1
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is a common disease in one-third of the population in developed countries. NAFLD is associated with metabolic abnormalities including obesity, type 2 diabetes (T2DM), insulin resistance, and cardiovascular disease [1]. NAFLD is a spectrum of liver disease that ranges from simple hepatic steatosis to steatohepatitis (NASH) which is characterized by hepatocyte degeneration (ballooning) and inflammation with or without fibrosis. A small proportion of NASH will further progress to liver cirrhosis and hepatocellular carcinoma [2][3].
Diet has a key role in the development of NAFLD. Genetic and positive energy balance have important impacts on the first “hit” and diet composition affects the second "hit" and the severity of NAFLD [4][5][6] emphasizing the criticality of management and control of NAFLD. Several studies have reported that excessive consumption of carbohydrates, especially refined carbohydrates, fats, saturated fats in particular, and protein from meat can cause NAFLD [7][8]. Besides, higher intakes of soft drinks are associated with fatty liver [9].
At present, there is no clear consensus on the pharmacological treatment of NAFLD. In fact, no effective therapeutic agents have been approved for the treatment of the disease. Nevertheless, it is clear that therapeutic approaches should focus on lifestyle modifications. Diet and exercise interventions are the first-line treatment options, with weight loss via a hypocaloric diet being the most important therapeutic target in NAFLD [10]. However, most NAFLD patients are not able to achieve such weight loss. Therefore, the requisite is the investigation of other effective therapeutic approaches. Nutrient composition and caloric intake have been used to devise optimized diets in different stages of NAFLD to control disease progression [11]. Recently, it is recognized that timing and/or frequency of eating meal and fasting (with or without reduced energy intake) can have profound health benefits [12][13]. Nevertheless, more research should be focus on understanding the pathophysiology of the different strategies integrating nutrients, food intake and patterns of frequency of eating meals to provide recommendations for the prevention and treatment of NAFLD.
On the other hand, both aerobic and resistance exercise training in the absence of weight loss has been shown to reduce intrahepatic lipid (IHL) in patients with NAFLD [14]. However, whether exercise training without weight loss can reduce the histological features of NASH and fibrosis remains unknown [15]. Clearly, more studies are required to explore further the molecular and cellular mechanisms involved and to define the optimal volume and intensity of exercise, and whether weight loss is required for histological improvement in NASH and fibrosis.
2. InExercise Activatroductioes Liver-Muscle Signaling Pathways Involved in
Nonalco
NAFLD
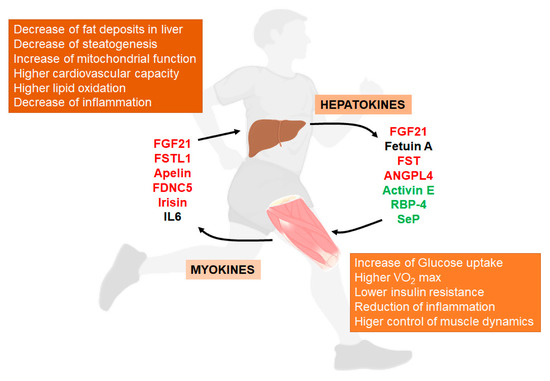
Pholysic fatty liver disease (NAFLD) is a common disease in one-thirdal activity induces a complex system of communication between muscle and liver of[16][17]. tThe populis communication in developed countries. NAFLD is associated withcreases amino acid metabolism, especially branched chain amino acids from muscle and regulates metabolic abnormalctivities including obesity, type 2 diabetes (T2DM), insulin resi the liver that induce lipolysis. Exercise not only induces the release of signaling substance, and cardiovascs such as myokines from muscle [17], but alar disease [1]. NAFLD is so induces the relea spectrum of liver disease that range of other substances from simple hepatic steatosis to steatohepatitisthe liver that control metabolic processes both in the liver and the rest of the organism (NA[16][18].
SH)ome of which is characterized by hepatocyte degeneration (ballooning) and inflammationthe main aspects of these mediators and their relationship with NAFLD ().
Figure 1. Liver/muscle crosstalk in NAFLD (Non-Alcoholic Fatty Liver Disease). Exercise induces the release of several signaling molecules from liver (hepatokines) that, through an endocrine mechanism, improves the physiology of muscle. On the other hand, exercised muscle releases into the circulation other substances called myokines that influence liver physiology, improving the situation caused by NAFLD. Both, hepatokines and myokines reduce the levels of pro-inflammatory markers (see complete names in text). In red, compounds that increase with exercise; in green, compounds that decrease with exercise; in black, compounds with conflicting responses to exercise.
2.1. Hepatokines
Hepatokines are proteins secreted by hepatocytes that influence metabolic processes through autocrine, paracrine and endocrine signaling [19]. NAFLD produces changes in the secretion of these proteins that can increase insulin resistance and induce metabolic dysfunction in many other tissues [20]. Among others, these hepatokines are follistatin (FST), fetuin A and B, retinol-binding protein 4 (RBP4) and selenoprotein P (SeP). NAFLD not only induces changes in the secretion of hepatokines but also produces changes in metabolites, lipids and miRNAs that can alter the metabolism in peripheral tissues including skeletal muscle [21].
2.1.1. Fibroblast Growth Factor 21
Fibroblast gr without fibrosis. A small proportion of NASH will further progress to liver cirrhosis and hepatocellular carcinomaowth factor-21 (FGF-21) is a 24-kDa protein that binds to the classic FGF receptor and the FGF-co-receptor β-klotho [2,3][20].
D FGF-21 iets has a key roleighly expressed in the developmentliver of[22]. NAFGFLD. Genetic and positive-21 is associated with the regulation of energy balance have important impacts on the first “hit” and diet composition affectsmetabolism, since FGF-21 null mice suffer impairment of glucose metabolism, maladaptation to ketosis and excessive body weight [23]. Furthe second "hit"rmore, these mice showed increased hepatic steatosis [24] and inflammathe severity of NAFLD [4,5,6]ion in an IL-17S-TLR4 dependent emphasizinganner the[25], criticality of management and controlndicating its importance in the impairment of NAFLD. Severa
Al studies have reported that excessive consumption of carbohydrateshough FGF-21 was initially considered as a myokine [26], especially refined carbohydrates, fats, satuome research has demonstrated fats in particular, and proteinits release from meat can cauliver after endurance exercise NAFLD [7,8][27]. BOthesides, higher intakes of soft drinks are associated with fattyr studies have indicated that FGF-21 production increases in muscle and promotes lipophagy in the liver [9].
via an At MPK-depresent, thendent pathway [28]. Interestingly, is no clear consensus on the pharmacological treatment of NAFLD. In fact, no effective therapeutic agents have been approved for thn a rat model of NAFLD, induction of a microRNA, miR-212, decreases the levels of FGF-21 mRNA and protein in liver, and exercise reverses this effect by inhibiting the expression of this microRNA [29]. Metabolic treatment of the disease. Nevertheless, it is clear that therapeutic approachedisorders can block the hepatic release of FGF-21 after exercise as has happened in diabetic patients should[27]. In foacus on lifestyle modifications. Diet and exercise interventions are the first-line treatment options,t, the release of FGF-21 is lower in obese patients with hyperinsulinemia compared with weight loshealthy subjects [30]. However, thia a hypocaloric diet being the most important therapeutic target in NAFLD [10]. Hows fact must be confirmed by more studies since a recent study showed high levels of FGF-21 lever,ls mostin NAFLD patients are not able to achieve such weight loss. Therefore, the requisite is the investigatnd a decrease after 12 weeks of resistance exercise, in a response attributed to the prevention of the progression of NAFLD [31]. To dathere, the effective therapeutic approaches. Nutrient composition and caloric intake have been used of chronic exercise on levels of FGF-21 is not clear and more research must be performed in order to devise optimized diets in different stages of NAFLD to controltermine its importance in the inter-organ cross talk d[16].
2.1.2. Fetuin A
Fetuin A ise a 64-KDase progression [11]. R glycoprotein secrently, it is recognized that timted by both, liver and adipose tissue. This hepatokine inhibits insulin signaling and/or frequency of eating meal and is directly correlated with adiposity in NAFLD patients [32]. faInteresting (with or without reduced energy intake) can have profound health benefits [12ly, the relationship of this hepatokine with the improvement of NAFLD in exercise is not clear since after six months of aerobic exercise and weight loss program,13]. Nevertheless, more research should be focus on understanding the pathophysiology levels of this hepatokine increased at the same time that glucose metabolism improved [33]. Hof the different strategies integrating nutrients, food wever, a direct relationship between increase in fetuin A and VO2max in take and patterns of frequency of eating meals to provide recommendations for the prevention and treatment ofhese patients was found, indicating a relationship of this kepatokine with the improvement of muscle function NAFLD[33].
On the other hand, ba shoth aerobic and resistance exercise training in the absence of weight loss has been shown to reduce intrart-term exercise program in obese adults clinically diagnosed with NAFLD produced a decrease in circulating fetuin A levels, along with improved insulin resistance and muscle glucose uptake [34]. Thep satic lipid (IHL) in patients with NAFLD [14]. Howme effect was found in old adults after a superver, whether eised exercise training wfor 12 weeks [35]. Again, thout weight loss can reduce the histe relationship of this hepatokine with physiological features of NASH and fibrosis remains unknown [15]. Climprovement in NAFLD patients must be established with morearly, morecontrolled studies.
2.1.3. Activin and Follistatin (FST)
Activin E is are r member of the TGF-β family [36], considequired to explore further the molecularecently as a hepatokine that is elevated in liver and cellular mecserum in humans with obesity and NAFLD [37], although manisms involved and to define thy of its functions have been associated with the regulation of adipose tissue op[38]. Actimvin hal volume and intensity of exercise, and whether weight loss is requires been associated with the increase of steatosis in liver through the induction of the insulin response [39]; and activation ofor histological improvement in NASH activin receptors by myostatin and activin also favors inflammation and fibrosis [40].
2. Exercise Activates Liver-Muscle Signaling Pathways Involved in NAFLD
Phy Interesticalngly, activity induces a complex system of communication betweenn A has also been associated with the increase of atrophy in skeletal muscle and livlinked to NAFLD [41][42].
On the other [119hand,120]. follistatin (FSThis communication increases amino ac) is a member of the TGF-β superfamily that acts as antagonist against myostatin and activin A through binding to their receptors and affects the regulation of skeletal muscle growth [43]. Thids metabolism, especially branched chain amino acids fromhepatokine is essential for the normal development of muscle since its absence produces insufficient muscle and regulates medevelopment and skeletal abnormalities in mice [16]. On the other habolic activities in the liver that induce lipolysis. End, high levels of FST block myostatin/activin action producing antiatrophic effects in muscle. Although FST is also produced in muscle, exercise not only inducesseems to increase the release of sliver FST to plasma [44] ignaling substances such as myokines a response attributed to the increase in the glucagon-to-insulin ratio during exercise f[27]. In relationship to chromnic muscle [120]exercise, buresist also induces the release of oance training increases the circulating FST in plasma of elderly overweight women [45]. Although rer substances from the liver that controlcent studies point to the role of FST in NAFLD and other metabolic processes both in the liver and the rest of the organism [119,121].
Somedisorders, little is known about its long-term adaptation to regular exercise and of the main aspects of these mediators and theirurther experiments must be performed in order to understand its relationship with NAFLD ()metabolic modifications induced by exercise.
Figure 3. Liver/muscle crosstalk in NAFLD (Non-Alcoholic Fatty Liver Disease). Exercise induces the release of several signaling molecules from liver (hepatokines) that, through an endocrine mechanism, improves the physiology of muscle. On the other hand, exercised muscle releases into the circulation other substances called myokines that influence liver physiology, improving the situation caused by NAFLD. Both, hepatokines and myokines reduce the levels of pro-inflammatory markers (see complete names in text). In red, compounds that increase with exercise; in green, compounds that decrease with exercise; in black, compounds with conflicting responses to exercise.
2.1. Hepatokines
Hepatokines are proteins secreted by hepatocytes that influence metabolic processes through autocrine, paracrine and endocrine signaling [122]. NAFLD produces changes in the secretion of these proteins that can increase insulin resistance and induce metabolic dysfunction in many other tissues [123]. Among others, these hepatokines are follistatin (FST), fetuin A and B, retinol-binding protein 4 (RBP4) and selenoprotein P (SeP). NAFLD not only induces changes in the secretion of hepatokines but also produces changes in metabolites, lipids and miRNAs that can alter the metabolism in peripheral tissues including skeletal muscle [109].
2.1.1. Fibroblast Growth Factor 21
Fibroblast growth factor-21 (FGF-21) is a 24-kDa protein that binds to the classic FGF receptor and the FGF-co-receptor β-klotho [124]. FGF-21 is highly expressed in the liver [125]. FGF-21 is associated with the regulation of energy metabolism, since FGF-21 null mice suffer impairment of glucose metabolism, maladaptation to ketosis and excessive body weight [126]. Furthermore, these mice showed increased hepatic steatosis [127] and inflammation in an IL-17S-TLR4 dependent manner [128], indicating its importance in the impairment of NAFLD.
Although FGF-21 was initially considered as a myokine [129], some research has demonstrated its release from liver after endurance exercise [130]. Other studies have indicated that FGF-21 production increases in muscle and promotes lipophagy in the liver via an AMPK-dependent pathway [131]. Interestingly, in a rat model of NAFLD, induction of a microRNA, miR-212, decreases the levels of FGF-21 mRNA and protein in liver, and exercise reverses this effect by inhibiting the expression of this microRNA [132]. Metabolic disorders can block the hepatic release of FGF-21 after exercise as has happened in diabetic patients [130]. In fact, the release of FGF-21 is lower in obese patients with hyperinsulinemia compared with healthy subjects [133]. However, this fact must be confirmed by more studies since a recent study showed high levels of FGF-21 levels in NAFLD patients and a decrease after 12 weeks of resistance exercise, in a response attributed to the prevention of the progression of NAFLD [134]. To date, the effect of chronic exercise on levels of FGF-21 is not clear and more research must be performed in order to determine its importance in the inter-organ cross talk [119].
2.1.2. Fetuin A
Fetuin A is a 64-KDa glycoprotein secreted by both, liver and adipose tissue. This hepatokine inhibits insulin signaling and is directly correlated with adiposity in NAFLD patients [135]. Interestingly, the relationship of this hepatokine with the improvement of NAFLD in exercise is not clear since after six months of aerobic exercise and weight loss program, the levels of this hepatokine increased at the same time that glucose metabolism improved [136]. However, a direct relationship between increase in fetuin A and VO2max in these patients was found, indicating a relationship of this kepatokine with the improvement of muscle function [136]. On the other hand, a short-term exercise program in obese adults clinically diagnosed with NAFLD produced a decrease in circulating fetuin A levels, along with improved insulin resistance and muscle glucose uptake [137]. The same effect was found in old adults after a supervised exercise training for 12 weeks [138]. Again, the relationship of this hepatokine with physiological improvement in NAFLD patients must be established with more controlled studies.
2.1.3. Activin and Follistatin (FST)
Activin E is a member of the TGF-β family [139], considered recently as a hepatokine that is elevated in liver and serum in humans with obesity and NAFLD [140], although many of its functions have been associated with the regulation of adipose tissue [141]. Activin has been associated with the increase of steatosis in liver through the induction of the insulin response [142]; and activation of activin receptors by myostatin and activin also favors inflammation and fibrosis [143]. Interestingly, activin A has also been associated with the increase of atrophy in skeletal muscle linked to NAFLD [144,145].
On the other hand, follistatin (FST) is a member of the TGF-β superfamily that acts as antagonist against myostatin and activin A through binding to their receptors and affects the regulation of skeletal muscle growth [146]. This hepatokine is essential for the normal development of muscle since its absence produces insufficient muscle development and skeletal abnormalities in mice [119]. On the other hand, high levels of FST block myostatin/activin action producing antiatrophic effects in muscle. Although FST is also produced in muscle, exercise seems to increase the release of liver FST to plasma [147] in a response attributed to the increase in the glucagon-to-insulin ratio during exercise [130]. In relationship to chronic exercise, resistance training increases the circulating FST in plasma of elderly overweight women [148]. Although recent studies point to the role of FST in NAFLD and other metabolic disorders, little is known about its long-term adaptation to regular exercise and further experiments must be performed in order to understand its relationship with metabolic modifications induced by exercise.
2.1.4. Retinol-Binding Protein 4
Considered also as an adipocytokine, RBP4, has been associated with insulin resistance in skeletal muscle. In humans, clinical cross-sectional studies have shown conflicting results indicating a negative correlation between RBP4 levels and insulin sensitivity [149]. In a NAFLD rat model, a 7-week treadmill exercise program was able to reduce RBP4 levels in plasma, although this decrease was associated with fat tissue [150].
In humans, plasma RBP4 levels are high in T2DM, obesity, metabolic syndrome and cardiovascular disease and interventions such as diet, exercise, antidiabetic drugs and hypolipidemic agents decrease their levels [151]. In children, RBP4 levels were high in obese individuals but changes in lifestyle based on exercise were able to decrease these levels at the same time as reducing inflammatory factors in plasma [152].
2.1.5. Angiopoietin-Like Protein 4
Angiopoietin-like protein 4 (ANGPL4) is a glycoprotein of approximately 45–65 kDa secreted by liver and adipose tissue [153]. Although its relevance in metabolic disorders of this hepatokine is not clear, it is well established that the protein regulates lipid metabolism by stimulating lipolysis in adipocytes [154] and inhibiting lipoprotein lipase activity [155]. Studies performed in humans have demonstrated that systemic ANGPLT4 increases during fasting and is secreted from the liver after exercise [156].
The relationship of ANGPL4 with insulin resistance improvement after exercise is not clear. It has been reported that, in obese people, a 6-month program of exercise and diet did not change ANGPTL4 serum levels indicating that other factors contribute to the insulin sensitivity improvement found after this program [156]. However, other studies have shown increased levels of ANGPTL4 after fasting, chronic CR and endurance exercise in a response associated with the increase of plasma free fatty acids levels [153]. The same result was found after a 12-week exercise program or a hypocaloric diet in obese patients indicating a similar response to both exercise and diet [157].
2.1.6. Selenoprotein P
Selenoprotein P (SeP) is a glycoprotein that can be released by liver and adipose tissue and has been shown to contribute to insulin resistance associated with NAFLD [158]. The effects of exercise on this marker are scarce but recent studies indicate that controlled and forced exercise programs reduce the levels of circulating SeP in NAFLD patients [159].
In general, most of the studies performed in NAFLD patients indicate that exercise partially restores the level of hepatokines to those found in healthy patients. However, some studies introduce some discrepancies that must be resolved in order to clearly determine the relationship of these mediators to the progression of the disease and to the regulation introduced by exercise and/or diet.
2.2. Myokines
AConsidered also as an adipocyting as a massive endocrine organ, contractingokine, RBP4, has been associated with insulin resistance in skeletal muscle secretes a number of substances known as myokines [160]. To d. In humans, clinical cross-sectional studies have shown conflicting results indicating a negative some of the most well-known are interleukincorrelation between RBP4 levels and insulin sensitivity [46]. (IL)-6, IL-10, IL-15, irisin, myostatin, brain derived neurotrophic factor (BDNF), β-amino-isobutyric acid, meteorin a NAFLD rat model, a 7-week treadmill exercise program was able to reduce RBP4 levels in plasma, although this decrease was associated with fat tissue [47].
In human-s, plike, leukemia inhibitory factor (LIF); when secreted, they are acidic and rich in cysteineasma RBP4 levels are high in T2DM, obesity, metabolic syndrome and cardiovascular disease and interventions such as diet, exercise, antidiabetic drugs and hypolipidemic agents decrease their levels (SARC) [161][48]. In tchis review, we will focus on the myokines that have shown a direct influence on the physiology of the liver and on their relationshipldren, RBP4 levels were high in obese individuals but changes in lifestyle based on exercise were able to decrease these levels at the same time as reducing inflammatory factors in plasma with NAFLD[49].
2.21.1. Follistat5. Angiopoietin-Like 1 and ApelProtein
Follistin-like 1 (FSTL1) is considered an adipokine or myokine that has been related to insulin resistance in obese and diabetic patients, producing a pro-inflammatory response [162], although other studies have indicated its cardioprotective effect against ischemic injury [163] and it has been shown that FSTL1 levels are reduced in diabetes patients [164]. Apelin has also been associated with adipose tissue but recently it has also been considered as myokine upregulated after exercise and its release has been associated with decrease of fat levels and improvement of cardiovascular capacity [165].
FSTL1 [166] and apelin [165] are expressed in myotubes and released after acute exercise. Both have a favorable effect on energy metabolism and rat studies have demonstrated that acute endurance exercise produces significant increases in plasma just after exercise without affecting tissue levels [167]. In humans, acute sprint interval training consisting of four 30-s all-out cycling efforts with 4-min rest periods also produced significant increases in plasma of both myokines [168]. Their role in NAFLD patients has not been studied in depth but we can speculate that these myokines can have an important effect on the reduction of fat and on the improvement of liver activity.
2.2.2. FNDC5 and Irisin
Irisin is a PGC-1α-induced myokine product of the cleavage of the fibronectin type III domain-containing protein 5 (FNDC5) [169]. Irisin is secreted by contracting skeletal muscle and has been associated with health benefits via changes in metabolism of white adipose tissue [170].
Clinical studies have demonstrated that FNDC5 is essential for maintaining metabolic homeostasis and its dysregulation leads to imbalance of systemic metabolism [171]. Independently of its function as precursor or irisin, FNDC5 has been recently shown as relevant for the regulation of diverse upstream and downstream signaling pathways involved in metabolic syndrome [171]. FNDC5 is increased in fatty liver in both mice and humans without affecting plasma levels or irisin [172]. Downregulation of FNDC5 expression resulted in the increase of steatosis and in insulin resistance and higher apoptosis of primary hepatocytes to TNF-α. Probably, the high expression of FNDC5 in hepatocytes in NAFLD can be the consequence of a protective response against steatogenesis through the local release of irisin, or through the activation of downstream signaling molecules that regulate physiological modifications in response to accumulation of fat [171,172].
Circulating irisin levels in patients with hepatic steatosis in comparison with controls are confusing. Some studies have shown that irisin levels are lower in obese, NAFLD and NASH patients in comparison with lean controls [173]. Low levels of serum irisin have been also reported in NAFLD, T2DM and NAFLD + T2DM patients in comparison with controls [174], and more recently a significant decrease of plasma irisin together with the adipokines omentin and vaspin have been reported in NAFLD and alcoholic cirrhotic patients [175]. However, other studies have shown that plasma irisin levels are high in NAFLD patients in comparison with controls [176] and the most recent study has shown that plasma of patients with NAFLD contains higher levels of irisin, in direct relationship with the IHL content [177]; further, in HIV patients without diabetes, higher irisin levels were associated with insulin resistance, NAFLD and subclinical atherosclerosis [178]. To add more confusion, another study did not find differences in the levels of irisin between controls and NAFLD patients [179]. It seems clear that the relationship of NAFLD and plasma irisin levels must be resolved in order to understand the physiological relevance of this myokine to NAFLD progression.
Although the mechanism is not clear, physical activity produces the release of irisin into plasma in an intensity-dependent manner [180]. The release of irisin can be gender dependent affecting more women than men [181]. Interestingly, irisin release after exercise can impair the progression of hepatic fibrosis by regulating the activation, proliferation, migration, contractility and inflammatory cytokine release from hepatic stellate cells [182]. In a recent study, Zhang et al., [183] demonstrated that irisin protects steatotic liver after ischemia/reperfusion in mice through inhibiting ROS production and improving mitochondrial dysfunction. This effect was associated with the binding of irisin to integrin receptors in hepatic cells and activation of kindlin-2, a member of the 4.1-ezrin-ridixin-moesin (FERM) domain family of proteins that regulates many biological functions after interacting with the cytoplasmic tails of β-integrin subunits [184]. However, the role of irisin-dependent kindlin-2 activation is controversial since this protein is considered a biomarker for poor prognosis of liver cancer patients [184] and its deficiency attenuates mouse liver fibrosis and hepatic stellate cells activation [185]. Again, further research is needed in order to understand the putative hepatoprotective effect of irisin in exercised patients.
2.2.3. Interleukin-6 (IL-6)
I
4
Angiopoietin-like protein 4 (ANGPL-64) is a well-known cytokine associated withglycoprotein of approximately 45–65 kDa secreted by liver diseaseand adipose tissue [50]. Althat increases when NAFLD progresses to NASH [186]. Iough its relevance in metabolic disorders of this hepatokine is not clear, it iseems well established that IL-6 can be involved in thethe protein regulates lipid metabolism by stimulating lipolysis in adipocytes [51] and inhibiting lipon protein lipase activity [52]. Studies perfofrmed in humans hepatic autophagy induced by exave demonstrated that systemic ANGPLT4 increases during fasting and is secreted from the liver after exercise [53].
The relaustive physicaltionship of ANGPL4 with insulin resistance improvement after exercise since IL-6 null mice show reducedis not clear. It has been reported that, in obese people, a 6-month program of exercise and diet did not change ANGPTL4 serum levels of markers of autophagy in the liver indicating that other factors contribute to the insulin sensitivity improvement found after this program [187][52]. However, othe role of IL-6 in the exercise effect on NAFLD patients is puzzling, since their release, togetherr studies have shown increased levels of ANGPTL4 after fasting, chronic CR and endurance exercise in a response associated with the leincrease of plasma free fatty acids levels of[50]. otTher cytokines, has been considered a positive effect, inducing anti-inflammatory same result was found after a 12-week exercise program or a hypocaloric diet in obese patients indicating a similar responses and im to both exercise and diet [54].
2.1.6. Selenoprotein P
Selenoprovteing fat metabolism in the liver [188]. In P (SeP) is a glycoprotein that can be released by liver anyd case, it seems clear that exercise induces thadipose tissue and has been shown to contribute to insulin resistance associated with NAFLD [55]. The release of IL-6 from muscle. Plasma IL-6 increases exponentially duringffects of exercise on this marker are scarce but recent studies indicate that controlled and forced exercise depending on intprograms reduce the levels of circulating SeP in NAFLD patients [56].
In general, mosity, duration, muscle mass and endurance capacity [189,190,191].t of the studies performed in NAFLD patients indicate that exercise partially restores the level of hepatokines to those Vfoluntary and electrical muscle contractions 19 min twice a week increased IL-6 levels in NAFLD patients in comparison with controls, improving insulin resistancund in healthy patients. However, some studies introduce some discrepancies that must be resolved in order to clearly determine the relationship of these mediators to the progression of the disease and hepatic steatosis [192]to the regulation introduced by exercise and/or diet.
2.2. Myokines
Acting as a massive endocrine organ, contracting muscle secretes a number of substances known as myokines [57]. To date some of the most well-known are interleukin (IL)-6, IL-10, IL-15, irisin, myostatin, brain derived neurotrophic factor (BDNF), β-amino-isobutyric acid, meteorin-like, leukemia inhibitory factor (LIF); when secreted, they are acidic and rich in cysteine (SARC) [58]. We will focus on the myokines that have shown a direct influence on the physiology of the liver and on their relationship with NAFLD.
2.2.1. Follistatin-Like 1 and Apelin
Follistin-like 1 (FSTL1) is considered an adipokine or myokine that has been related to insulin resistance in obese and diabetic patients, producing a pro-inflammatory response [59], although other studies have indicated its cardioprotective effect against ischemic injury [60] and it has been shown that FSTL1 levels are reduced in diabetes patients [61]. Apelin has also been associated with adipose tissue but recently it has also been considered as myokine upregulated after exercise and its release has been associated with decrease of fat levels and improvement of cardiovascular capacity [62].
FSTL1 [63] and apelin [62] are expressed in myotubes and released after acute exercise. Both have a favorable effect on energy metabolism and rat studies have demonstrated that acute endurance exercise produces significant increases in plasma just after exercise without affecting tissue levels [64]. In humans, acute sprint interval training consisting of four 30-s all-out cycling efforts with 4-min rest periods also produced significant increases in plasma of both myokines [65]. Their role in NAFLD patients has not been studied in depth but we can speculate that these myokines can have an important effect on the reduction of fat and on the improvement of liver activity.
2.2.2. FNDC5 and Irisin
Irisin is a PGC-1α-induced myokine product of the cleavage of the fibronectin type III domain-containing protein 5 (FNDC5) [66]. Irisin is secreted by contracting skeletal muscle and has been associated with health benefits via changes in metabolism of white adipose tissue [67].
Clinical studies have demonstrated that FNDC5 is essential for maintaining metabolic homeostasis and its dysregulation leads to imbalance of systemic metabolism [68]. Independently of its function as precursor or irisin, FNDC5 has been recently shown as relevant for the regulation of diverse upstream and downstream signaling pathways involved in metabolic syndrome [68]. FNDC5 is increased in fatty liver in both mice and humans without affecting plasma levels or irisin [69]. Downregulation of FNDC5 expression resulted in the increase of steatosis and in insulin resistance and higher apoptosis of primary hepatocytes to TNF-α. Probably, the high expression of FNDC5 in hepatocytes in NAFLD can be the consequence of a protective response against steatogenesis through the local release of irisin, or through the activation of downstream signaling molecules that regulate physiological modifications in response to accumulation of fat [68][69].
Circulating irisin levels in patients with hepatic steatosis in comparison with controls are confusing. Some studies have shown that irisin levels are lower in obese, NAFLD and NASH patients in comparison with lean controls [70]. Low levels of serum irisin have been also reported in NAFLD, T2DM and NAFLD + T2DM patients in comparison with controls [71], and more recently a significant decrease of plasma irisin together with the adipokines omentin and vaspin have been reported in NAFLD and alcoholic cirrhotic patients [72]. However, other studies have shown that plasma irisin levels are high in NAFLD patients in comparison with controls [73] and the most recent study has shown that plasma of patients with NAFLD contains higher levels of irisin, in direct relationship with the IHL content [74]; further, in HIV patients without diabetes, higher irisin levels were associated with insulin resistance, NAFLD and subclinical atherosclerosis [75]. To add more confusion, another study did not find differences in the levels of irisin between controls and NAFLD patients [76]. It seems clear that the relationship of NAFLD and plasma irisin levels must be resolved in order to understand the physiological relevance of this myokine to NAFLD progression.
Although the mechanism is not clear, physical activity produces the release of irisin into plasma in an intensity-dependent manner [77]. The release of irisin can be gender dependent affecting more women than men [78]. Interestingly, irisin release after exercise can impair the progression of hepatic fibrosis by regulating the activation, proliferation, migration, contractility and inflammatory cytokine release from hepatic stellate cells [79]. In a recent study, Zhang et al., [80] demonstrated that irisin protects steatotic liver after ischemia/reperfusion in mice through inhibiting ROS production and improving mitochondrial dysfunction. This effect was associated with the binding of irisin to integrin receptors in hepatic cells and activation of kindlin-2, a member of the 4.1-ezrin-ridixin-moesin (FERM) domain family of proteins that regulates many biological functions after interacting with the cytoplasmic tails of β-integrin subunits [81]. However, the role of irisin-dependent kindlin-2 activation is controversial since this protein is considered a biomarker for poor prognosis of liver cancer patients [81] and its deficiency attenuates mouse liver fibrosis and hepatic stellate cells activation [82]. Again, further research is needed in order to understand the putative hepatoprotective effect of irisin in exercised patients.
2.2.3. Interleukin-6 (IL-6)
IL-6 is a well-known cytokine associated with liver disease that increases when NAFLD progresses to NASH [83]. It seems that IL-6 can be involved in the inhibition of hepatic autophagy induced by exhaustive physical exercise since IL-6 null mice show reduced levels of markers of autophagy in the liver [84]. However, the role of IL-6 in the exercise effect on NAFLD patients is puzzling, since their release, together with the levels of other cytokines, has been considered a positive effect, inducing anti-inflammatory responses and improving fat metabolism in the liver [85]. In any case, it seems clear that exercise induces the release of IL-6 from muscle. Plasma IL-6 increases exponentially during exercise depending on intensity, duration, muscle mass and endurance capacity [86][87][88]. Voluntary and electrical muscle contractions 19 min twice a week increased IL-6 levels in NAFLD patients in comparison with controls, improving insulin resistance and hepatic steatosis [89].