| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Farid Menaa | + 3105 word(s) | 3105 | 2020-11-16 10:28:44 | | | |
| 2 | Peter Tang | -338 word(s) | 2767 | 2020-12-08 12:18:32 | | |
Video Upload Options
Emerging studies suggested that the S-(-)-mitotane is more potent than the R-(+)-mitotane for AdrenoCortical Carcinoma (ACC) treatment. Currently, mitotane is the only FDA-approved drug . Therefore, we suggest that the production of pure and active S-(-)-mitotane might offer synergic or additive benefits for ACC patients, and even better if combined to solid lipid-based nanocarriers, and smart/advanced nanocarriers.
1. Adreno-Cortical Carcinoma: an Update
1.1. Epidemiology and Etiology
In contrast to benign adreno-cortical adenomas (ACA) that occur in at least 3% of the population aged over 50 years, adrenocortical carcinoma (ACC) is a rare endocrine malignancy [1][2][3]. Indeed, the worldwide incidence of this orphan disease is estimated to be around 0.5-2 cases per million/year and its prevalence about 4-12 cases per million population [1][2][3]. Exceptionally, in some regions of the world such as southern Brazil, the annual incidence in the children population - under 15 years old - is about 3.4-4.2 cases per million, which is considerable comparatively to the worldwide incidence [4]. A bimodal age distribution has been observed in ACC patients with peaks in childhood, before the age of 5, and in the fourth to fifth decades of life [5][6]. Moreover, women are slightly more predisposed to the disease than men (ratio 1.5) [2][7][8].
The majority of ACCs are sporadic neoplasms of undetermined etiology while familial predisposition can occur. Interestingly, somatic mutations in genes predisposing to some syndromes associated with the increased susceptibility of cancer (e.g. Li-Fraumeni, Beckwith-Wiedemann), have also been identified in either benign or malignant sporadic adreno-cortical tumors (ACTs) [9]. Thereby, inactivating mutations at the 17p13 locus including the TP53 - protein considered as the "guardian of the genome" [10], as well as alterations of the 11p15 locus leading to IGF-II (type II Insulin Growth Factor) over-expression and adrenal cancer cell proliferation, were frequently observed [11].
1.2. Prognosis, Diagnosis and Therapy
ACC is characterized by a poor prognosis and a high risk of recurrence post-therapy. Indeed, the recurrence is about 49% after adjuvant chemotherapy and up to 85% after surgery without adjuvant treatment [12][13]. In about 40% of the cases, the disease relapse is manifested by the development of metastatic disease to the lungs, liver or bone at the diagnosis or within 6-24 months of surgical resection [7][8][12][13][14]. Moreover, the unsatisfactory overall 5-year survival ranges between 23% and 60% [2][6][8][15]. Three main prognostic parameters are significantly associated with a shorter patient's survival: (i) older age at diagnosis; (ii) tumor stage at presentation (i.e. stage III aka involvement of local lymph nodes and, stage IV aka local organ invasion or distant metastases), which can slightly differs according to the staging system used (i.e. MacFarlane-Sullivan [16] or European Network for the Study of Adrenal Tumors (ENSAT) [17]); (iii) hypersecretion of cortisol, a major adrenal steroid hormone [18].
At present, early diagnosis of this aggressive malignancy is mainly monitored by cortisol levels, which are too high in 60% of patients - especially in children (about 90%) - frequently leading to Cushing's syndrome with or without virilization [1][2][5].
Complete surgical resection of the tumor (i.e. ipsilateral adrenalectomy with or without nephrectomy and/or splenectomy) is the only therapy that has consistently shown to prolong patient's survival, particularly if disease is detected at early stages (I and II) but this, usually concerns less than 60% of the patients [1][19][20]. Indeed, median survival in patients with unresectable tumors or incomplete tumor resection is usually less than 12 months (about 3 to 9 months) [14][21][22] whereas, after complete resection, the median survival is generally improved (13 to 28 months) as shown in retrospective studies [19][23][24][25]. Radiotherapy is only indicated as palliative treatment for patients with bone (and brain) metastases [1], and international prospective randomized studies are still insufficient to evaluate its benefit in the treatment of unresectable disease [26]. Finally, chemotherapy mainly consists of using mitotane, the only Food and Drug Administration (FDA)-approved drug against ACC, and can be administrated as follows: (i) alone; (ii) as combined regimens (e.g. mitotane plus etoposide-doxorubicincisplatin (EDP/M)) preferably in patients with incomplete, not possible or not successful tumor resection; (iii) as adjuvant in patients with high risk of recurrence at presentation or at relapse [1][20][27].
Interestingly, adjuvant treatment (i.e. chemotherapy and/or radiotherapy after surgery) might significantly decrease the disease recurrence after surgery and, increase the overall survival of the patients [12][13][18][28][29][30][31]. Nevertheless, the potential benefits of adjuvant treatment have not been confirmed in some other studies [32][33][34], most likely because of incomplete surgery and variable drug metabolism [1][35][36]. Besides, current available systemic therapies provided incomplete efficient responses (< 50%) in cases of advanced ACC and, remain severely limited mainly because of the rarity of ACC disease that had hampered the ability to undertake international randomized clinical studies to identify the most effective first- and second-line cytotoxic regimens [37]. Consequently, in spite of its relative efficacy, mitotane drug therapy remains the cornerstone, mainly in metastatic stage [38][39].
Hopefully, the two most recent international randomized clinical studies, FIRM-ACT (First International Randomized trial in locally advanced and Metastatic Adrenocortical Carcinoma Treatment) and, ADIUVO (an international prospective, randomized, open-label, and controlled phase III trial for patients with ACC after radical resection), endorsed by the ENSAT [40], will show interesting data. Briefly, FIRM-ACT aims to assess the efficacy of mitotane combined to other drugs (e.g. EDP/M) as first line treatment versus Streptozotocin plus mitotane (Sz/M), while ADIUVO consist to evaluate the efficacy of mitotane as adjuvant treatment versus observation in patients with ACC at low-intermediate risk of recurrence after radical resection [27][37]. However, until results from all randomized clinical trials become available, healthcare professionals will be challenged by an uncertainty.
Alternatively, the better understanding of the molecular pathogenesis of ACC, such as IGF signaling pathway, might allow the design of promising therapeutic targets [20][41].
Eventually, the rapid emergence of the nanotechnology and state-of-art chromatography systems, shall significantly contribute to the development of mitotane chiral nano-formulations, which might present greater therapeutic features than the free mitotane drug formulation (i.e. lower toxicity, significant efficacy to lower the disease progression, capability to enhance patient's survival and patient's quality of life). However, the development of generic mitotane formulations shall be avoided due to the Narrow Therapeutic Index (NTI), and inadequate glucocorticoids administration must be prevented in order to limit the risk of adverse effects.
2. Mitotane: Synthesis, Structure and Drug Properties
2.1. Synthesis Route
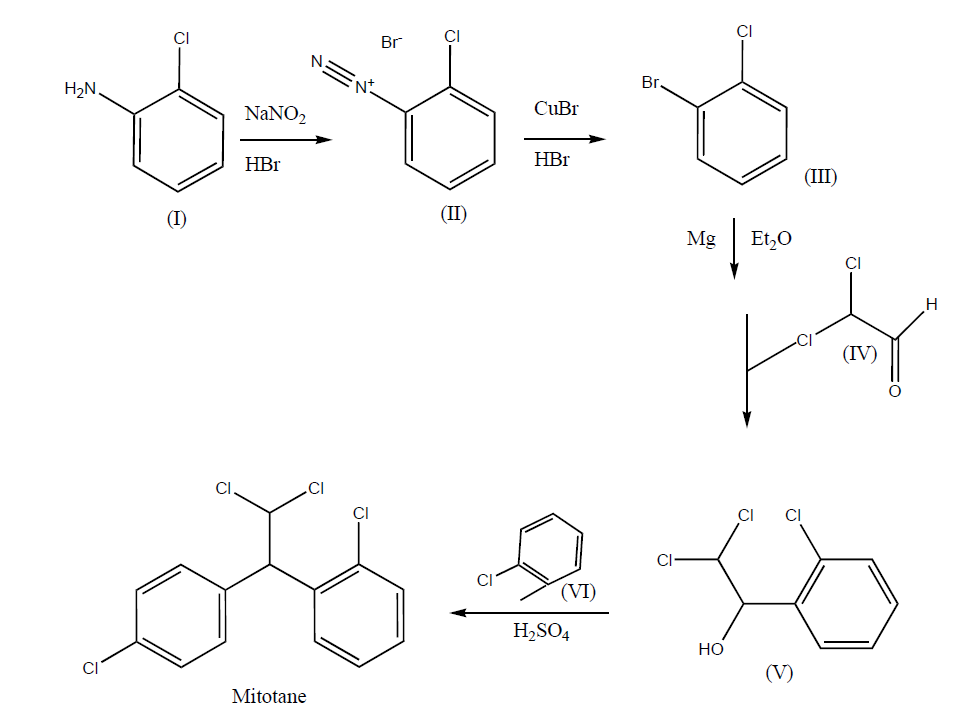
The synthesis route of mitotane (o,p'-DDD aka 1-(2-chlorophenyl)-1-(4-chlorophenyl)-2,2-dichloroethane, or 1-chloro-2-[2,2-dichloro-1(4-chlorophenyl)ethyl]benzene, or 1,1-(o,p'-dichlorodiphenyl)-2,2-dichoroethane, or 1,1-dichloro-2-[o-chlorophenyl]-2-[p-chlorophenyl]ethane) has been more recently reviewed [42]. The manufacturing process is simply carried out in 5 steps Fig. 1 - three of them include chemical reactions and the other two correspond to recrystallizations -, and gas chromatography coupled to mass spectroscopy (GC-MS) is generally sufficient to characterize mitotane during this process or in the final product. Thereby, the classical synthesis route starts with the diazotisation of 2-chloroaniline (I) with NaNO2 and HBr in H2O that furnishes 2-chlorobenzenediazonium bromide (II), which is then brominated with CuBr and HBr to afford 1-bromo-2-chlorobenzene (III) [43]. 1-bromo-2-chlorobenzene (III) is treated with Mg in ether, and the obtained Grignard reagent is condensed with dichloroacetaldehyde (IV) in ether, yielding 2,2-dichloro-1-(2-chlorophenyl)ethanol (V), which finally is condensed with chlorobenzene (VI) in the presence of H2SO4 to provide mitotane [44].
Figure 1. Synthesis route of mitotane (Lysodren®; C14H10Cl4). Diazotisation of 2-chloroaniline (I) with Sodium nitrite (NaNO2) and hydrogen bromide (HBr) in water (H2O) furnishes 2-chlorobenzenediazonium bromide (II), which is then brominated with copper bromide (CuBr) and HBr to afford 1-bromo-2-chlorobenzene (III). Treatment of 1-bromo-2-chlorobenzene (III) with magnesium (Mg) in ether (ET2O), and condensing the obtained Grignard reagent with dichloroacetaldehyde (IV) in ether yielding 2,2-dichloro-1-(2-chlorophenyl)ethanol (V), which finally condenses with chlorobenzene (VI) in the presence of sulfuric acid (H2SO4) to provide mitotane. Adapted with permission from [42].
2.2. Structure and Physical-Chemical Properties
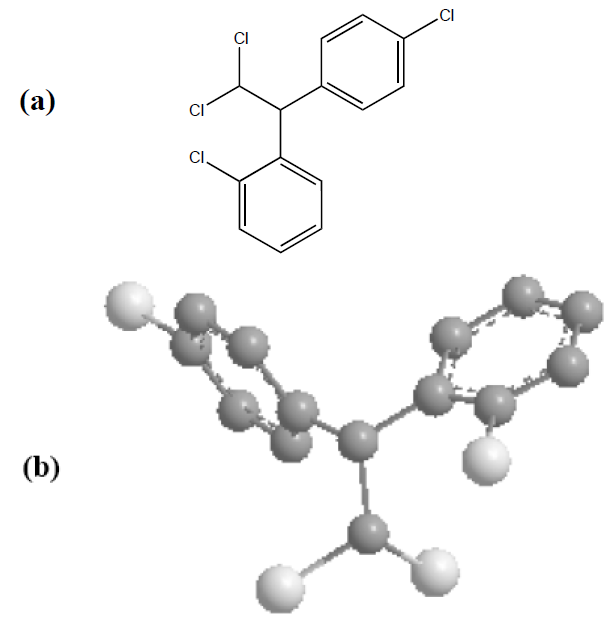
This oral antineoplastic agent is best known by its trivial name, o,p'-DDD. Its systematic chemical name, according to IUPAC (International Union of Pure and Applied Chemistry) nomenclature, is 1-chloro-2-[2,2-dichloro-1(4chlorophenyl)ethyl]benzene [45]. The 2D and 3D chemical structures of mitotane (C14H10Cl4) are shown in Fig. 2.
Figure 2. Chemical structures of mitotane (C14H10Cl4). (a) 2D structural view; (b) 3D structural view, where carbons from aromatic rings are in grey and chlorine atoms in white. In both cases (a) and (b), the hydrogen bonds have been omitted for clarity.
According to the US Pharmacopeia (USP) [46], Lysodren® must present the major following chemical-physical and pharmaceutical features: (i) a monoisotopic mass /molecular weight (MW) of about 318-320 Da; (ii) a melting point ranging between 75-81ºC; (iii) a dosing of 500 mg of the active substance mitotane; (iv) a white granular solid composed of clear colorless crystals; (v) a tasteless and slight pleasant aromatic odor; (vi) a low solubility in water, an acceptable solubility in either ethanol, ether, hexane, isooctane, carbon tetrachloride, fixed oils or fats; (vii) inactive ingredients represented by Avicel (matrix of microcrystalline cellulose), Polyethylene Glycol (PEG) 3350, colloidal silicon dioxide, and corn starch; (viii) an optimal stability when stored at 25°C (77°F), with excursions permitted to 15°C-30°C (59°F-86°F); (ix) absence of chromophores that absorb at wavelengths > 290 nm, to avoid direct photolysis by sunlight; (x) an estimated usual half-life of 90 days, taking into consideration the possible atmosphere-degradation by a reaction involving photochemically-produced hydroxyl radicals.
These properties underlining the effect of the particle size and physical form on the dissolution, hence the bioavailability of the active substance – consequently need to be tightly controlled to ensure the clinical safety and efficacy of the medicinal product [46].
2.3. Biological Effects and Pharmacological Properties
Mitotane (Lysodren®), developed in 1960, is an isomer of DDD (dichloro-diphenyl-dichloro-ethane), a derivative of the pesticide DDT (dichloro-diphenyl-trichloro-ethane) which was shown to produce adrenal atrophy in dogs in 1948 and, represents up-to-date the only Food and Drug Administration (FDA)-approved drug for the treatment of ACC [47][48]. Mitotane acts both as an inhibitor of steroidogenesis and an adrenolytic agent. Mechanistically, it inhibits directly 11β-hydroxylase, and cholesterol side-chain cleavage (SCC) in the mitochondria of steroidogenic cells together with antagonizing chemotherapy drug efflux, therefore blocking cortisol synthesis and reducing multidrug resistance (MDR), respectively [49]. Interestingly, mitotane has been shown to induce a p53-independent irreversible G2-arrested in cultured adrenocortical cell lines when combined to radiotherapy, as well as an increase of the radiotherapy cell growth inhibitory effect [50]. Furthermore, recent findings established a critical role of IGF signaling in ACC pathophysiology and provide rationale for use of targeted IGF-1R (type I Insulin-like Growth Factor Receptor) antagonists - especially when combined with mitotane - to treat ACC in future clinical trials [51].
Pharmacological analysis of oral Lysodren® in humans showed that about 40% only is absorbed, and approximately 10% of the administered dose is recovered in the urine as a water-soluble metabolite [52]. A variable amount of metabolite (1%-17%) is excreted in the bile within 24 hours and, because of its lipophilicity, the balance is apparently stored in the tissues (e.g. mainly adipose, liver, brain and adrenal tissues) [52]. Peak plasma Lysodren® concentrations occur 3-5 hours after a single oral dose of the drug and distribution of the drug between plasma and tissues is completed within 12 hours [52]. Consequently, cumulative high dose administration of mitotane is often required (up to 4-6 g/day during 3-5 months) which, subsequently, can lead to higher toxicity events [52][53]. Following discontinuation of Lysodren®, the plasma terminal half-life has ranged from 18 to 159 days, but can even last longer in some tissues (e.g. storing tissues such as fat ones) [52]. It is not known whether Lysodren® or its metabolites are able to cross the placenta or distribute into milk. The NTI of mitotane anti-tumor activity is achieved at the plasma concentration of 14 mg/L [52][53][54][55][56], and significant side effects have been noticed in more than 80% of all patients particularly when systemic levels of mitotane were greater than 20 mg/L [1][57]. The adverse effects include the gastro-intestinal system (e.g. nausea, vomiting, diarrhea) or the central nervous system (e.g. lethargy, ataxia, depression), which can be reversible after cessation of mitotane [1][57][58][59][60]. According to the World Health Organization (WHO) criteria, the overall response rate in 72 patients was 49%, including five patients with complete response [27]. This inter-patient variability, low response and considerable drug toxicity might be explained by many factors and mechanisms (e.g. genetics, epigenetics, ability of human tumor cells to efflux the drug or to metabolize it) and, underlines the importance of personalized medicine for mitotane dose titration as well as close clinical supervision.
In fact, mitotane metabolism is being studied for almost four decades to better understand its pharmacokinetics and pharmacodynamics and so, its molecular activity, which would help in carrying out the treatment [61][62][63][64][65][66][67][68][69][70]. Several approaches, using chromatography and/or spectrometry, have been developed to quantitatively determine mitotane and its metabolites in body fluids (e.g. serum, plasma, and urine) as well as in feces, and associate them with clinical outcomes [61][66][67][68][69][70]. Among the major mitotane metabolites, we can cite o,p'-DDE (i.e. 1,1-(o,p'-dichlorodiphenyl)-2,2 dichloroethane aka 1,1-dichloro-2-[p-chlorophenyl]-2-[o-chloro phenyl]ethane) [61], and o,p'-DDA (1,1-(o,p'-dichlorodiphenyl) acetic acid) [62] which has been identified through a proposed route involving the adrenal mitochondrial cytochrome P450-catalyzed hydroxylation of mitotane at the β-carbon [63][64]. Subsequent dehydrochlorination of the hydroxylated product forms the corresponding acyl chloride that, in the presence of water, formed the acidic metabolite, o,p'-DDA, although it could alternatively bind to tissue nucleophiles [65]. Interestingly, the synthesis route of β-3H-mitotane has been reported few years ago for use in a assay for mitotane metabolism [65], and consisted in the reduction of 1-(2-chlorophenyl)-1-(4- chlorophenyl)-2,2,2-trichloroethane (o,p'-DDT) by an aluminium-Hg2Cl2 couple in the presence of tritied water (3H20). Thereby, the determination of the 3H+, released to the aqueous media after organic solvent extraction, constituted a specific, faster (about 2-3 hours), and more sensitive assay for mitotane metabolic activation mediated by β-hydroxylation than 14Cmitotane- high-performance liquid chromatography (HPLC) [65]. Initial experiments in rats using 14C-labeled mitotane along with thin-layer chromatography (TLC), gas-liquid chromatography (GLC) and MS, reported that most of metabolites (87.8%) was concentrated in the feces (e.g. o,p'-DDA and its hydroxy-derivates such as 4-hydroxy-, 3-hydroxy-, and 3,4-dihydroxy-substituted o,p'-DDA, as well as o,p'-DDE) [66]. Interestingly, it was shown using GC-MS/Selected Ion Monitoring (SIM) that o,p'-DDA is present in the plasma at a concentration about 10 times higher than the levels of o,p'-DDD (mitotane) and o,p'-DDE [67]. This finding was later confirmed by other approaches using HPLC separation [61][68]. The clinical significance of such high plasmatic o,p'-DDA levels is not established yet. Nevertheless, a relatively recent study that explored the relationship between the plasma levels of mitotane and its metabolites, o,p'-DDA and o,p'-DDE determined by HPLC, with the efficacy of mitotane therapy during a long-term follow-up of pediatric and adult patients with adrenal cancer, suggested that plasmatic o,p'-DDE concentrations could be more closely related to clinical improvement or remission than the mitotane levels [69]. Indeed, higher o,p’-DDE and o,p’-DDE/o,p’-DDD seemed to be associated with a good/favorable prognosis during the prolonged mitotane therapy [69], and so might consitute interesting/important factors in clinical practice.
3. Conclusions and Perspectives
Adrenocortical carcinoma (ACC) is a rare but aggressive malignancy with a poor prognosis. Owing to some of its physicalchemical and pharmacological properties (e.g. hydrophobicity, lability, and subsequent very low systemic bioavailability), mitotane (Lysodren®), the only FDA-approved adrenolytic drug offers modest response rates in ACC cancer patients while causing significant health side-effects when chronic doses are employed. Thus, the management of ACC patients, particularly those with advanced ACC, requires multidisciplinary and innovative approaches to overcome these current therapeutic limitations. In this manuscript, we highlighted a possible "two-in-one" solution to efficiently treat patients with ACC, based on the recent and emerging investigations that suggest a favorable use of: (i) nanostructured lipid carriers (NLC) to load mitotane with greater features compared to other polymeric particles (e.g. in terms of safety, efficacy and cost of production), and (ii) S-(-)-mitotane, considered to be more potent than R-(+)-mitotane (Lysodren®) in cancer patients. Therefore, pure active S-(-)-mitotane loaded into NLC might offer better clinical results than S-(-)-mitotane as a free drug and, synergic or additive beneficial health effects for a larg number of ACC patients comparatively to the use of R-(+)-mitotane loaded in the same experimental conditions into NLC. Eventually, more studies (e.g. in vivo, clinical, epidemiological ones) are needed to assess the pharmacological, physical and chemical properties as well as the risk/benefits ratio of such possible novel mitotane drug formulations (e.g. triggered release, long-term stability, safety/toxicity, efficacy) for the patients before tempting a large scale production that can be greatly conducted using "green" methods.
References
- Bruno Allolio; Martin Fassnacht; Adrenocortical Carcinoma: Clinical Update. The Journal of Clinical Endocrinology & Metabolism 2006, 91, 2027-2037, 10.1210/jc.2005-2639.
- E. Michalkiewicz; R. Sandrini; B. Figueiredo; E.C.M. Miranda; E. Caran; A. G. Oliveira-Filho; R. Marques; M.A.D. Pianovski; L. Lacerda; L. M. Cristofani; et al.J. JenkinsC. Rodriguez-GalindoR. C. Ribeiro Clinical and Outcome Characteristics of Children With Adrenocortical Tumors: A Report From the International Pediatric Adrenocortical Tumor Registry. Journal of Clinical Oncology 2004, 22, 838-845, 10.1200/jco.2004.08.085.
- NIH consensus and state-of-the-science statements; NIH state-of-the-science statement on management of the clinically inapparent adrenal mass ("incidentaloma").. NIH consensus and state-of-the-science statements 2004, 19, 1-25.
- Mara A. D. Pianovski; Eliane M. C. P. Maluf; Denise S. De Carvalho; Raul C. Ribeiro; Carlos Rodriguez-Galindo; Paolo Boffetta; Patrícia Zancanella; Bonald Figueiredo; Mortality rate of adrenocortical tumors in children under 15 years of age in Curitiba, Brazil. Pediatric Blood & Cancer 2006, 47, 56-60, 10.1002/pbc.20624.
- A.-C. Koschker; M. Fassnacht; Stefanie Hahner; D. Weismann; Bruno Allolio; Adrenocortical Carcinoma - Improving Patient Care by Establishing New Structures. Experimental and Clinical Endocrinology & Diabetes 2006, 114, 45-51, 10.1055/s-2006-923808.
- B L Wajchenberg; M A Albergaria Pereira; B B Medonca; A C Latronico; P Campos Carneiro; V A Alves; M C Zerbini; B Liberman; G Carlos Gomes; M A Kirschner; et al. Adrenocortical carcinoma: clinical and laboratory observations.. Cancer 2000, 88, 711-736.
- Jean-Pierre Luton; Sonia Cerdas; Line Billaud; Guy Thomas; Brigitte Guilhaume; Xavier Bertagna; Marie-Hélène Laudat; Albert Louvel; Yves Chapuis; Philippe Blondeau; et al.André BonninHenri Bricaire Clinical Features of Adrenocortical Carcinoma, Prognostic Factors, and the Effect of Mitotane Therapy. New England Journal of Medicine 1990, 322, 1195-1201, 10.1056/nejm199004263221705.
- Philippe Icard; Pierre Goudet; Cyril Charpenay; Bernard Andreassian; Bruno Carnaille; Yves Chapuis; Patrick Cougard; Jean-François Henry; Charles Proye; Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World Journal of Surgery 2001, 25, 891-897, 10.1007/s00268-001-0047-y.
- Patsy S. Soon; Kerrie L. McDonald; Bruce G. Robinson; Stan B. Sidhu; Molecular Markers and the Pathogenesis of Adrenocortical Cancer. The Oncologist 2008, 13, 548-561, 10.1634/theoncologist.2007-0243.
- Raul Ribeiro; Fabiano Sandrini; Bonald Figueiredo; Gerard P. Zambetti; Edson Michalkiewicz; Antony R. Lafferty; Luiz DeLacerda; Mark Rabin; Craig Cadwell; Gilberto Sampaio; et al.Israil CatConstantine A. StratakisRomolo Sandrini An inherited p53 mutation that contributes in a tissue-specific manner to pediatric adrenal cortical carcinoma. Proceedings of the National Academy of Sciences 2001, 98, 9330-9335, 10.1073/pnas.161479898.
- Ch. Fottner; Andreas Hoeflich; Eckhard Wolf; M. M. Weber; Role of the Insulin-like Growth Factor System in Adrenocortical Growth Control and Carcinogenesis. Hormone and Metabolic Research 2004, 36, 397-405, 10.1055/s-2004-814563.
- T.J. Fahey; Adjuvant Mitotane Treatment for Adrenocortical Carcinoma. Yearbook of Surgery 2008, 2008, 192-193, 10.1016/s0090-3671(08)79077-3.
- Alfredo Berruti; Martin Fassnacht; Eric Baudin; Gary Hammer; Harm Haak; Sophie Leboulleux; Britt Skogseid; Bruno Allolio; Massimo Terzolo; Adjuvant Therapy in Patients With Adrenocortical Carcinoma: A Position of an International Panel. Journal of Clinical Oncology 2010, 28, e401-e402, 10.1200/jco.2009.27.5958.
- P Icard; A Louvel; Y. Chapuis; Survival rates and prognostic factors in adrenocortical carcinoma.. null 1992, 16, 753-758.
- D.E. Schteingart; Adrenocortical Carcinoma in the United States: Treatment Utilization and Prognostic Factors. Yearbook of Endocrinology 2009, 2009, 223-224, 10.1016/s0084-3741(09)79396-5.
- DeLellis, R.A.; Lloyd, R.V.; Heitz, P.U.; Eng, C.. Pathology and Genetics of Tumours of Endocrine Organs; DeLellis, Lloyd, Heitz, Eng, Eds.; IARC: Lyon, France, 2004; pp. 1-136.
- Martin Fassnacht; Sarah Johanssen; Marcus Quinkler; Peter Bucsky; Holger S. Willenberg; Felix Beuschlein; Massimo Terzolo; Hans-Helge Mueller; Stefanie Hahner; Bruno Allolio; et al.for the German Adrenocortical Carcinoma Registry Group and the European Network for the Study of Adrenal Tumors Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma. Cancer 2008, 115, 243-250, 10.1002/cncr.24030.
- Irina Veytsman; Lynnette Nieman; Tito Fojo; Management of Endocrine Manifestations and the Use of Mitotane As a Chemotherapeutic Agent for Adrenocortical Carcinoma. Journal of Clinical Oncology 2009, 27, 4619-4629, 10.1200/jco.2008.17.2775.
- D E Schteingart; G M Doherty; P G Gauger; T J Giordano; G D Hammer; M Korobkin; F P Worden; Management of patients with adrenal cancer: recommendations of an international consensus conference. Endocrine-Related Cancer 2005, 12, 667-680, 10.1677/erc.1.01029.
- Daniela F Maluf; Brás H De Oliveira; Enzo Lalli; Therapy of adrenocortical cancer: present and future. American journal of cancer research 2010, 1, 222-232.
- Jeffrey E. Lee; David H. Berger; Adel K. El-Naggar; Robert C. Hickey; Rena Vassilopoulou-Sellin; Robert F. Gagel; M. Andrew Burgess; Douglas B. Evans; Surgical management, DNA content, and patient survival in adrenal cortical carcinoma. Surgery 1995, 118, 1090-1098, 10.1016/s0039-6060(05)80119-9.
- F Crucitti; R Bellantone; A Ferrante; M Boscherini; P Crucitti; The Italian Registry for Adrenal Cortical Carcinoma: analysis of a multiinstitutional series of 129 patients. The ACC Italian Registry Study Group.. Surgery 1996, 119, 161-170.
- R Bellantone; A Ferrante; M Boscherini; C P Lombardi; P Crucitti; F Crucitti; G Favia; D Borrelli; L Boffi; L Capussotti; et al.G CarboneM CasacciaA CavallaroA Del GaudioG DettoriV Di GiovanniA MazziottiD MarranoE MasentiP MiccoliF MoscaA MussaR PetronioG PiatL Marazano Role of reoperation in recurrence of adrenal cortical carcinoma: results from 188 cases collected in the Italian National Registry for Adrenal Cortical Carcinoma.. Surgery 1997, 122, 1212-1218.
- J. Christian Jensen; Harvey I. Pass; William F. Sindelar; Jeffrey A. Norton; Recurrent or Metastatic Disease in Select Patients With Adrenocortical Carcinoma. Archives of Surgery 1991, 126, 457-461, 10.1001/archsurg.1991.01410280059008.
- Richard D. Schulick; Murray F. Brennan; Long-Term Survival After Complete Resection and Repeat Resection in Patients With Adrenocortical Carcinoma. Annals of Surgical Oncology 1999, 6, 719-726, 10.1007/s10434-999-0719-7.
- I. G. C. Hermsen; Y. E. Groenen; M. W. Dercksen; J. Theuws; H. R. Haak; Response to radiation therapy in adrenocortical carcinoma. Journal of Endocrinological Investigation 2010, 33, 712-714, 10.1007/bf03346675.
- Alfredo Berruti; Massimo Terzolo; Paola Sperone; Anna Pia; Silvia Della Casa; David J Gross; Carlo Carnaghi; Paolo Casali; Francesco Porpiglia; Franco Mantero; et al.Giuseppe ReimondoAlberto AngeliLuigi Dogliotti Etoposide, doxorubicin and cisplatin plus mitotane in the treatment of advanced adrenocortical carcinoma: a large prospective phase II trial. Endocrine-Related Cancer 2005, 12, 657-666, 10.1677/erc.1.01025.
- Anna A. Kasperlik-Zalułska; Barbara M. Migdalska; Stefan Zgliczyński; Anna M. Makowska; Adrenocortical carcinoma. A clinical study and treatment results of 52 patients. Cancer 1995, 75, 2587-2591, 10.1002/1097-0142(19950515)75:10<2587::aid-cncr2820751028>3.0.co;2-5.
- Martin Fassnacht; Sarah Johanssen; Wiebke Fenske; Dirk Weismann; Ayman Agha; Felix Beuschlein; Dagmar Führer; Christian Jurowich; Marcus Quinkler; Stephan Petersenn; et al.Martin SpahnStefanie HahnerBruno Allolioon behalf of the German ACC Registry Group Improved Survival in Patients with Stage II Adrenocortical Carcinoma Followed Up Prospectively by Specialized Centers. The Journal of Clinical Endocrinology & Metabolism 2010, 95, 4925-4932, 10.1210/jc.2010-0803.
- B. Wangberg; A Khorram-Manesh; S Jansson; B Nilsson; O Nilsson; C E Jakobsson; S Lindstedt; A Odén; H. Ahlman; The long-term survival in adrenocortical carcinoma with active surgical management and use of monitored mitotane. Endocrine-Related Cancer 2010, 17, 265-272, 10.1677/erc-09-0190.
- Aaron Sabolch; Mary Feng; Kent Griffith; Gary Hammer; Gerard Doherty; Edgar Ben-Josef; Adjuvant and Definitive Radiotherapy for Adrenocortical Carcinoma. International Journal of Radiation Oncology*Biology*Physics 2011, 80, 1477-1484, 10.1016/j.ijrobp.2010.04.030.
- Rena Vassilopoulou-Sellin; Mary J. Klein; Pamela N. Schultz; Naguib A. Samaan; Vincent F. Guinee; Sarah H. Taylor; Kenneth R. Hess; Impact of adjuvant mitotane on the clinical course of patients with adrenocortical cancer. Cancer 1993, 71, 3119-3123, 10.1002/1097-0142(19930515)71:10<3119::aid-cncr2820711037>3.0.co;2-8.
- Jerome Bertherat; Joël Coste; Xavier Bertagna; Adjuvant Mitotane in Adrenocortical Carcinoma. New England Journal of Medicine 2007, 357, 1256-1259, 10.1056/nejmc076267.
- T.J. Fahey; Recurrence of Adrenal Cortical Carcinoma Following Resection: Surgery Alone Can Achieve Results Equal to Surgery Plus Mitotane. Yearbook of Surgery 2011, 2011, 143-144, 10.1016/j.ysur.2011.04.005.
- David E. Schteingart; Adjuvant Mitotane Therapy of Adrenal Cancer — Use and Controversy. New England Journal of Medicine 2007, 356, 2415-2418, 10.1056/nejme078087.
- Massimo Terzolo; Alfredo Berruti; Adjunctive treatment of adrenocortical carcinoma. Current Opinion in Endocrinology, Diabetes & Obesity 2008, 15, 221-226, 10.1097/med.0b013e3282fdf4c0.
- Alfredo Berruti; Anna Ferrero; Paola Sperone; Fulvia Daffara; Giuseppe Reimondo; Mauro Papotti; Luigi Dogliotti; Alberto Angeli; Massimo Terzolo; Emerging drugs for adrenocortical carcinoma. Expert Opinion on Emerging Drugs 2008, 13, 497-509, 10.1517/14728214.13.3.497.
- Wandoloski, M.; Bussey, K.J.; Demeure, M.J.; Adrenocortical Cancer. Surg. Clin. North. Am. 2009, 89, 1255-1267.
- André Lacroix; Approach to the Patient with Adrenocortical Carcinoma. The Journal of Clinical Endocrinology & Metabolism 2010, 95, 4812-4822, 10.1210/jc.2010-0990.
- European Network for the Study of Adrenal Tumors (ENSAT) . ens@t. Retrieved 2020-12-8
- Lawrence S. Kirschner; Emerging Treatment Strategies for Adrenocortical Carcinoma: A New Hope. The Journal of Clinical Endocrinology & Metabolism 2006, 91, 14-21, 10.1210/jc.2005-1739.
- R.S. Vardanyan; V.J. Hruby; Synthesis of Essential Drugs. Synthesis of Essential Drugs 2006, 1, 411, 10.1016/b978-0-444-52166-8.x5000-6.
- Snyder, H.R.; Wicks, Z. Jr.; o-Chlorobromobenzene. Org. Synth. Coll. 1955, 3, 185.
- H. L. Haller; Paul D. Bartlett; Nathan L. Drake; Melvin S. Newman; Stanley J. Cristol; Charles M. Eaker; Robert A. Hayes; Glen W. Kilmer; Barney Magerlein; George P. Mueller; et al.Abraham SchneiderWilliam Wheatley The Chemical Composition of Technical DDT1. Journal of the American Chemical Society 1945, 67, 1591-1602, 10.1021/ja01225a058.
- International Union of Pure and Applied Chemistry (IUPAC) . Queen Mary University of London. Retrieved 2020-12-8
- United States Pharmacopeia (USP) . USP–NF. Retrieved 2020-12-8
- A A Nelson; G Woodard; Adrenal cortical atrophy and liver damage produced in dogs by feeding 2,2-bis-(parachloro-phenyl)-1,1-dichloroethane.. Federation proceedings 1948, 7, 277.
- Delbert M. Bergenstal; Roy Hertz; Mortimer B. Lipsett; Richard H. Moy; CHEMOTHERAPY OF ADRENOCORTICAL CANCER WITH o,p′DDD. Annals of Internal Medicine 1960, 53, 672-682, 10.7326/0003-4819-53-4-672.
- Yvan Touitou; Andre Bogdan; Jean-Pierre Luton; Changes in corticosteroid synthesis of the human adrenal cortex in vitro, induced by treatment with o,p'-DDD for cushing's syndrome: evidence for the sites of action of the drug. Journal of Steroid Biochemistry 1978, 9, 1217-1224, 10.1016/0022-4731(78)90015-8.
- L Cerquetti; B Bucci; R Marchese; Silvia Misiti; U De Paula; R Miceli; A Muleti; D Amendola; P Piergrossi; E Brunetti; et al.V ToscanoA Stigliano Mitotane increases the radiotherapy inhibitory effect and induces G2-arrest in combined treatment on both H295R and SW13 adrenocortical cell lines. Endocrine-Related Cancer 2008, 15, 623-634, 10.1677/erc.1.1315.
- Ferdous M. Barlaskar; Aaron C. Spalding; Joanne H. Heaton; Rork Kuick; Alex C. Kim; Dafydd G. Thomas; Thomas J. Giordano; Edgar Ben-Josef; Gary D. Hammer; Preclinical targeting of the type I insulin-like growth factor receptor in adrenocortical carcinoma.. The Journal of Clinical Endocrinology & Metabolism 2008, 94, 204-12, 10.1210/jc.2008-1456.
- Peter Heilmann; Philipp Wagner; Peter Nawroth; Reinhard Ziegler; Therapie des Nebennierenrindenkarzinoms mit Lysodren (o,p’-DDD) Erfahrungen mit der Therapiesteuerung durch Monitoring der Serumspiegel von o,p’-DDD. Medizinische Klinik 2001, 96, 371-377, 10.1007/pl00002218.
- Alexandria T. Phan; Adrenal Cortical Carcinoma—Review of Current Knowledge and Treatment Practices. Hematology/Oncology Clinics of North America 2007, 21, 489-507, 10.1016/j.hoc.2007.04.007.
- van Slooten, H; Moolenaar, A.J.; van Seters, A.P.; Smeenk, D. The treatment of adrenocortical carcinoma with o,p_-DDD: prognostic implications of serum level monitoring. Eur J Cancer Clin Oncol., 1984, 20, 47-53.
- Baudin, E.; Pellegriti, G.; Bonnay, M.; Penfornis, A.; Laplanche, A.; Vassal, G.; Schlumberger, M. Impact of monitoring plasma 1,1 dichlorodiphenildichloroethane (o,p_DDD) levels on the treatment of patients with adrenocortical carcinoma. Cancer, 2001, 92, 1385-1392.
- Hermsen, I.G.; Fassnacht, M.; Terzolo, M.; Houterman, S.; den Hartigh, J.; Leboulleux, S.; Daffara, F.; Berruti, A.; Chadarevian, R.; Schlumberger, M.;Allolio, B.; Haak, H.R.; Baudin, E. Plasma concentrations of o,p'DDD, o,p'DDA, and o,p'DDE as predictors of tumor response to mitotane in adrenocortical carcinoma: results of a retrospective ENS@T multicenter study. J Clin Endocrinol Metab., 2011, 96, 1844-1851.
- Lanser, J.B.; van Seters, A.P.; Moolenaar, A.J.; Haak, H.R.; Bollen, E.L. Neuropsychologic and neurologic side effects of mitotane and reversibility ofsymptoms. J Clin Oncol, 1992, 10, 1504.
- Schteingart, D.E.; Motazedi, A.; Noonan, R.A.; Thompson, N.W. Treatment of adrenal carcinomas. Arch Surg., 1982, 117, 1142-1146.
- Hutter, Jr A.M.; Kayhoe, D.E. Adrenal cortical carcinoma. Results of treatment with o,p´DDD in 138 patients. Am J Med., 1966, 41, 581-592.
- Lubitz, J.A.; Freeman, L.; Okun, R. Mitotane use in inoperable adrenal cortical carcinoma. JAMA, 1973, 223, 1109-1112.
- Andersen, A.; Kasperlik-Zaluska, A.A.; Warren, D.J. Determination of mitotane (o,p-DDD) and its metabolites o,p-DDA and o,p-DDE in plasmaby high-performance liquid chromatography. Ther Drug Monit., 1999, 21, 355-359.
- Reif, V.D., Sinsheimer, J.E.; Ward, J.C.; Schteingart, D.E. Aromatic hydroxylation and alkyl oxidation in metabolism of mitotane (o,p'-DDD) inhumans. J Pharm Sci., 1974, 63, 1730-1736.
- Schteingart, D.E.; Sinsheimer, J.E.; Counsell, R.E.; Abrams, G.D.; McClellan, N.; Djanegara, T.; Hines, J.; Ruangwises, N.; Benitez, R.; Wotring, L.L. Comparison of the adrenalytic activity of mitotane and a methylated homolog on normal adrenal cortex and adrenal cortical carcinoma. Cancer Chemother Pharmacol., 1993, 31, 459-466.
- Hart, M.M.; Straw, J.A. Studies on the site of action of o,p'-DDD in the dog adrenal cortex. 1. Inhibition of ACTH-mediated pregnenolone synthesis.Steroids, 1971, 17, 559-574.
- Piñeiro-Sánchez, M.L.; Vaz A.D.N.; Counsell, R.E.; Ruyan, M.; Schteingart, D.E.; Sinsheimer, J.E. Synthesis of ?-3H-Mitotane for Use in a Rapid Assay for Mitotane Metabolism. Journal of Labelled Compounds and Radiopharmaceuticals, 1995, 36, 121-127.
- Reif, V.D.; Sinsheimer, J.E. Metabolism of 1-(0-chlorophenyl)-1-(pchlorophenyl)-2,2-dichloroethane (o,p'-DDD) in rats. Drug Metab Dispos,1975, 3, 15-25.
- Inouye, M.; Mio, T.; Sumino, K. Use of GC/MS/SIM for rapid determination of plasma levels of o,p'-DDD, o,p'-DDE and o,p'-DDA. Clin Chim Acta,1987, 170, 305-314.
- Garg, M.B.; Sakoff, J.A.; Ackland, S.P. A simple HPLC method for plasma level monitoring of mitotane and its two main metabolites in adrenocorticalcancer patients. J Chromatogr B Analyt Technol Biomed Life Sci., 2011, 879, 2201-2205.
- Kasperlik-Zaluska, A.A.; Cichocki, A. Clinical role of determination of plasma mitotane and its metabolites levels in patients with adrenal cancer: results of a long-term follow-up. J Exp Ther Oncol., 2005, 5, 125-132.
- Ana Mornar; Miranda Sertić; Niksa Turk; Biljana Nigović; Mirko Koršić; Simultaneous analysis of mitotane and its main metabolites in human blood and urine samples by SPE-HPLC technique. Biomedical Chromatography 2012, 26, 1308-1314, 10.1002/bmc.2696.