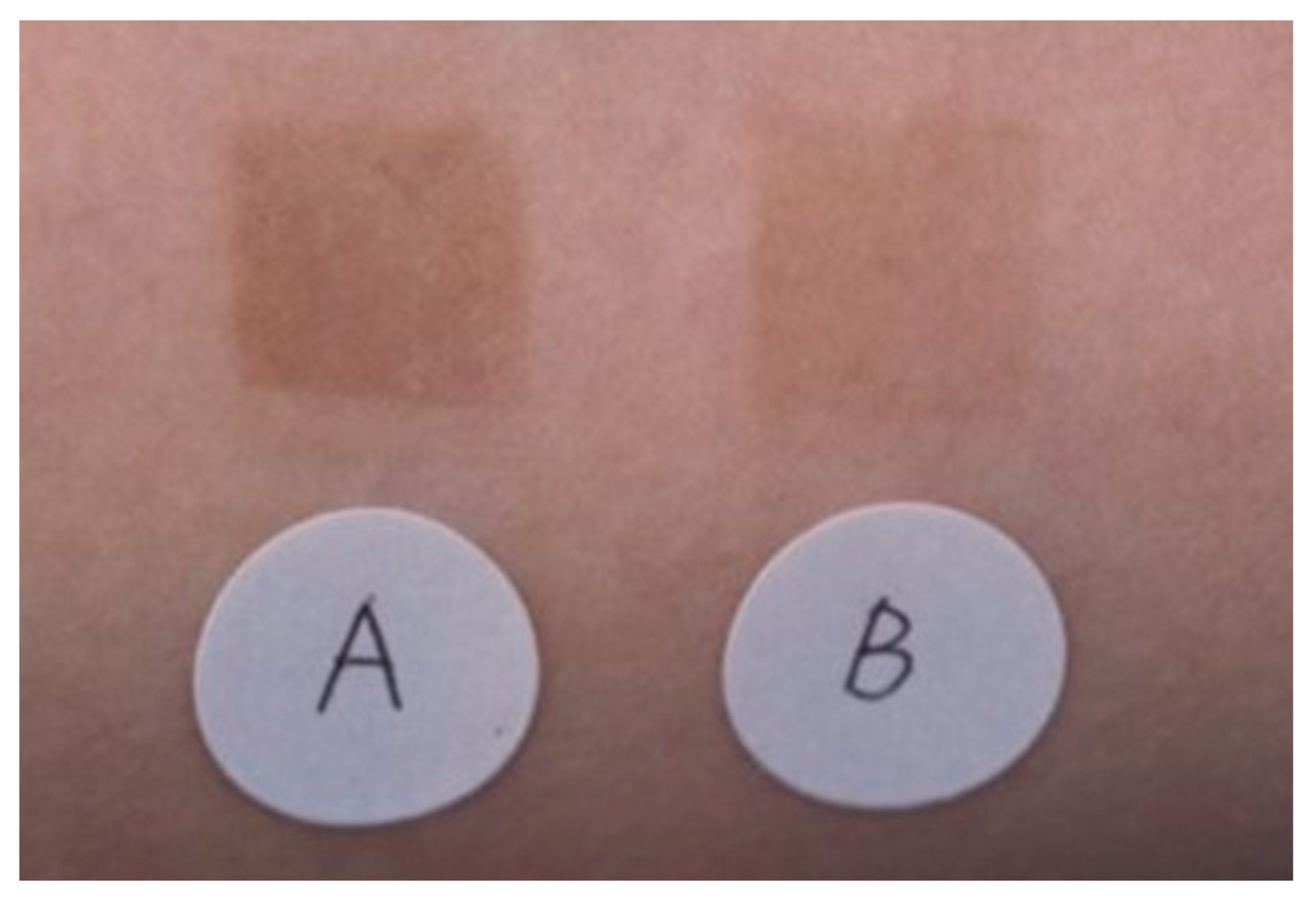
3. Effectiveness of TXA for Sun-Induced Hyperpigmentation
4. Mechanism of Action of TXA
This entry is adapted from the peer-reviewed paper 10.3390/cosmetics9050108
References
- Maeda, K. Large melanosome complex is increased in keratinocytes of solar lentigo. Cosmetics 2017, 4, 49.
- Maeda, K. New method of measurement of epidermal turnover in humans. Cosmetics 2017, 4, 47.
- Maeda, K. Timeline of the development of skin-lightening active ingredients in Japan. Molecules 2022, 27, 4774.
- Abiko, Y.; Iwamoto, M. Plasminogen-plasmin system: VII. Potentiation of antifibrinolytic action of a synthetic inhibitor, tranexamic acid, by α2-macroglobulin antiplasmin. Biochim. Biophys. Acta 1970, 214, 411–418.
- Japanese Pharmacopoeia and Related Informations. The Japanese Pharmacopoeia 18th Edition, Tranexamic Acid, 1850~1851. Available online: https://jpdb.nihs.go.jp/kyokuhou/indexe.html (accessed on 15 September 2022).
- Dai, L.; Bevan, D.; Rangarajan, S.; Sørensen, B.; Mitchell, M. Stabilization of fibrin clots by activated prothrombin complex concentrate and tranexamic acid in FVIII inhibitor plasma. Haemophilia 2011, 17, e944–e948.
- Zhang, X.Y.; Yang, X.H.; Yang, H.; Yang, Y.P. Study of inhibitory effect of acidum tranexamicum on melanin synthesis. Chin. J. Dermatovenerol. Int. Tradit. West. Med. 2003, 2, 227–229.
- Kim, M.S.; Bang, S.H.; Kim, J.H.; Shin, H.J.; Choi, J.H.; Chang, S.E. Tranexamic acid diminishes laser-induced melanogenesis. Ann. Dermatol. 2015, 27, 250–256.
- Lindgren, A.L.; Austin, A.H.; Welsh, K.M. The Use of Tranexamic acid to prevent and treat post-inflammatory hyperpigmentation. J. Drugs Dermatol. 2021, 20, 344–345.
- Passeron, T. Melasma pathogenesis and influencing factors—An overview of the latest research. J. Eur. Acad. Dermatol. Venereol. 2013, 27 (Suppl. 1), 5–6.
- Lee, A.Y. Recent progress in melasma pathogenesis. Pigment Cell Melanoma Res. 2015, 28, 648–660.
- Hernández-Barrera, R.; Torres-Alvarez, B.; Castanedo-Cazares, J.P.; Oros-Ovalle, C.; Moncada, B. Solar elastosis and presence of mast cells as key features in the pathogenesis of melasma. Clin. Exp. Dermatol. 2008, 33, 305–308.
- Kim, E.H.; Kim, Y.C.; Lee, E.S.; Kang, H.Y. The vascular characteristics of melasma. J. Dermatol. Sci. 2007, 46, 111–116.
- Kim, S.J.; Park, J.Y.; Shibata, T.; Fujiwara, R.; Kang, H.Y. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin. Exp. Dermatol. 2016, 41, 480–485.
- Kawashima, M.; Kawada, A.; Takiwaki, H.; Mizuno, A.; Torii, H.; Hayashi, N.; Nogita, T.; Akiyoshi, E.; Yoshikawa, N.; Watanabe, C.; et al. Clinical efficacy of DH-4243 for Chloasma: A multi-center randomized controlled trial. Jpn. J. Clin. Dermatol. 2007, 61, 735–743. (In Japanese)
- Na, J.I.; Choi, S.Y.; Yang, S.H.; Choi, H.R.; Kang, H.Y.; Park, K.C. Effect of TXA on melasma: A clinical trial with histological evaluation. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 1035–1039.
- Lee, H.C.; Thing, T.G.; Goh, C.L. Oral Tranexamic acid (TA) in the treatment of melasma: A retrospective analysis. J. Am. Acad. Dermatol. 2016, 75, 385–392.
- Del Rosario, E.; Florez-Pollack, S.; Zapata, L., Jr.; Hernandez, K.; Tovar-Garza, A.; Rodrigues, M. Randomized, placebo-controlled, double-blind study of oral tranexamic acid in the treatment of moderate to severe melasma. J. Am. Acad. Dermatol. 2018, 78, 63–369.
- Tse, T.W.; Hui, E. Tranexamic acid: An important adjuvant in the treatment of melasma. J. Cosmet. Dermatol. 2013, 12, 57–66.
- Cho, H.H.; Choi, M.; Cho, S.; Lee, J.H. Role of oral tranexamic acid in melasma patients treated with IPL and low fluence QS Nd: YAG laser. J. Dermatolog. Treat. 2013, 24, 292–296.
- Wu, S.; Shi, H.; Wu, H.; Yan, S.; Guo, J.; Sun, Y.; Pan, L. Treatment of melasma with oral administration of tranexamic acid. Aesthetic Plast. Surg. 2012, 36, 964–970.
- Kato, H.; Araki, J.; Eto, H.; Doi, K.; Hirai, R.; Kuno, S.; Higashino, T.; Yoshimura, K. A prospective randomized controlled study of oral tranexamic acid for preventing postinflammatory hyperpigmentation after Q-switched ruby laser. Dermatol. Surg. 2011, 37, 605–610.
- Kondo, S.; Okada, Y.; Tomita, Y. Clinical study of effect of tranexamic acid emulsion on melasma and freckles. Skin Res. 2007, 6, 309–315. (In Japanese)
- Ebrahimi, B.; Naeini, F.F. Topical tranexamic acid as a promising treatment for melasma. J. Res. Med. Sci. 2014, 19, 753–757.
- Banihashemi, M.; Zabolinejad, N.; Jaafari, M.R.; Salehi, M.; Jabari, A. Comparison of therapeutic effects of liposomal tranexamic acid and conventional hydroquinone on melasma. J. Cosmet. Dermatol. 2015, 14, 174–177.
- Na Ayuthaya, P.K.; Niumphradit, N.; Manosroi, A.; Nakakes, A. Topical 5% tranexamic acid for the treatment of melasma in Asians: A double-blind randomized controlled clinical trial. J. Cosmet. Laser Ther. 2012, 14, 150–154.
- Xu, Y.; Ma, R.; Juliandri, J.; Wang, X.; Xu, B.; Wang, D.; Lu, Y.; Zhou, B.; Luo, D. Efficacy of functional microarray of microneedles combined with topical tranexamic acid for melasma: A randomized, self-controlled, split-face study. Medicine 2017, 96, e6897.
- Shihab, N.; Prihartono, J.; Tovar-Garza, A.; Agustin, T.; Legiawati, L.; Pandya, A.G. Randomised, controlled, double-blind study of combination therapy of oral tranexamic acid and topical hydroquinone in the treatment of melasma. Australas. J. Dermatol. 2020, 61, 237–242.
- Igarashi, M.; Tomita, Y.; Seiji, M. Hydroquinone therapy for chloasma. Rinsho Derma 1977, 19, 761–765. (In Japanese)
- Shin, J.U.; Park, J.; Oh, S.H.; Lee, J.H. Oral tranexamic acid enhances the efficacy of low-fluence 1064-nm Q-switched neodymium-doped yttrium aluminum garnet laser treatment for melasma in Koreans: A randomized, prospective trial. Dermatol. Surg. 2013, 39, 435–442.
- Qu, Y.; Wang, F.; Liu, J.; Xia, X. Clinical observation and dermoscopy evaluation of fractional CO2 laser combined with topical tranexamic acid in melasma treatments. J. Cosmet. Dermatol. 2021, 20, 1110–1116.
- Agamia, N.; Apalla, Z.; Salem, W.; Abdallah, W. A comparative study between oral tranexamic acid versus oral tranexamic acid and Q-switched Nd-YAG laser in melasma treatment: A clinical and dermoscopic evaluation. J. Dermatolog. Treat. 2021, 32, 819–826.
- Maeda, K. Tranexamic acid. Mon. Book Derma 2005, 98, 35–42. (In Japanese)
- Higashi, N. Treatment of melasma with oral tranexamic acid. Ski. Res. 1988, 30, 676–680. (In Japanese)
- Kita, Y.; Sugai, T. Effect of bleach agents on chloasma. Ski. Res. 1992, 34, 142–146. (In Japanese)
- Zhu, C.Y.; Li, Y.; Sun, Q.N.; Takada, A.; Kawada, A. Analysis of the effect of different doses of oral tranexamic acid on melasma: A multicentre prospective study. Eur. J. Dermatol. 2019, 29, 55–58.
- Miescher, G.; Minder, H. Untersuchungen über die durch langwelliges Ultraviolett hervorgerufene Pigmetdunkelung. Strahlentherapie 1939, 66, 6–23.
- Pathak, M.A.; Stratton, K. Free radicals in human skin before and after exposure to light. Arch. Biochem. Biophys. 1968, 123, 468–476.
- Mizuno, N. Behavior of melanocyte after single ultraviolet irradiation. Jpn. J. Clin. Dermatol. 1968, 22, 131–143. (In Japanese)
- Eller, M.S.; Yaar, S.M.; Gilchrest, B.A. DNA damage and melanogenesis. Nature 1994, 372, 413–414.
- Abdel-Malek, Z.A. Endocrine factors as effectors of integumental pigmentation. In Dermatologic Clinics; Nordlund, J.J., Ed.; W.B. Saunders: New York, NY, USA, 1988; Volume 6, pp. 175–183.
- Nordlund, J.J.; Abdel-Malek, Z.A.; Boissy, R.E.; Rheins, L.A. Pigment cell biology: An historical review. J. Invest. Dermatol. 1989, 92, 53S–60S.
- Gilchrest, B.A.; Park, H.-Y.; Eller, M.S.; Yaar, M. Mechanisms of ultraviolet light-induced pigmentation. Photochem. Photobiol. 1996, 63, 1–10.
- Tomita, Y.; Maeda, K.; Tagami, H. Mechanisms for hyperpigmentation in postinflammatory pigmentation, ulticaria pigmentosa and sunburn. Dermatologica 1989, 179, 49–53.
- Nordlund, J.J.; Collins, C.E.; Rheins, L.A. Prostaglandin E2 and D2 but not MSH stimulate the proliferation of pigment cells in the pinnal epidermis of the DBA/2 mouse. J. Invest. Dermatol. 1986, 86, 433–437.
- Tomita, Y.; Maeda, K.; Tagami, H. Melanocyte-stimulating properties of arachidonic acid metabolites: Possible role in postinflammatory pigmentation. Pigment. Cell Res. 1992, 5, 357–361.
- Maeda, K.; Naganuma, M. Melanocyte-stimulating properties of secretory phospholipase A2. Photochem. Photobiol. 1997, 65, 145–149.
- Birchall, N.; Orlow, S.J.; Kupper, T.; Pawelek, J. Interactions between ultraviolet light and interleukin-1 on MSH binding in both mouse melanoma and human sequamous carcinoma cells. Biochem. Biophys. Res. Commun. 1991, 175, 839–845.
- Schauer, E.; Trautinger, F.; Köck, A.; Schwarz, A.; Bhardwaj, R.; Simon, M.; Ansel, J.C.; Schwarz, T.; Luger, T.A. Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J. Clin. Invest. 1994, 93, 2258–2262.
- Halaban, R.; Langdon, R.; Birchall, N.; Cuono, C.; Baird, A.; Scott, G.; Moellmann, G.; McGuire, J. Basic fibroblast growth factor from human keratinocytes is a natural mitogen for melanocytes. J. Cell Biol. 1988, 107, 1611–1619.
- Imokawa, G.; Yada, Y.; Miyagishi, M. Endothelins secreted from human keratinocytes are intrinsic mitogens for human melanocytes. J. Biol. Chem. 1992, 267, 24675–24680.
- Tomita, Y.; Maeda, K.; Tagami, H. Stimulatory effect of histamine on normal human melanocytes in vitro. Tohoku J. Exp. Med. 1998, 155, 209–210.
- Chang, W.C.; Shi, G.Y.; Chow, Y.H.; Chang, L.C.; Hau, J.S.; Lin, M.T.; Jen, C.J.; Wing, L.Y.; Wu, H.L. Human plasmin induces a receptor-mediated arachidonate release coupled with G proteins in endothelial cells. Am. J. Physiol. 1993, 264, C271–C281.
- Ishihara, Y.; Kitamura, S.; Kosaka, K.; Harasawa, M. Tranexamic acid no prostaglandin gouseisogai ni kansuru kenkyu. Jpn. Pharmacol. Ther. 1978, 6, 398–402. (In Japanese)
- Weide, I.; Tippler, B.; Syrovets, T.; Simmet, T. Plasmin is a specific stimulus of the 5-lipoxygenase pathway of human peripheral monocytes. Thromb. Haemost. 1996, 76, 561–568.
- Sasaki, H.; Akamatsu, H.; Matoba, Y.; Ri, S.; Ito, A.; Asada, Y. Effects of Tranexamic Acid on Neutrophil Chemotaxis, Phagocytosis and Reactive Oxygen Species Generation in vitro. Jpn. Pharmacol. Ther. 1994, 22, 1429–1435. (In Japanese)
- Toki, N.; Takasugi, S.; Fujii, K. Basic research of histaminergic drugs and antihistaminergic drugs. Med. Consult. New Remedies 1981, 18, 1195–1202. (In Japanese)
- Xing, X.; Xu, Z.; Chen, L.; Jin, S.; Zhang, C.; Xiang, L. Tranexamic acid inhibits melanogenesis partially via stimulation of TGF-β1 expression in human epidermal keratinocytes. Exp. Dermatol. 2022, 31, 633–640.
- Zhu, J.W.; Ni, Y.J.; Tong, X.Y.; Guo, X.; Wu, X.P.; Lu, Z.F. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int. J. Med. Sci. 2020, 17, 903–911.
- Tomita, Y.; Maeda, K.; Tagami, H. Leukotrienes and thromboxane B2 stimulate normal human melanocytes in vitro: Possible inducers of postinflammatory pigmentation. Tohoku J. Exp. Med. 1988, 156, 303–304.
- Morelli, J.G.; Hake, S.S.; Murphy, R.C.; Norris, D.A. Leukotriene B4-induced human melanocyte pigmentation and leukotriene C4-induced human melanocyte growth are inhibited by different isoquinolinesulfonamides. J. Invest. Dermatol. 1992, 98, 55–58.
- Maeda, K.; Naganuma, M. Topical trans-4-aminomethylcyclohexanecarboxylic acid prevents ultraviolet radiation-induced pigmentation. J. Photochem. Photobiol. B Biol. 1998, 47, 136–141.
- Nakano, T.; Fujita, H.; Kikuchi, N.; Arita, H. Plasmin converts pro-form of group I phospholipase A2 into receptor binding, active forms. Biochem. Biophys. Res. Commun. 1994, 198, 10–15.
