1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is a common disease in one-third of the population in developed countries. NAFLD is associated with metabolic abnormalities including obesity, type 2 diabetes (T2DM), insulin resistance, and cardiovascular disease [1]. NAFLD is a spectrum of liver disease that ranges from simple hepatic steatosis to steatohepatitis (NASH) which is characterized by hepatocyte degeneration (ballooning) and inflammation with or without fibrosis. A small proportion of NASH will further progress to liver cirrhosis and hepatocellular carcinoma [2][3].
Diet has a key role in the development of NAFLD. Genetic and positive energy balance have important impacts on the first “hit” and diet composition affects the second "hit" and the severity of NAFLD [4][5][6] emphasizing the criticality of management and control of NAFLD. Several studies have reported that excessive consumption of carbohydrates, especially refined carbohydrates, fats, saturated fats in particular, and protein from meat can cause NAFLD [7][8]. Besides, higher intakes of soft drinks are associated with fatty liver [9].
At present, there is no clear consensus on the pharmacological treatment of NAFLD. In fact, no effective therapeutic agents have been approved for the treatment of the disease. Nevertheless, it is clear that therapeutic approaches should focus on lifestyle modifications. Diet and exercise interventions are the first-line treatment options, with weight loss via a hypocaloric diet being the most important therapeutic target in NAFLD [10]. However, most NAFLD patients are not able to achieve such weight loss. Therefore, the requisite is the investigation of other effective therapeutic approaches. Nutrient composition and caloric intake have been used to devise optimized diets in different stages of NAFLD to control disease progression [11]. Recently, it is recognized that timing and/or frequency of eating meal and fasting (with or without reduced energy intake) can have profound health benefits [12][13]. Nevertheless, more research should be focus on understanding the pathophysiology of the different strategies integrating nutrients, food intake and patterns of frequency of eating meals to provide recommendations for the prevention and treatment of NAFLD.
On the other hand, both aerobic and resistance exercise training in the absence of weight loss has been shown to reduce intrahepatic lipid (IHL) in patients with NAFLD [14]. However, whether exercise training without weight loss can reduce the histological features of NASH and fibrosis remains unknown [15]. Clearly, more studies are required to explore further the molecular and cellular mechanisms involved and to define the optimal volume and intensity of exercise, and whether weight loss is required for histological improvement in NASH and fibrosis.
2. Exercise Activates Liver-Muscle Signaling Pathways Involved in NAFLD
Physical activity induces a complex system of communication between muscle and liver [16][17]. This communication increases amino acid metabolism, especially branched chain amino acids from muscle and regulates metabolic activities in the liver that induce lipolysis. Exercise not only induces the release of signaling substances such as myokines from muscle [17], but also induces the release of other substances from the liver that control metabolic processes both in the liver and the rest of the organism [16][18].
Some of the main aspects of these mediators and their relationship with NAFLD (Figure 1).
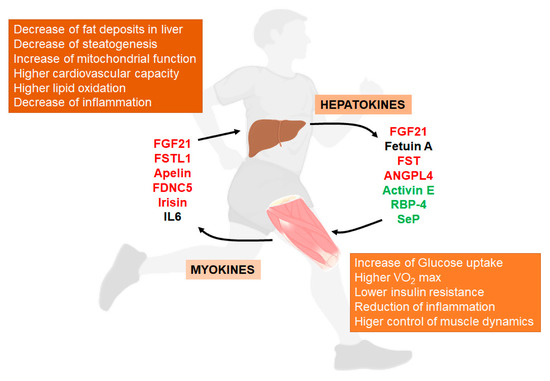
Figure 1. Liver/muscle crosstalk in NAFLD (Non-Alcoholic Fatty Liver Disease). Exercise induces the release of several signaling molecules from liver (hepatokines) that, through an endocrine mechanism, improves the physiology of muscle. On the other hand, exercised muscle releases into the circulation other substances called myokines that influence liver physiology, improving the situation caused by NAFLD. Both, hepatokines and myokines reduce the levels of pro-inflammatory markers (see complete names in text). In red, compounds that increase with exercise; in green, compounds that decrease with exercise; in black, compounds with conflicting responses to exercise.






