
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kishor Kumar Sadasivuni | + 2042 word(s) | 2042 | 2020-11-10 09:08:58 | | | |
| 2 | Vicky Zhou | Meta information modification | 2042 | 2020-11-24 03:06:45 | | |
Video Upload Options
Simultaneous detection of analytes that together exist in biological organisms necessitates the development of effective and efficient non enzymatic electrodes in sensing. In this regard, development of sensing elements for detecting glucose and hydrogen peroxide (H2O2) is significant. The non-enzymatic sensing is more economical and has longer lifetime than enzymatic electrochemical sensing, but it has several drawbacks such as high working potential, slow electrode kinetics, poisoning from intermediate species and weak sensing parameters. Here is a comprehensive overview of the recent developments in non-enzymatic glucose and H2O2 (NEGH) sensing, by focusing mainly on sensing performance, electro catalytic mechanism, morphology and design of electrode materials. A comparison of glucose and H2O2 sensing parameters using same electrode materials is outlined to predict the efficient sensing performances of advanced nanomaterials with metal/metal oxides and hybrid metallic nanocomposites.
1. Introduction
Non-enzymatic sensing has been in the research limelight, and most sensors based on nanomaterials are designed to detect single analytes. The simultaneous detection of analytes that together exist in biological organisms necessitates the development of effective and efficient non-enzymatic electrodes in sensing. In this regard, the development of sensing elements for detecting glucose and hydrogen peroxide (H2O2) is significant. Non-enzymatic sensing is more economical and has a longer lifetime than enzymatic electrochemical sensing, but it has several drawbacks, such as high working potential, slow electrode kinetics, poisoning from intermediate species and weak sensing parameters.
2. Importance of Non-Enzymatic Electrochemical Sensing
Glucose is an essential carbohydrate involved in major catabolic pathways, including oxidative phosphorylation and glycolysis for the creation of proteins, glycogens, and lipids [1][2]. Glucose is absorbed through the intestines, and, converted by the liver into a more stable form of glycogen, regulated by the hormone insulin [3][4]. Diabetes mellitus (DM) has been termed the “invisible killer” as a consequence of both hyperglycemia and hypoglycemia [5]. A fasting blood glucose concentration less than 100 mg/dl (5.6 mmol/L) is normal, a level from 100 to 125 mg/dL (5.6 to 6.9 mmol/L) is considered prediabetes and greater than 126 mg/dL (7 mmol/L) on two separate tests allows the diagnosis of diabetes. Hypoglycemia is defined by a blood glucose concentration <70 mg/dl (3.9 mmol/L) and concentrations of both <54 mg/dL (3.0 mmol/L) and <50mg/dL (2.8 mmol/L) cause defective glucose counterregulation and impaired awareness of hypoglycemia. Hyperglycemia can result in multiple metabolic abnormalities associated with long term microvascular and macrovascular complications [6][7][8][9][10]. The global prevalence of diabetes in 2019 was estimated at 463 million people, and has been predicted to rise 10.2% by 2030 and 10.9% by 2045. The prevalence is higher in developed countries (10.4%) than in developing countries (4.0%). Furthermore, one in two people living with diabetes do not know that they have diabetes. The rising burden of diabetes in low- and middle-income countries may cause financial strain on individuals and health systems. Among all countries worldwide, the United States and China have the highest diabetes related medical expenditure. Between 2019 and 2045, the global expenditure for diabetes treatment is expected to grow from USD 760 billion to USD 845 billion. Diagnosis and management of diabetes require accurate, sensitive, reliable, rapid, and attentive monitoring of glucose in day to day life [11][12]. Generally, H2O2 is generated during enzyme/glucose reactions and so the monitoring of H2O2 is also of great importance. H2O2 is an unstable compound found in nature that plays a vital role as an intermediate in several biological reactions such as the metabolism of proteins, carbohydrates, cell signaling, and immune responses [13][14]. However, excess H2O2 can damage DNA or proteins via the generation of reactive oxygen species [15]. Hence, the monitoring of both H2O2 and glucose with a novel sensing approach in humans and the environment is of great significance. Such non-enzymatic glucose and H2O2 (NEGH) sensors have applications in biomedical devices, catalysis, and the environment.
Several analytical approaches have been reported to quantify glucose and H2O2 levels, namely calorimetric, titrimetric analysis, spectrometry, fluorescence, chemiluminescence, and high-pressure liquid chromatography [16][17][18][19][20]. However, these methods have certain limitations, such as cumbersome fabrication processes, low reproducibility, matrix interference, high cost, and short shelf time. Hence, there is a need for the development of more efficient techniques for glucose and H2O2 quantification, and, in this context, electrochemical methods have much influence. Electrochemical techniques for glucose and H2O2 sensing have good accuracy, specificity, response time, simplicity, lower detection limits, high physical and chemical stability, enhanced electron transfer rate, practical detectability, easy to scale up, and biocompatibility [21]. The first enzyme-based glucose sensors were explored in 1960, and have served to drive work in this area for many researchers. Thereafter, first, second, and third generation enzyme-based glucose biosensors have been established. Third-generation sensors are still in their infancy, but those based on nano-mesoporous electrode surfaces show promise but with some drawbacks [22][23]. The mechanism of these sensors is based on the detection of oxygen or H2O2, the electron mediator, or the enzyme. Immobilized glucose oxidase (GOx) sensing results in the detection of gluconolactone and H2O2 [24]. Hence, the sensing of both glucose and H2O2 exists in correlation and has significance in food, pharmaceutical, clinical, and environmental studies [25][26]. However, enzymatic glucose and H2O2 sensors (EGHS) have certain limitations, including enzyme denaturation due to environmental changes (pH, humidity, and temperature), digestion by proteases, expensive preparation, time-consuming purification, high cost, thermo-chemical deformation, poor reproducibility, lack of stability, and tedious enzyme immobilization techniques [27][28]. These disadvantages of EGHS, as mentioned, can be adequately defined by nanomaterial assisted electrochemical processes through NEGH sensing.
The most significant challenges faced while designing NEGH sensing are the high working potential, unpredicted redox reactions, slow electro kinetics, intermediate poisoning and weak sensing parameters [29]. Therefore, recent efforts have been devoted primarily on discovering novel nanomaterials with high conductivity, efficient catalytic activity, and excellent physical and chemical strength for the construction of non-enzymatic sensors [30][31]. Nanomaterials have a large surface area, applied potential window, low charge transfer resistance, and flexibility, which makes them ideal electrode materials [32][33]. These novel nanomaterials include metal/metal oxide, carbon, and polymer nanocomposites in different nano morphologies such as crystals, rods, wires, fibers, twisters, core shell, and quantum dots (Figure 1) [34].
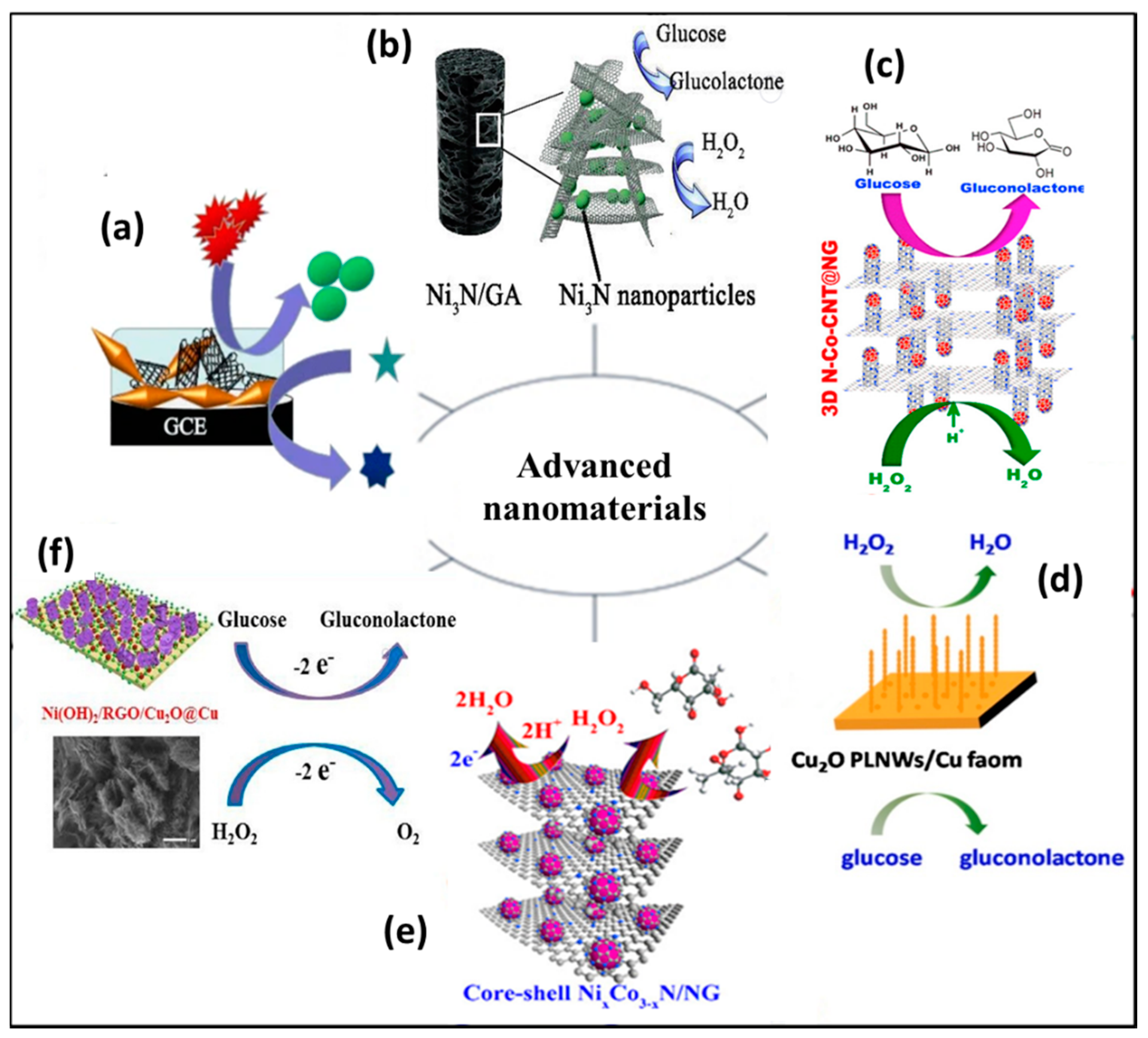
A wide variety of nanomaterials are fabricated; however, only a limited number of nanomaterials have been utilized for NEGH sensing due to their enhanced conductivity, surface area, electro kinetics, and the electro catalytic activity in acid, and base media. The nanoparticle concentration, synergistic effect, charge carrier type, surface charge, bandgap, mobility and density of electrons on the surface of a nanomaterial can be tuned by considering a combination of materials, and efficient preparation method, which has enabled their applications in a wide range of electrochemical devices [41][42][43]. Significant research effort was dedicated to the development of NEGH sensing with advanced nanomaterials to obtain high conductivity, suitably applied potential, and portable sensing of glucose and H2O2.
3. Future Perspectives
Limited research development has been made with regard to the fabrication of advanced nanomaterials with bifunctional property for NEGHS. Further improved research and development are necessary to make the commercialization of implantable in vivo and portable in vitro NEGHS devices, which require the improvement of practical, affordable, advanced nanomaterial-based electrocatalysts with multifunctional reactivity. The current research review addresses multiple directions for the achievement of non-enzymatic bifunctional electrode platforms. Electrochemical sensing parameters of advanced nanomaterial with bifunctional electrodes are dependent upon the electrode potential, bandgap, surface defects, synergetic effect, and surface area of the nanocomposites. However, the influence of these issues on NEGH sensing is not addressed in the literature and provides opportunities for the future development of biodevices. Since the multienzymatic properties of nanomaterials have attracted wider research interest, the catalytic (glucose) and peroxidase (H2O2) activity of nanomaterial should be effectively optimized and promoted for the best performance of NEGH sensors. The essential electrochemical mechanism in NEGH sensing with the same electrode material should be established using theoretical and analytical models with relevant laboratory experiments. Current studies on NEGH sensors mostly focus on the electrocatalyst performance of advanced nanomaterials and limit the understanding of the influence of nanomaterial morphology on glucose and H2O2 quantification and the interaction with bio-analytes. To overcome this, researchers should focus on the development of nanomaterials in different morphologies, such as dots, tubes, fibers, spheres, and core-shells, and a detailed study should be undertaken to improve the surface area and conductivity, which could have a positive influence on the development of NEGH sensors. The modified electrodes show catalytic activity in acidic or basic conditions, which limit the practical application of NEGH sensors. In this context, studies must be done on the oxidation and reduction mechanisms at neutral pH conditions by considering novel nanomaterials. The use of biopolymers as bio-catalytic centers are tolerable to achieve highly sensitive and selective NEGH sensors, and distinct consideration should be given to building electrode platforms with improved robustness and enhanced electro catalytic activity. NEGH sensor-based nanomaterials as catalysts have been demonstrated to be very reasonable; conversely, it is essential to design new schemes for the synthesis, functionalization, and fabrication of nanomaterials to acquire more accurate quantification of glucose and H2O2. Several sequential steps involved in the preparation of electrodes for a conventional modified electrode based on NEGH sensing, including cumbersome electrode cleaning, polishing and washing, binder and solvent selection, catalyst preparation, and loading process, have increased the time and cost of NEGH sensing electrodes. Furthermore, to establish contact between the working electrode and catalyst using a binder remains another challenge for the performance of NEGH sensing. This could be avoided by developing binder-free, freestanding bare electrodes, ink/screen printed electrodes and the in situ fabrication/modification of advanced nanomaterials as modified electrodes that make possible the preparation of disposable NEGH sensing electrodes. Moreover, another compelling research direction is in the preparation of metal/metal oxide morphologies with emerging carbon materials (g-C3N4, graphene, CNTs, black phosphorous, and activated carbon, etc.) to form new functional materials. For commercialization, an important prospect is the prolongation of lifetime of the sensors, even though the non-enzymatic sensors are more stable than enzymatic sensors, they lack in the corrosion property/unstable in humid conditions, which requires researchers to focus on anticorrosive nanomaterials.
Current challenges in improving efficiency of the NEGH sensors can be overcome by optimizing the selectivity, working potential, linearity, sensitivity and working pH conditions. Though some NEGH sensors are good in neutral pH conditions with low detection limits, their linear range of detection may be questionable. The low detection range sensors are not useful in day-to-day diabetes management and hence few reports have been applied in various real-time applications such as sensing in antibiotic lotions, milk, and glucose-based fuel cells, etc. The selectivity of NEGH remains a huge problem, which means that the oxidation of interference compounds such as AA, DA, and UA chlorine ions and other carbohydrates at the same working potential affects the glucose and H2O2 determinations. Transition metal/metal-oxide-based sensors have shown significant progress in selectivity issues and electrode fouling problems due to reasonable isoelectric point values. From the reported literature on NEGH sensors the sensitivity was improved using different strategies and the novel combination of nanomaterials. Sensor sensitivity is dependent on on working potential, electro kinetics and electrolyte conditions. However, different research groups have performed sensing under their own optimized conditions, which necessitates a uniform protocol for sensing operations. In addition, to improving the sensitivity by optimizing the properties of advanced nanomaterials, the selectivity performance should be more focused to achieve stability, repeatability, and practical evaluation of glucose and H2O2. The dual in-line sensor requires a clear mechanism with suitable working conditions in neutral pH. The use of the same electrode material for multiple applications is essential to reduce the cost and will make commercialization easy. The dual sensor requires a clear electro catalytic mechanism for sustainable development, and it can be achieved by operating the electrodes at the same working potential (positive/negative). In short, the bifunctional, electro-catalyst-based NEGH sensing technology must be extended from the laboratory to the field by proper implementation to boost sustainable electronic devices.
References
- Pandey, P.; Tripathi, R.P.; Srivatava, R.; Goswami, S. Alternative therapies useful in the management of diabetes: A systematic review. J. Pharm. Bioallied Sci. 2011, 3, 504–512.
- Zaidi, S.A.; Shin, J.H. Recent developments in nanostructure based electrochemical glucose sensors. Talanta 2016, 149, 30–42.
- Niu, X.; Li, X.; Pan, J.; He, Y.; Qiu, F.; Yan, R. Recent advances in non-enzymatic electrochemical glucose sensors based on non-precious transition metal materials, opportunities and challenges. RSC Adv. 2016, 6, 84893–84905.
- Aziz, A.; Asif, M.; Ashraf, G.; Azeem, M.; Majeed, I.; Ajmal, M.; Wang, J.; Liu, H. Advancements in electrochemical sensing of hydrogen peroxide, glucose and dopamine by using 2D nanoarchitectures of layered double hydroxides or metal dichalcogenides A review. Microchim. Acta 2019, 186, 671.
- Tian, K.; Prestgard, M.; Tiwari, A. A review of recent advances in nonenzymatic glucose sensors. Mater. Sci. Eng. C 2014, 41, 100–118.
- Bilal, S.; Ullah, W.; Ali Shah, A.U.H. Polyaniline@CuNi nanocomposite: A highly selective, stable and efficient electrode material for binder free non-enzymatic glucose sensor. Electrochim. Acta 2018, 284, 382–391.
- Justice Babu, K.; Sheet, S.; Lee, Y.S.; Gnana Kumar, G. Three-dimensional dendrite Cu–Co/reduced graphene oxide architectures on a disposable pencil graphite electrode as an electrochemical sensor for nonenzymatic glucose detection. ACS Sustain. Chem. Eng. 2018, 6, 1909–1918.
- Gopalan, A.I.; Muthuchamy, N.; Komathi, S.; Lee, K.P. A novel multicomponent redox polymer nanobead based high performance non-enzymatic glucose sensor. Biosens. Bioelectron 2016, 84, 53–63.
- Keen, O.S.; Baik, S.; Linden, K.G.; Aga, D.S.; Love, N.G. Enhanced Biodegradation of Carbamazepine after UV/H2O2 Advanced Oxidation. Environ. Sci. Technol. 2012, 46, 6222–6227.
- Wei, Y.; Zhang, Y.; Liu, Z.; Guo, M. A Novel Profluorescent Probe for Detecting Oxidative Stress Induced by Metal and H2O2 in Living Cells. Chem. Commun. 2010, 46, 4472–4474.
- Pramanik, D.; Dey, S.G. Active Site Environment of Hemebound Amyloid Peptide Associated with Alzheimer’s Disease. J. Am. Chem. Soc. 2011, 133, 81–87.
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative Diseases and Oxidative Stress. Nat. Rev. Drug Discov. 2004, 3, 205–214.
- Finkel, T.; Serrano, M.; Blasco, M.A. The Common Biology of Cancer and Ageing. Nature 2007, 448, 767–774.
- Chen, X.; Wu, G.; Cai, Z.; Munetaka Oyama, X. Chen Advances in enzyme-free electrochemical sensors for hydrogen peroxide, glucose, and uric acid. Microchim. Acta 2014, 181, 689–705.
- Chen, S.; Yuan, R.; Chai, Y.; Hu, F. Electrochemical sensing of hydrogen peroxide using metal nanoparticles: A review. Microchim. Acta 2013, 180, 15–32.
- Yuan, L.; Lin, W.; Xie, Y.; Chen, B.; Zhu, S. Single Fluorescent Probe Responds to H2O2, NO, and H2O2/NO with Three Different Sets of Fluorescence Signals. J. Am. Chem. Soc. 2012, 134, 1305–1315.
- Yang, P.; Tong, X.; Wang, G.; Gao, Z.; Guo, X.; Qin, Y. NiO/SiC nanocomposite prepared by atomic layer deposition used as a novel electrocatalyst for nonenzymatic glucose sensing. ACS Appl. Mater. Interfaces 2015, 7, 4772–4777.
- Su, L.; Feng, J.; Zhou, X.; Ren, C.; Li, H.; Chen, X. Colorimetric detection of urine glucose based ZnFe2O4 magnetic nanoparticles. Anal. Chem. 2012, 84, 5753–5758.
- Mohammed, N.; Baidya, A.; Murugesan, V.; Kumar, A.A.; Ganayee, M.A.; Mohanty, J.S.; Tam, K.C.; Pradeep, T. Diffusion Controlled Simultaneous Sensing and Scavenging of Heavy Metal Ions in Water Using Atomically Precise Cluster Cellulose Nanocrystal Composites. ACS Sustain. Chem. Eng. 2016, 4, 6167–6176.
- Akhtar, N.; El-Safty, S.A.; Abdelsalam, M.E.; Shenashen, M.A.; Kawarada, H. Radially oriented nanostrand electrodes to boost glucose sensing in mammalian blood. Biosens. Bioelectron. 2016, 77, 656–665.
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 2010, 102, 29–45.
- Ekin, S.; Zeynep, A. Significance of nanomaterials in electrochemical glucose sensors: An updated review (2016–2020). Biosens. Bioelectron. 2020, 112165.
- Scognamiglio, V. Nanotechnology in glucose monitoring: Advances and challenges in the last 10 years. Biosens. Bioelectron. 2013, 47, 12–25.
- Chen, A.C.; Chatterjee, S. Nanomaterials based electrochemical sensors for biomedical applications. Chem. Soc. Rev. 2013, 42, 5425–5438.
- Aydogdu, G.; Zeybek, D.K.; Pekyardimci, S.; Kilic, E. A novel amperometric biosensor based on ZnO nanoparticles-modified carbon paste electrode for determination of glucose in human serum. Artif. Cells Nanomed. Biotechnol. 2013, 41, 332–338.
- Cash, K.J.; Clark, H.A. Nanosensors and nanomaterials for monitoring glucose in diabetes. Trends Mol. Med. 2010, 16, 584–593.
- Xue, B.; Li, K.; Feng, L.; Lu, J.; Zhang, L. Graphene wrapped porous Co3O4/NiCo2O4 double-shelled nanocages with enhanced electrocatalytic performance for glucose sensor. Electrochim. Acta 2017, 239, 36–44.
- Jiang, D.; Chu, Z.; Peng, J.; Luo, J.; Mao, Y.; Yang, P.; Jin, W. One-step synthesis of three-dimensional Co(OH)2/rGO nano-flowers as enzyme-mimic sensors for glucose detection. Electrochim. Acta 2018, 270, 147–155.
- Mao, Y.; Mei, Z.; Liang, L.; Zhou, B.; Tian, Y. Robust and magnetically recoverable dual-sensor particles: Real-time monitoring of glucose and dissolved oxygen. Sens. Actuators B Chem. 2018, 262, 371–379.
- Li, Y.; Niu, X.; Tang, J.; Lan, M.; Zhao, H. A comparative study of nonenzymatic electrochemical glucose sensors based on Pt-Pd nanotube and nanowire arrays. Electrochim. Acta 2014, 130, 1–8.
- Zang, G.; Hao, W.; Li, X.; Huang, S.; Gan, J.; Luo, Z.; Zhang, Y. Copper nanowires-MOFs-graphene oxide hybrid nanocomposite targeting glucose electro-oxidation in neutral medium. Electrochim. Acta 2018, 277, 176–184.
- Xu, H.; Xia, C.; Wang, S.; Han, F.; Akbarib, M.K.; Hai, Z.; Zhuiykov, S. Electrochemical non-enzymatic glucose sensor based on hierarchical 3D Co3O4/Ni heterostructure electrode for pushing sensitivity boundary to a new limit. Sens. Actuators B Chem. 2018, 267, 93–103.
- Jia, L.; Wei, X.; Lv, L.; Zhang, X.; Duan, X.; Xua, Y.; Liu, K.; Wang, J. Electrodeposition of hydroxyapatite on nickel foam and further modification with conductive polyaniline for non-enzymatic glucose sensing. Electrochim. Acta 2018, 280, 315–322.
- Lv, J.; Wei, X.; Lv, L.; Zhang, X.; Duan, X.; Xu, Y.; Liu, K.; Wang, J. Facile synthesis of novel CuO/Cu2O nanosheets on copper foil for high sensitive nonenzymatic glucose biosensor. Sens. Actuators B Chem. 2017, 248, 630–638.
- He, M.; Xuedong, W.; Tai, Z.; Ling, H.; Qun, W.; Daoping, R.; Tongliang, H.; Falin, T.; Huimin, W.; Jimin, G. A nanocomposite consisting of gold nanobipyramids and multiwalled carbon nanotubes for amperometric nonenzymatic sensing of glucose and hydrogen peroxide. Mikrochim. Acta 2019, 186, 235.
- Yin, D.; Bo, X.; Liu, J.; Guo, L. A novel enzyme free glucose and H2O2 sensor based on 3D graphenme aerogels with Ni3N nanoparticles. Anal. Chim. Acta 2018, 1038, 11–20.
- Balamurugan, J.; Thanh, T.D.; Karthikeyan, G.; Lee, N.H.K.J.H. A novel hierarchical 3D N-Co-CNT@NG nanocomposite electrode for non-enzymatic glucose and hydrogen peroxide sensing applications. Biosens. Bioelectron. 2017, 89, 970–977.
- Lu, W.; Sun, Y.; Dai, H.; Ni, P.; Jiang, S.; Wang, Y.; Li, Z.; Li, Z. Direct growth of pod like Cu2O nanowires arrays on copper foam: Highly sensitive and efficient non enzymatic glucose and H2O2 biosensor. Sens. Actuators B 2016, 231, 860–866.
- Deepalakshmi, T.; Tran, D.T.; Kim, N.H.; Chong, K.T.; Lee, J.H. Nitrogen-Doped Graphene-Encapsulated Nickel Cobalt Nitride as a Highly Sensitive and Selective Electrode for Glucose and Hydrogen Peroxide Sensing Applications. ACS Appl. Mater. Interfaces 2018, 10, 35847–35858.
- Wu, X.; Li, F.; Zhao, C.; Qian, X. One-step construction of hierarchical Ni(OH)2/RGO/Cu2O on Cu foil for ultra-sensitive non-enzymatic glucose and hydrogen peroxide detection. Sens. Actuators B Chem. 2018, 274, 163–171.
- Zhang, E.; Xie, Y.; Ci, S.; Jia, J.; Wen, Z. Porous Co3O4 hollow nanododecahedra for nonenzymatic glucose biosensor and biofuel cell. Biosens. Bioelectron. 2016, 81, 46–53.
- Liu, L.; Wang, Z.; Yang, J.; Liu, G.; Li, J.; Guo, L.; Chen, S.; Guo, Q. NiCo2O4 nanoneedle-decorated electrospun carbon nanofiber nanohybrids for sensitive non-enzymatic glucose sensors. Sens. Actuators B Chem. 2018, 258, 920–928.
- Yoon, H.; Xuan, X.; Jeong, S.; Park, J.Y. Wearable, robust, non-enzymatic continuous glucose monitoring system and its in vivo investigation. Biosens. Bioelectron. 2018, 117, 267–275.




